Abstract
Background and aim
Enhanced recovery after surgery (ERAS) programs, following a variety of perioperative treatments with evidence‐based medical evidence, has indicated its validity to accelerate rehabilitation in a wide variety of surgical procedures. This randomized controlled trial (RCT) study was implemented to verify the safety and efficacy of the perioperative effects in patients undergoing hepatectomy with ERAS or with conventional surgery (CS).
Methods
From August 2016 to November 2017, according to the inclusion criteria, 160 patients with liver diseases were suitable for participating in this experiment. Patients before liver resection were randomized into ERAS group (n = 80) and CS group (n = 80), and then the outcome measures were compared between the two groups.
Results
Enhanced recovery after surgery group had significantly less complications than CS group (P = .009). Compared with CS group, patients in ERAS group had low peak of WBCs in postoperative day (POD1), ALT in POD1 and POD3 (P < .05), high value of ALB in POD3 and POD5 (P < .05), less pain and higher patient satisfaction (P < .001), earlier exhaust, oral feeding, ambulation and extubation time (P < .05),and also had less hospital stay and cost (P < .001). There were no significant differences in readmission rate (<30 days) between two groups (P = .772).
Conclusions
Enhanced recovery after surgery programs applied to patients undergoing hepatectomy can safely and effectively relieve stress response, reduce the incidence of complications, improve patient satisfaction, accelerate patient recovery, reduce financial burden, and bring economic benefits.
Keywords: enhanced recovery after surgery, hepatectomy, perioperative treatments, randomized controlled trial
Abbreviations
- ALB
albumin
- ALT
alanine transaminase
- ASA
American Society of Anesthesiologists
- CS
conventional surgery
- ERAS
enhanced recovery after surgery
- POD
postoperative day
- RCT
randomized controlled trial
- TBIL
total bilirubin
- WBCs
white blood cells
1. INTRODUCTION
Enhanced recovery after surgery (ERAS) was first introduced by Kehlet in 1990s and was shown to reduce the complication rate and hospital stay duration after colorectal surgery.1 Its core concept has been used in a variety of perioperative treatments to relieve the physical and psychological stress, including preoperative education, epidural or regional anesthesia, perioperative fluid management, minimally invasive techniques, optimal pain control, early initiation of oral feeding, and mobilization. In recent years, ERAS protocols have been applied to various types of surgery at many institutes worldwide, including gastric,2 colorectal,3 gynecologic,4 vascular,5 and urologic6 procedures.
In liver disease, such as complicated hepatolithiasis and liver neoplasm, partial hepatectomy always acted as the preferred treatment. As the concepts of precise hepatectomy and anatomical hepatectomy were put forward, the minimally invasive surgery was gradually applied in hepatectomy. Nowadays, laparoscopic small range hepatectomy was coming to be a standard surgery. Laparoscopic partial hepatectomy had the advantages of minor trauma, lighter body reaction and accelerated rehabilitation, which reflected more highlights of ERAS programs. In China, ERAS has been applied to many hepatobiliary surgery centers in the past 3 years.7, 8 Since ERAS project started in August 2016, ERAS has been actively implemented to hepatectomy in our department.
Several studies and meta‐analyses have shown that patients obtained many benefits such as less pain, earlier intestinal function recovery, shorter hospital stay, less medical expenses, and higher medical satisfaction by implementing the ERAS programs.9, 10, 11, 12 To provide further evidence for effectiveness of ERAS programs in hepatectomy, a RCT was carried out to compare the perioperative effects between ERAS group and CS group in patients undergoing hepatectomy.
2. METHODS
2.1. Participants
From August 2016 to November 2017, all patients aged 18‐70 years who had received partial hepatectomy caused by various liver diseases at the First Affiliated Hospital of University of South China were included in this study. Operation in all patients with liver resection were performed by the same team of surgeons with the experience of over 500 cases of hepatectomy. The team were skilled in partial hepatectomy in our department including laparoscopic or open left lateral hepatectomy, left hemihepatectomy, and anatomical segment hepatectomy. The inclusion criteria included (1) No mental disease and physical activity disorder; (2) No serious heart, lung, brain or renal dysfunction; (3) No history of malignancy; (4) Child‐Pugh class A/B liver function; (5) Complete data, informed consent and cooperation.
The study was approved by the ethics committee of the First Affiliated Hospital of University of South China. All patients signed informed consent to participate in the randomized treatment.
2.2. Perioperative procedures
Patients randomly assigned to the ERAS group received the perioperative procedures of ERAS programs strictly, and the CS group patients received the traditional care. The process took the project as the main line including preoperative, intraoperative, and postoperative procedures (Table 1), which were designed with reference to ERAS guidelines.13 The surgery was implemented as soon as possible after random allocation. The minimally invasive surgery should be preferential considered. The choice of incision should have the advantage of a good surgical field and an accurate space to complete the operation.
Table 1.
Perioperative procedures
| ERAS group | CS group |
|---|---|
| Preoperative | |
| Perioperative information about ERAS education | Educate in standard manner |
| Assess nutritional status by NRS‐2002 and give enteral nutrition | No |
| No routine bowel preparation | Routine bowel preparation |
| Oral carbohydrates 400 mL 2 h before operation | No |
| Intraoperative | |
| Middle‐ thoracic epidural anesthesia (local anesthetic + low‐dose opioid) combined tracheal intubation and general anesthesia | Routine tracheal intubation and general anesthesia |
| Target oriented fluid infusion and low central venous pressure | Standard mode |
| Wear stretch hose | No |
| Routine medical insulation blanket and heated transfusion | No |
| No nasogastric tube or removed as early as possible | Routine nasogastric tube drainage |
| Minimal use of abdominal drain | Standard use of abdominal drains |
| Postoperative | |
| Adopt preventive, timely and multimodal analgesia | On‐demand analgesia |
| Drinking 6 h, 24 h feeding fluid and gradually transition to normal diet | Eat after anus exhausts |
| 12 h after surgery: mobilize out of bed at least four times per day 24 h after surgery: mobilization 4 times daily 48 h after surgery to discharge date: normal mobilization | No mobilization plan |
| Remove catheter 12 h after surgery | Remove catheter after mobilization |
| Early remove abdominal drainage tube if no fistula | No |
| Check the discharge criteria and leave hospital | Leave hospital as standard time |
CS, conventional surgery; ERAS, enhanced recovery after surgery.
2.3. Outcome measures
The primary outcome measures were the rate of complications (evaluation by Clavien–Dindo classification14), hospital stay and cost. The secondary outcome measures were postoperative laboratory indexes (POD1, POD3, and POD5), the time of postoperative exhaust, oral feeding, ambulation, and drainage tube removal, the rate of readmission (<30 days), pain score, and patient satisfaction. The postoperative laboratory indexes included white blood cells (WBCs) and hepatic function of postoperative day 1 (POD1), POD3, POD5. The hepatic function contained the value of POD1, POD3, and POD5 alanine transaminase (ALT), total bilirubin (TBIL), and albumin (ALB). The Wong‐Baker faces pain rating scale (Score>4 points) was used for pain assessment as the moderate or above pain.15 The inpatient satisfaction questionnaire was used to assess patient satisfaction following the questionnaire of Peking Union Medical College Hospital.16
The discharge criteria in groups followed the recommendations by van Dam RM et al17 of Maastricht University Medical Centre: (1) Normal or decreasing serum bilirubin; (2) Good pain control with oral analgesic only; (3) Tolerance of solid food; (4) No intravenous fluids; (5) Patient mobility being independent or at the preoperative level; (6) Patients wish to go home.
The termination criteria in each group included (1) Severe complications or deaths occurred in the study; (2) The patient had no experimental compliance; (3) The patient requested to withdraw from the study.
2.4. Sample size calculation
The purpose of this clinical study was to investigate the safety and effectiveness of the ERAS programs applied to the hepatectomy, and reduce the incidence rate of complications. According to the published literature,18, 19 compared with the CS group, the ERAS group has predicted 18% difference in the overall complication rates. Assuming a type‐I error of 5% (ɑ = 0.05), a power of 80% for a two‐tailed log‐rank test (β = 0.2), the minimum sample size required for each group was 72 cases, and about 10% termination of the experiment, each sample size was actually set to 80 cases.
2.5. Random grouping method
The computer‐generated random numbers were equally allocated to two groups. A nurse who was not in experimental study prepared a random digital envelope and sent it to patient. After the patients were randomly assigned to the ERAS group or the CS group, we arranged a doctor and another nurse in our department to inform the patients about the perioperative procedures and collect relevant data to make sure the procedures were correctly implemented and the data were objective.
2.6. Statistical treatment
The statistical information including patient general data, clinical indicators, and perioperative index were collected accurately. Statistical analysis was performed by the SPSS (version 19.0) software. The measurement data were represented by mean standard deviation (Mean ± SD), the independent sample t test was used to compare the measurement data between groups. Chi‐square test and Fisher's exact test were used to compare the counting data between groups. P value < .05 was considered to be statistically significant.
3. RESULTS
3.1. Experimental complete situation
During the experimental period, hepatectomy was carried out in 195 patients. According to the inclusion criteria, only 160 patients were suitable for participating in this experiment. Before randomization, 35 patients were excluded (5 patients were due to age limitation, 9 patients have been carried out emergency surgery, 11 patients have got terminal hepatic malignancy, and 10 patients declined to participate). After randomization, no patient was excluded to the study, the details were shown in Figure 1.
Figure 1.
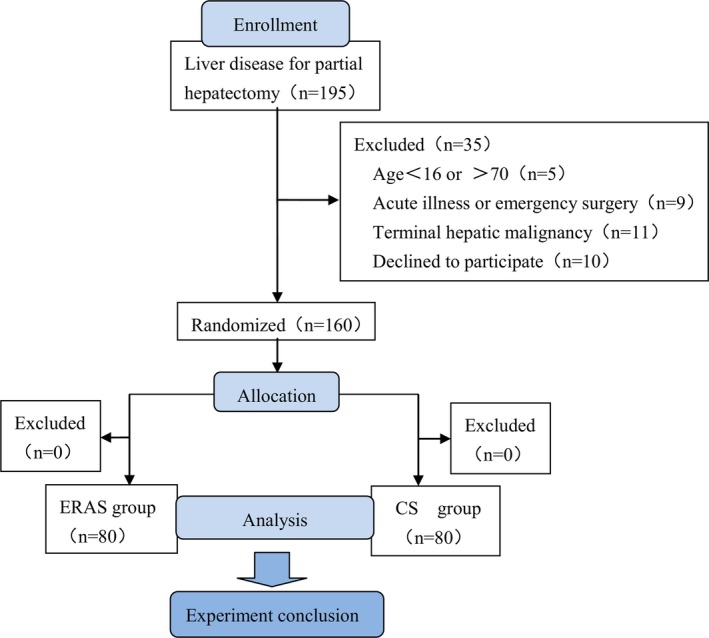
Flow chart. ERAS, enhanced recovery after surgery; CS, conventional surgery
3.2. Comparability of two groups
The general information of the two groups was shown in Table 2. There were no significant differences in age, gender, hepatitis B carrier, Child‐Pugh class, ASA grade, and type of hepatopathy between two groups (P > .05). There were also no significant differences in operation time, bleeding volume between two groups (P > .05). In both groups, the range of hepatectomy including hepatectomy segments > 2 and hepatectomy segments ≤ 2 had no significant differences (P = .623). In ERAS group, the rate of laparoscopic hepatectomy was 48.75% (39 of 80 patients, 4 patients converted to open hepatectomy), and in CS group was 38.75% (31 of 80 patients, 3 patients converted to open hepatectomy), there were also no significant differences in the rate of laparoscopic hepatectomy (P = .362).
Table 2.
Comparison of preoperative and intraoperative conditions
| ERAS group (n = 80) | CS group (n = 80) | P | |
| Preoperative | |||
| Age (years)a | 53.7 ± 9.8 | 55.4 ± 9.2 | .247 |
| Gender (male/female) | 47/33 | 40/40 | .267 |
| Hepatitis B carrier | 22 | 28 | .306 |
| Child‐Pugh class (A/B) | 8/72 | 13/67 | .242 |
| ASA grade (II/III) | 10/60 | 12/68 | .646 |
| Type of hepatopathy | |||
| Liver tumor | 36 | 32 | .522 |
| hepatolith | 44 | 48 | |
| Intraoperative | |||
| Operation time (hours)a | 4.9 ± 1.5 | 5.3 ± 1.4 | .100 |
| Bleeding volume (deciliters)a | 1.7 ± 1.5 | 2.0 ± 1.3 | .273 |
| Range of hepatectomy | |||
| Hepatectomy segments > 2 | 28 | 31 | .623 |
| Hepatectomy segments ≤ 2 | 52 | 49 | |
| Type of hepatectomy | |||
| Laparoscopic hepatectomy | 39 | 31 | .362 |
| Open hepatectomy | 37 | 46 | |
| Conversion to open hepatectomy | 4 | 3 | |
ASA, American society of anesthesiologists; CS, conventional surgery; ERAS, enhanced recovery after surgery.
Mean ± SD.
3.3. Comparison of postoperative clinical indexes
The complications were defined according to the grading system by Clavien–Dindo showed in Table 3. There was no perioperative mortality in the two groups. The morbidity rate of complications was 28.75% (23 of 80 patients) in the ERAS group and 48.75% (39 of 80 patients) in the CS group (P = .009). No patient in both groups suffered a deep vein thrombosis. The postoperative complications Grade II‐V in ERAS group and CS group was 18.75% vs 32.5%, respectively (P = .046). One patient in the ERAS group and two patients in the CS group underwent reoperation because of hemorrhage. There were no grade IV and grade V complications in both groups.
Table 3.
Surgical complications by Clavien–Dindo classification
| ERAS group (n = 80) | CS group (n = 80) | P | |
|---|---|---|---|
| Overrall complications | 23 (28.75) | 39 (48.75) | .009 |
| Grade I | |||
| Nausea/vomiting | 5 (6.25) | 9 (11.25) | .263 |
| Wound infection | 3 (3.75) | 4 (5) | .687 |
| Deep vein thrombosis | 0 (0.0) | 0 (0.0) | N/A |
| Grade II | |||
| Liver failure | 3 (3.75) | 5 (6.25) | .468 |
| iGrade IIIa | |||
| Pleural effusion | 5 (6.25) | 10 (12.5) | .175 |
| Bile leakage | 3 (3.75) | 7 (8.75) | .191 |
| Intraperitoneal inflammation | 3 (3.75) | 2 (2.5) | .650 |
| Grade IIIb | |||
| Hemorrhage > 1000 mL and reoperation | 1 (1.25) | 2 (2.5) | .560 |
| Grade IVa | 0 (0.0) | 0 (0.0) | N/A |
| Grade IVb | 0 (0.0) | 0 (0.0) | N/A |
| Grade V | 0 (0.0) | 0 (0.0) | N/A |
| Complications Grade II‐V | 15 (18.75) | 26 (32.5) | .046 |
Values are presented as n (%).
CS, conventional surgery; ERAS, enhanced recovery after surgery.
Comparison of laboratory indexes in POD1, POD3, and POD5 were shown in Figure 2. Compared with CS group, the ERAS group had lower number of WBCs in POD1 (P < .001), ALT in POD1 and POD3 (P < .05), TBIL in POD5 (P = .003), but had higher number of ALB in POD3 and POD5 (P < .05).
Figure 2.
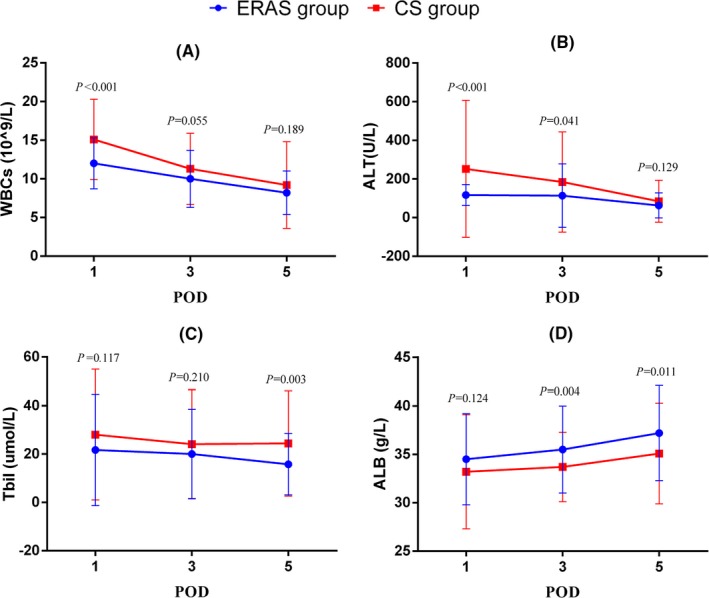
Comparison of postoperative laboratory indexes, including POD1, POD3, POD5 value of WBCs (A) ALT (B), TBIL (C), and ALB (D). ERAS, enhanced recovery after surgery; CS, conventional surgery; POD, postoperative day
Comparison of pain score was shown in Figure 3A. In ERAS group, most patients scored below three points, but the patients in CS group scored more than four points, which indicated that more patients in CS group had already suffered moderate to severe pain (P < .001). Comparison of patient satisfaction was shown in Figure 3B. The satisfaction in ERAS group was significantly higher than the CS group (P < .001).
Figure 3.
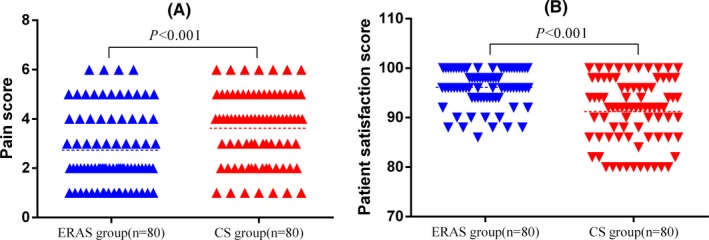
(A) Comparison of pain score (B) Comparison of patient satisfaction. ERAS, enhanced recovery after surgery; CS, conventional surgery
Comparison of outcomes were shown in Table 4. The anus exhaust time in ERAS group was faster than the CS group (P < .001). In ERAS group, the oral enteral nutrition was earlier than the CS group (P = .003), most patients in ERAS group drinked some water eight hours after surgery. Most patients in ERAS group got out of bed earlier than patients in CS group (P < .001). The peritoneal drainage tube was removed much earlier in ERAS group than CS group of patients (P = .013), no patient required a reinsertion of drainage tube in both two groups. The median hospital stay was 16.9 ± 3.4 days in the ERAS group, which was significantly shorter than that in the CS group (21.6 ± 6.8 days; P < .001). The median cost of hospitalization was $7795 ± 1526 in ERAS group and $9138 ± 2115 in CS group (P < .001). In ERAS group, 6 patients (6.86%) were readmitted for wound dehiscence (2 patients), bile leak (2 patients) and ileus (2 patients). While in CS group, 7 patients (8.75%) were readmitted for wound dehiscence (1 patient), bile leak (2 patients), ileus (2 patients), and pain (2 patients). There were no significant differences in readmission rate (<30 days) between two groups (P = .772). No readmission was related to an acute life‐threatening condition in both groups.
Table 4.
Comparison of outcomes
| ERAS group (n = 80) | CS group (n = 80) | P | |
|---|---|---|---|
| Feeding time (hours)a | 64.0 ± 17.9 | 77.0 ± 26.4 | <.001 |
| Exhaust time (hours)a | 63.5 ± 16.9 | 73.4 ± 23.7 | .003 |
| Ambulation time (hours)a | 47.1 ± 20.1 | 65.9 ± 32.6 | <.001 |
| Extubation time (days)a | 7.9 ± 2.3 | 9.0 ± 3.4 | .013 |
| Hospital stay (days)a | 16.9 ± 3.4 | 21.6 ± 6.8 | <.001 |
| Cost (US dollar)a | 7795 ± 1526 | 9138 ± 2115 | <.001 |
| Readmission (<30 days)b | 6 (6.86) | 7 (8.75) | .772 |
ERAS, enhanced recovery after surgery, CS, conventional surgery.
Mean ± SD.
n (%).
4. DISCUSSION
In our study, we randomly selected 160 patients with different liver diseases, such as liver tumors and hepatolithiasis, so the sample size and diseases were convincing. Also, we made the ERAS implementation process of hepatectomy with project as the main line, which including preoperative, intraoperative, and postoperative procedures, every patient should be strictly followed, so the experiment could be completed in a planned way. Additionally, we used the inpatient satisfaction questionnaire to assess patient satisfaction following the questionnaire of Peking Union Medical College Hospital, which could ensure that patients have an objective evaluation of medical services.
But our study also has some limitations. First, we carried out the RCT only in a single center, the results of this study might not be very high level of evidence, so multi‐center RCT study should be carried out. Second, we had just completed a single‐blind trial, data collectors might have a certain orientation, thus the test results would be influenced, so double‐blind clinical trial also should be implemented. Finally, more clinical index should be studied which could reflect the superiority of ERAS, such as indocyanine green test (ICG), 3D printing, and other diagnostic technique. More study would be carried out in our center that the validity of ERAS could be ulteriorly verified.
The results of this RCT has showed the safety and efficacy of ERAS programs carried out in patients undergoing hepatectomy. As the morbidity rate of overall complications decreased in ERAS group after implementing the ERAS programs, which proved that ERAS programs can safely reduce surgical stress and accelerate patient recovery. We can see that compared with CS group, the patients in ERAS group had low peak of WBCs and ALT,also had higher number of ALB in postoperative days, which indicated that the less perioperative stress and liver function impairment. The pain scores were used to evaluate the effect of postoperative analgesia, the patient satisfaction reflected effectiveness of ERAS programs. With multimodal analgesia, most patients in ERAS group had a less pain than in CS group. ERAS can shorten exhaust, feeding and ambulation time, and effectively reduce stress response and make patients feel better in postoperative days, the patient satisfaction improve at the same time. All patients in ERAS group had lower hospital stay and cost compared with CS group, which indicated that with a low medical care expenditure ERAS programs could bring economic benefits and reduce social burden. The readmission rate (<30 days) was similar in ERAS and CS groups, which indicated that the scientific discharge criteria in ERAS programs would not increase the readmission rate. The results of RCT were consistent with the research of ERAS protocol in laparoscopic colorectal surgery by Pędziwiatr et al.20, 21, 22, 23
The advantages of ERAS concept manifest in more adequate preoperative preparation before surgery. The detailed preoperative education can reduce patient anxiety through communication.24, 25 The nutritional evaluation and support can ensure the nutritional status of the body for the surgery.26 Pulmonary exercise can accelerate the recovery of lung function after surgery.27 No bowel preparation as usual can prevent dehydration and disturbance of electrolyte.28 Oral carbohydrates 400 milliliters two hours before operation can relieve thirst, reduce anxiety, lower insulin resistance, and promote postoperative intestinal function recovery.29, 30
The advanced concept of surgery, anesthesia, infusion, heat preservation, and analgesia during the operation reflect the superiority of ERAS programs. The combined anesthesia can reduce surgical stress and relieve postoperation pain very well.31, 32 The minimally invasive surgery can reduce surgical trauma, but the good exposure of the surgical field and accurate completion of the surgical procedure should be considered first.33 Anatomical hepatectomy should be used which can help to clear the lesion thoroughly, reduce surgical bleeding, remain the maximum liver volume and relief surgical stress response. However, to ensure safe implementation of ERAS programs, the indications of laparoscopic hepatectomy must be strictly observed. Post‐anesthesia catheterize and no normally placed the stomach tube can reduce the pain and stress caused by the operation, and accelerate the recovery of the patients after surgery.34
ERAS can generate better postoperative pain relief and rehabilitation therapy model. Adopt preventive, timely and multimodal analgesia can relieve the pain caused by the surgery effectively.35 Early ambulation and oral nutrition can better maintain muscle function and promote the recovery of gastrointestinal peristalsis.36, 37 Early respiratory function training can promote the recovery of lung function.38 On the premise of ensuring safety, early extubation can relief postoperative pain and mental disturbances, also encourage patients to get out of bed earlier.39 The scientific discharge criteria can speed up the recovery of the patients’ physiology and psychology, reduce the postoperative complications, hospitalization time and expenses, reduce the financial burden on families and societies, bring economic benefits and increase the patient satisfaction degree of medical service.17
ERAS can be always improved, all patients undergoing surgery can benefit from this programs.40 For patients who have liver diseases and require hepatectomy, whether laparoscopic surgery or laparotomy, liver tumor or hepatolithiasis, segmental hepatectomy or hemihepatectomy, ERAS programs can effectively accelerate the perioperative rehabilitation.9, 41, 42 However, the ERAS programs cannot be successfully implemented without multidisciplinary collaboration, and it is essential to have teamwork from the departments of surgery, anesthesia, nursing, nutrition, rehabilitation, and other departments.43, 44
5. CONCLUSION
The presented study showed that ERAS programs were safe and effective for patients undergoing hepatectomy. Specifically, the ERAS programs can help patients relieve postoperative stress and pain, reduce the incidence of complications, cut down the hospital stay and expenses, not to increase the readmission rate, accelerate postoperative recovery, improve patient satisfaction, reduce financial burden, and bring economic benefits.
Trial Registration: The RCT study was retrospectively registered in the Chinese Clinical Trial Registry, the registration number was ChiCTR‐INR‐17012865.
CONFLICT OF INTEREST
The authors have no funding or conflict of interests to disclose.
Qi S, Chen G, Cao P, et al. Safety and efficacy of enhanced recovery after surgery (ERAS) programs in patients undergoing hepatectomy: A prospective randomized controlled trial. J Clin Lab Anal. 2018;32:e22434 10.1002/jcla.22434
Shuo Qi and Guodong Chen contributed equally to this work.
Contributor Information
Jun He, Email: hejunjxb@163.com.
Xiuda Peng, Email: xiudapengusc@163.com.
REFERENCES
- 1. Kehlet H, Slim K. The future of fast‐track surgery. Br J Surg. 2012;99:1025‐1026. [DOI] [PubMed] [Google Scholar]
- 2. Shrikhande SV, Pai E. Enhanced recovery after surgery in laparoscopic gastric cancer surgery: many questions, few answers. J Minim Access Surg. 2014;10:105‐106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alfonsi P, Slim K, Chauvin M, et al. French guidelines for enhanced recovery after elective colorectal surgery. J Visc Surg. 2014;151:65‐79. [DOI] [PubMed] [Google Scholar]
- 4. Modesitt SC, Sarosiek BM, Trowbridge ER, et al. Enhanced recovery implementation in major gynecologic surgeries: effect of care standardization. Obstet Gynecol. 2016;128:457‐466. [DOI] [PubMed] [Google Scholar]
- 5. Rockley M, Chu K, Bayne J. Current perioperative practice in Canadian vascular surgery. Can J Surg. 2015;58:374‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Merelli E, Sabbatini A, Zagallo E, et al. Enhanced recovery protocol after urological surgery and nutrition: initial experience of the European Institute of Oncology (IEO) in Milan. Clin Nutr ESPEN. 2016;12:e40. [Google Scholar]
- 7. Liang X, Ying H, Wang H, et al. Enhanced recovery program versus traditional care in laparoscopic hepatectomy. Medicine (Baltimore). 2016;95:e2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu X, Wang Y, Feng T, et al. Nonstrict and individual enhanced recovery after surgery (ERAS) in partial hepatectomy. Springerplus. 2016;5:2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Connor S, Cross A, Sakowska M, et al. Effects of introducing an enhanced recovery after surgery programme for patients undergoing open hepatic resection. HPB (Oxford). 2013;15:294‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hughes MJ, McNally S, Wigmore SJ. Enhanced recovery following liver surgery: a systematic review and meta‐analysis. HPB (Oxford). 2014;16:699‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Małczak P, Pisarska M, Piotr M, et al. Enhanced recovery after bariatric surgery: systematic review and meta‐analysis. Obes Surg. 2017;27:226‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ni TG, Yang HT, Zhang H, et al. Enhanced recovery after surgery programs in patients undergoing hepatectomy: a meta‐analysis. World J Gastroenterol. 2015;21:9209‐9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melloul E, Hubner M, Scott M, et al. Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg. 2016;40:2425‐2440. [DOI] [PubMed] [Google Scholar]
- 14. Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien‐Dindo classification of surgical complications: five‐year experience. Ann Surg. 2009;250:187‐196. [DOI] [PubMed] [Google Scholar]
- 15. Garra G, Singer AJ, Domingo A, et al. The Wong‐Baker pain FACES scale measures pain, not fear. Pediatr Emerg Care. 2013;29:17‐20. [DOI] [PubMed] [Google Scholar]
- 16. Sun J, Hu G, Ma J, et al. Consumer satisfaction with tertiary healthcare in China: findings from the 2015 China National Patient Survey. Int J Qual Health Care. 2017;29:213‐221. [DOI] [PubMed] [Google Scholar]
- 17. van Dam RM, Hendry PO, Coolsen MM, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg. 2008;95:969‐975. [DOI] [PubMed] [Google Scholar]
- 18. Schultz NA, Larsen PN, Klarskov B, et al. Evaluation of a fast‐track programme for patients undergoing liver resection. Br J Surg. 2013;100:138‐143. [DOI] [PubMed] [Google Scholar]
- 19. Page AJ, Gani F, Crowley KT, et al. Patient outcomes and provider perceptions following implementation of a standardized perioperative care pathway for open liver resection. Br J Surg. 2016;103:564‐571. [DOI] [PubMed] [Google Scholar]
- 20. Pędziwiatr M, Pisarska M, Kisielewski M, et al. Is ERAS in laparoscopic surgery for colorectal cancer changing risk factors for delayed recovery? Med Oncol. 2016;33:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pisarska M, Pędziwiatr M, Małczak P, et al. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study Int J Surg 2016;36: 377‐382. [DOI] [PubMed] [Google Scholar]
- 22. Pędziwiatr M, Pisarska M, Kisielewski M, et al. ERAS protocol in laparoscopic surgery for colonic versus rectal carcinoma: are there differences in short‐term outcomes? Med Oncol. 2016;33:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pędziwiatr M, Wierdak M, Nowakowski M, et al. Cost minimization analysis of laparoscopic surgery for colorectal cancer within the enhanced recovery after surgery (ERAS) protocol: a single‐centre, case‐matched study. Wideochir Inne Tech Maloinwazyjne. 2016;11:14‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foss M. Enhanced recovery after surgery and implications for nurse education. Nurs Stand. 2011;25:35‐39. [DOI] [PubMed] [Google Scholar]
- 25. Lewicka M, Makara‐Studzinska M, Sulima M, et al. Intensification of anxiety and depression, and personal resources among women during the peri‐operative period. Ann Agric Environ Med. 2014;21:91‐97. [PubMed] [Google Scholar]
- 26. Lohsiriwat V. The influence of preoperative nutritional status on the outcomes of an enhanced recovery after surgery (ERAS) programme for colorectal cancer surgery. Tech Coloproctol. 2014;18:1075‐1080. [DOI] [PubMed] [Google Scholar]
- 27. Pouwels S, Fiddelaers J, Teijink JA, et al. Preoperative exercise therapy in lung surgery patients: a systematic review. Respir Med. 2015;109:1495‐1504. [DOI] [PubMed] [Google Scholar]
- 28. Miralpeix E, Nick AM, Meyer LA, et al. A call for new standard of care in perioperative gynecologic oncology practice: impact of enhanced recovery after surgery (ERAS) programs. Gynecol Oncol. 2016;141:371‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inoda A, Nagata H, Otsuka K, et al. Effect of oral rehydration therapy before general anesthesia on satisfaction, stress response, and hemodynamics in surgical patients for laparoscopic colectomy. Masui. 2015;64:285‐293. [PubMed] [Google Scholar]
- 30. Tamura T, Yatabe T, Kitagawa H, et al. Oral carbohydrate loading with 18% carbohydrate beverage alleviates insulin resistance. Asia Pac J Clin Nutr. 2013;22:48‐53. [DOI] [PubMed] [Google Scholar]
- 31. Fawcett WJ, Baldini G. Optimal analgesia during major open and laparoscopic abdominal surgery. Anesthesiol Clin. 2015;33:65‐78. [DOI] [PubMed] [Google Scholar]
- 32. Sun HZ, Song YL, Wang XY. Effects of different anesthetic methods on cellular immune and neuroendocrine functions in patients with hepatocellular carcinoma before and after surgery. J Clin Lab Anal. 2016;30:1175‐1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ratti F, Cipriani F, Reineke R, et al. Impact of ERAS approach and minimally‐invasive techniques on outcome of patients undergoing liver surgery for hepatocellular carcinoma. Dig Liver Dis. 2016;48:1243‐1248. [DOI] [PubMed] [Google Scholar]
- 34. Ichida H, Imamura H, Yoshimoto J, et al. Randomized controlled trial for evaluation of the routine use of nasogastric tube decompression after elective liver surgery. J Gastrointest Surg. 2016;20:1324‐1330. [DOI] [PubMed] [Google Scholar]
- 35. Wong‐Lun‐Hing EM, van Dam RM, Welsh FK, et al. Postoperative pain control using continuous i.m. bupivacaine infusion plus patient‐controlled analgesia compared with epidural analgesia after major hepatectomy. HPB (Oxford) 2014;16:601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kato T, Miyata G, Kamei T. Early ambulation after surgery and perioperative rehabilitation. Nihon Geka Gakkai Zasshi. 2015;116:254‐259. [PubMed] [Google Scholar]
- 37. Abunnaja S, Cuviello A, Sanchez JA. Enteral and parenteral nutrition in the perioperative period: state of the art. Nutrients. 2013;5:608‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sebio Garcia R, Yanez Brage MI, Gimenez Moolhuyzen E, et al. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta‐analysis. Interact Cardiovasc Thorac Surg. 2016;23:486‐497. [DOI] [PubMed] [Google Scholar]
- 39. Sanchez‐Urdazpal Gonzalez L, Salido Fernandez S, Alday Munoz E, et al. Implementation of an ERAS program in liver surgery. Nutr Hosp. 2015;31(Suppl 5):16‐29. [DOI] [PubMed] [Google Scholar]
- 40. Slim K, Vignaud M. Enhanced recovery after surgery: the patient, the team, and the society. Anaesth Crit Care Pain Med. 2015;34:249‐250. [DOI] [PubMed] [Google Scholar]
- 41. He F, Lin X, Xie F, et al. The effect of enhanced recovery program for patients undergoing partial laparoscopic hepatectomy of liver cancer. Clin Transl Oncol. 2015;17:694‐701. [DOI] [PubMed] [Google Scholar]
- 42. Page AJ, Ejaz A, Spolverato G, et al. Enhanced recovery after surgery protocols for open hepatectomy–physiology, immunomodulation, and implementation. J Gastrointest Surg. 2015;19:387‐399. [DOI] [PubMed] [Google Scholar]
- 43. Hoffmann H, Kettelhack C. Fast Track Surgery ‐ conditions and challenges in post‐surgical treatment. Ther Umsch. 2012;69:9‐13. [DOI] [PubMed] [Google Scholar]
- 44. Pearsall EA, Meghji Z, Pitzul KB, et al. A qualitative study to understand the barriers and enablers in implementing an enhanced recovery after surgery program. Ann Surg. 2015;261:92‐96. [DOI] [PubMed] [Google Scholar]


