Abstract
Background
Hemolysis, Icterus, and Lipemia constituting the HIL index, are the most common causes of interference with accurate measurement in biochemistry. This study focuses on bilirubin interference, aiming to identify the analyses impacted and proposing a way to predict nominal interference‐free analyte concentrations, based on both analyte level and Icterus Index (I ict).
Methods
Sixteen common analytes were studied: alanine aminotransferase (ALT), albumin (ALB), alkaline phosphatase (ALP), amylase (AMY), aspartate aminotransferase (AST), total cholesterol (CHOLT), creatinine (CREA, enzymatic method), fructosamine (FRUC), gamma‐glutamyl transferase (GGT), HDL cholesterol (HDLc), total iron (Iron), lipase (LIP), inorganic phosphorus (Phos), total protein (PROT), triglycerides (TG), and uric acid (UA). Both the traditional 10% change in concentrations from baseline and the Total Change Level (TCL) were taken as acceptance limits. Nineteen pools of sera covering a wide range of values were tested on the Cobas® 6000 (Roche Diagnostics). I ict ranged from 0 to 60.
Results
Eight analytes increased (FRUC and Phos) or decreased (CHOLT, CREA, HDLc, PROT, TG, and UA) significantly when I ict increased. FRUC, HDLc, PROT, and UA showed a linear relationship when I ict increased. A non‐linear relationship was found for TG, CREA, and for CHOLT; this also depended on analyte levels. Others were not impacted, even at high I ict.
Conclusions
A method of estimating an interference‐free value for FRUC, HDLc, PROT, Phos, UA, TG, and CREA, and for CHOLT in cases of cholestasis, is proposed. I ict levels are identified based on analytical performance goals, and equations to recalculate interference‐free values are also proposed.
Keywords: bilirubin, biochemistry, correction, icterus index, interference
1. Introduction
Biochemical analysis of body fluids provides clinically useful information. Hemolysis, icterus, and lipemia (HIL) are the most common causes of blood sample inadequacy and interfere with the accurate measurement of various analytes.1, 2, 3, 4, 5
Icterus interferes via bilirubin (BIL), in two ways: physical interference (light absorption) and chemical interference (with reagent components as H2O2).6 For every assay method, a cut‐off value for Icterus (Icterus Index: I ict) is established by the manufacturer and indicated on the insert sheet,7 or recognized through laboratory experience. Therefore, a sample may be rejected if the I ict value exceeds this threshold. In the case of hemolysis (except if in vivo hemolysis), a new sample – without hemolysis – is required for accurate assay measurements. However, in some pathological circumstances, such as newborn jaundice or hepatic diseases like viral hepatitis or cirrhosis, a new sample without interference is not available. Yet these are precisely the cases where every chemical analysis counts for the physician, and rejection of assays above the cut‐off value could hinder treatment.
More recently, sophisticated chemical analyzers have automatically detected HIL status and have reported HIL index values. The HIL alert index (also known as the threshold level) is defined as the lowest concentration of HIL that interferes with chemical analyses, yielding a bias >10%.6
The aim of this study is to determine which analyses are impacted when I ict increases and to propose a method of predicting, when possible, nominal interference‐free analyte concentrations as a function of measured I ict.
2. Materials and Methods
This study was considered a quality assessment project and was therefore deemed exempt from ethics committee approval. It was conducted at La Conception Hospital, Biochemistry Laboratory, Marseille, France. To evaluate the effect of bilirubin on routine analytes, we analyzed the most common analytes reported by manufacturers to be affected by this interferent. We chose the most common, manufacturer‐reported interferents and tested different levels of bilirubin. We provide a comprehensive report on the effect of this interference on the analytes listed in Table 1.
Table 1.
Icterus interference cut‐off from Roche7 observed values and TCL (see abbreviation in text). For each analyte (column A), the tested range (column C) with the corresponding units (column B) is done. Roche's icterus interference cut‐off at 10% (column D) is compared to the value observed when there is 10% variation (column E). At least, I ict observed at TCL level (column F) is done. The values differ when TCL is not close to 10% (column G). Therefore, the interpretation is adapted to the analytical performance goals of the laboratory
| Column A | Column B | Column C | Column D | Column E | Column F | Column G |
|---|---|---|---|---|---|---|
| Tests | Units | Range tested (min‐max) | I ict 10% proposal from Roche | I ict observed at 10% variation | TCL (%) | I ict observed at TCL (column F) |
| ALT | U/L | 12‐359 | 60 | >60 | 11.3 | >60 |
| ALB | g/L | 22.8‐37.9 | 60 | >60 | 5.3 | 45 |
| ALP | U/L | 48‐381 | 60 | >60 | 7.1 | 58 |
| AMY | U/L | 30‐158 | 60 | >60 | 6.3 | >60 |
| AST | U/L | 14‐734 | 60 | >60 | 9 | >60 |
| CHOLT | mmol/L | 2.06‐6.72 | 14 | 14 | 4.7 | 7 |
| CREA | μmol/L | 55‐912 | 15 | 13 | 6.0 | 6 |
| FRUC | mmol/L | 139‐228 | 5 | 5 | 9.5 | 5 |
| GGT | U/L | 26‐179 | 20 | >60 | 8.9 | >60 |
| HDLc | mmol/L | 0.5‐1.74 | 30 | 20 | 6.9 | 12 |
| Iron | μmol/L | 5.14‐27 | 60 | >60 | 14.3 | >60 |
| LIP | U/L | 21‐410 | 50 | >60 | 13.1 | >60 |
| Phos | mmol/L | 0.73‐1.95 | 60 | >60 | 5.2 | 34 |
| PROT | g/L | 45.2‐67.5 | 20 | 16 | 5.2 | 6 |
| TG | mmol/L | 0.81‐3.64 | 10 | 20 | 11 | 21 |
| UA | μmol/L | 204‐670 | 40 | 43 | 5.3 | 20 |
We studied the effect of icterus interference on alanine aminotransferase (ALT, IFCC with pyridoxal phosphate), albumin (ALB, bromocresol green), alkaline phosphatase (ALP, IFCC), amylase (AMY, IFCC), aspartate aminotransferase (AST, IFCC with pyridoxal phosphate), total cholesterol (CHOLT, enzymatic), creatinine (CREA, enzymatic), fructosamine (FRUC, nitroblue tetrazolium), gamma‐glutamyl transferase (GGT, IFCC/Szasz), HDL cholesterol (HDLc, direct method), total iron (Iron, ferrozine), lipase (LIP, diglyceride substrat), inorganic phosphorus (Phos, molybdate, UV), total protein (PROT, Biuret), triglycerides (TG, enzymatic), uric acid (UA, uricase). Samples were tested on the Cobas® 6000 (Roche Diagnostics, Meilan, France). All assays were performed according to manufacturer's instructions. Nineteen pools were realized covering a wide range of values. As a preliminary, to be pooled, each serum was analyzed for triglycerides and tested to ensure that it was without detectable concentrations of free hemoglobin and bilirubin (HIL≤1).
A 10 mmol/L bilirubin stock solution was prepared with physiological sera from of commercially available human conjugated bilirubin (ref B850 from Frontier Scientific, Logan, UT, USA) and stored away from light at −20°C. Each pool was aliquoted (about 1 mL) and spiked with 100 μL of an extemporaneous dilution of bilirubin stock solution (with physiological sera) in order to obtain I ict ranging from 0 to 60 in 16 samples and immediately measured for the analytes listed above, as well as for total bilirubin (TBIL) and I ict.
Given the lack of studies on icterus interference using Roche Diagnostics analyzers and the conflicting published results, our three objectives were: (1) to verify I ict quality specifications on Roche Diagnostics analyzers for chemistry testing, considering both the traditional 10% change in concentration from baseline and the Total Change Level (TCL) as acceptance limits,8, 9 (2) to determine the proportion of tests impacted by icterus beyond these allowable limits and (3) to propose a method of predicting, when possible, nominal interference‐free analyte concentrations as a function of measured I ict.
Two approaches were used to establish analytical performance goals:
The mean percentage deviation was compared to the Acceptable Change Limit (ACL), according to ISO 5725‐6.10 The ACL for interpreting a measured difference is based on the analytical imprecision (CVa), using the formula ACL = 2.77 CVa.
The second approach took into account acceptable imprecision, based on intra‐individual biological variation. According to the College of American Pathologists recommendations, the imprecision of a method, for individual single and multipoint testing, should be equal or less than one‐half of the average within‐subject variation (CVb), and this should be the goal for short‐term laboratory imprecision (≤0.5 CVb). The CVb of each analyte was taken from the listing of biological variation for 316 analytes by Ricos et al.11 This database was most recently updated in 2014, but some analytes are still missing.12
To monitor changes in an analyte from the same sample for the same individual, we combined the two approaches (analytical and intra‐individual imprecisions) by estimating the square root of the sum of the squared analytical and biological imprecisions, defined as the total change limit (TCL):
The TCL is the square root of the sum of squared analytical reproducibility (CVa) (obtained from Roche Diagnostics QC) and biological imprecision (CVb) (found in “Ricos database”11, 12):
Based on the results observed, a function between I ict and the variation in the analyte (in %) is estimated and optimized, depending on whether a linear or differing relationship to level is found.
We define X′ as the interference‐free calculated value, X as value of the analyte at I ict, and X0 as the original value of the analyte (when I ict≤1) corresponding to the unspiked pool. The analytes variation (in %) when I ict increase is D for the observed values and Z for the calculated values after correction.
Working from the I ict held in the laboratory, this relationship is used to estimate X′ from all the observed values and the I ict.
Based on results, the observed variation (D) in the analytes (in %) is D = 100 (X–X0)/X0. Whereas, D is also a function of I ict and is generally a linear equation, so D = a + b × I ict.
X′ is calculated from I ict and X. After changing X0 for X′, the interference‐free value is calculated from the relationship: X′ = X/(D + 1). Therefore, knowing X and I ict makes it possible to estimate X′.
The % of difference between X′ and X0 can be calculated. The goal is reached if less than 5% of Z (where Z = 100 (X′−X0)/X0)) gives a value below the TCL.
Data analysis was performed in Excel 2013 (Microsoft Inc., Redmond, WA, USA).
3. Results
As I ict was determinate by a simple measure of two wavelengths (at 505 and 480 nm) with hemoglobin and lipemia correction, however, it is not a direct determination of TBIL level. So, we observe that there is a linear correlation between I ict and TBIL (Figure 1). The same correlation is found with I ict and direct bilirubin (data not shown). Nevertheless, a better correlation is observed with a second degree equation, where TBIL = 3.89.10−2 × (I ict)2 + 10.6 × I ict + 5.21 (R 2 = 0.9954).
Figure 1.
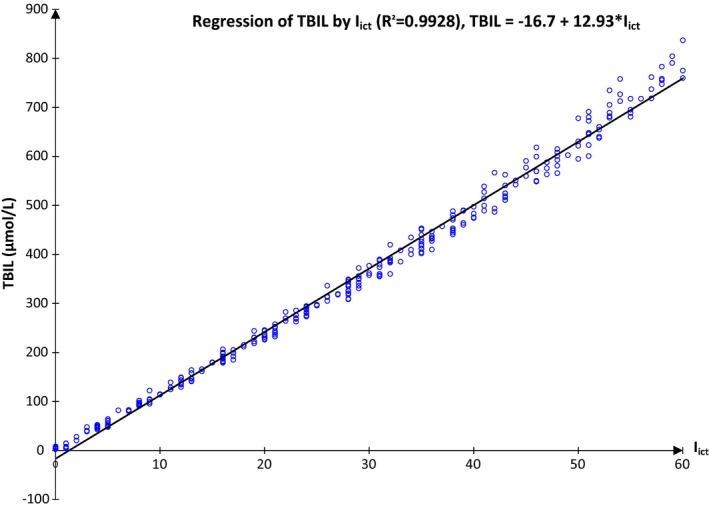
Relation between I ict and TBIL (μmol/L; see abbreviation in text). A linear relation is observed between I ict and TBIL. As I ict is always available (and not TBIL), a relation between I ict and the interference on the analytes should be studied
Different types of relation between an analyte and I ict are observed (Figure 2) as positive interference a negative interference and non‐linear variation and two‐level interference.
Figure 2.
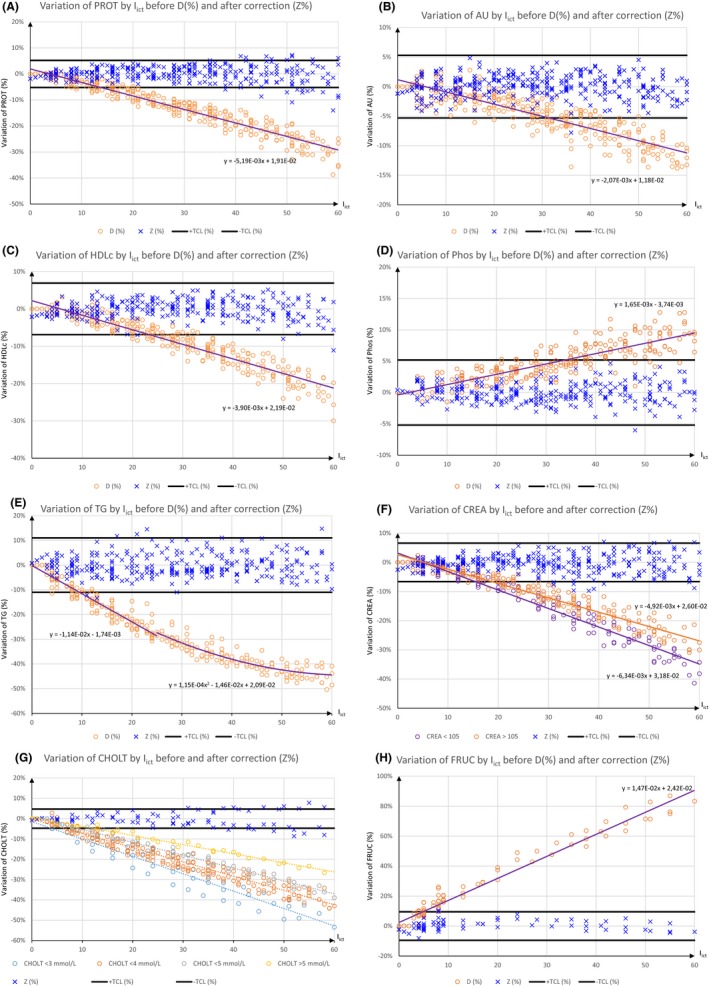
Relation between I ict and analytes. Observed variation before D (%) and after correction Z (%), allowable cut‐off as ±TCL (%) and equations between I ict and D (%) are shown for PROT (A), AU (B), Phos (C), HDLc (D),TG (E), CREA (F), CHOLT (G) and FRUC (H). CHOLT is subdivided from <3 mmol/L to >5 mmol/L. The interference with CHOLT depends on level of this analyte. For CHOL (G), Z (%) is valid only when CHOLT >4 mmol/L and I ict≤40. In this last case (G), equation is 8.38 × 10−3 − 5.41 × 10−3x (see abbreviation in text)
The range of tested analytes and the 10% icterus interference cut‐off from Roche Diagnostics and the cut‐off from the observed values and from TCL are included in Table 1. Eight analytes increase or decrease significantly when I ict increases. CHOLT, CREA, HDLc, PROT, TG, and UA decrease, while FRUC and Phos increase. Figure 2 shows also for these eight analytes the equations between I ict and D. The allowed cutoff, TCL, is included and the figure shown the impact of I ict before (D) and after correction (Z).
Between CHOLT and I ict, the relation is more complex (Figure 2). For CHOLT, for the same decrease, 20% for example, I ict ranges from 20 to 50 depending on CHOLT level. In the same manner, for the same I ict, 30 for example, CHOLT decrease ranges from −12.8% to −24.3%. Therefore, Figure 2g show the complexity of the relation, based on I ict but also on CHOLT level. In this case, Z is done only for CHOLT>4. In this condition, if I ict≤40, the Z bias is less than 5%, when TCL is used as acceptable limit.
4. Discussion and Conclusion
While previous studies indicate that the chemical properties of bilirubin, whether conjugated (cBIL) or unconjugated (ucBIL), do not contribute to differences in interference,5 Roche Diagnostics gives differing levels of interference for I ict with cBIL and ucBIL. Bilirubin may be present in some pathological circumstances, and therefore sera analysis could be impacted.
Our conclusion here is that Roche Diagnostics suggested icterus interference cut‐off yield a bias ≥10%, except for GGT and TG (Table 1). For GGT, Roche Diagnostics indicates an I ict cut‐off at 20, as no influence was observed in our study, which is in agreement with.13 For TG, Roche Diagnostics suggested cut‐off is half the value we observed. Roche Diagnostics consistently refers to Glick et al. 14 on HIL interference. In this publication, TG seems to increase when bilirubin is added. However, data show the opposite (Figure 2). In the latest available dataset,7 Roche Diagnostics indicates that a decrease is observed when I ict is above 10. Moreover, Roche Diagnostics even refers to Glick et al. on HIL interference for FRUC, HDLc and Iron, which are not tested in Glick's publication. For CREA, although there is now an enzymatic method, Roche Diagnostics still refers to the same study (CREA with Jaffé method). Manufacturer's data are not always confirmed in laboratory practice.1, 2, 15 The conclusion differs when TCL is used as acceptance limit. Interference cut‐off is therefore recalculated and generally emerges as lower than the arbitrary 10% cut‐off. However, even with TCL cut‐off, we observed that some analytes are not impacted by plasmatic bilirubin, even at high concentrations. Parameters not impacted by plasmatic bilirubin are ALP, ALT, AMY, AST, GGT, Iron, and LIP (Figure 2). These results are in agreement with the Roche Diagnostics dataset 7 and other studies.3, 5, 16 One parameter is affected (ALB), but only at high I ict, above 45, which corresponds to about 563 μmol/L of bilirubin (Figure 1).
For analytes that decrease, we found that the degree of icterus interference differs according to analyte. A linear relationship was found for FRUC, HDLc, PROT, and UA. A non‐linear relationship (Figure 2) was found for TG, and for two analytes (CREA and CHOLT), where it also depended on analyte concentrations.17 For five analytes, FRUC, HDLc, PROT, Phos and UA, a simple correction with a linear equation gives the interference‐free value (X′). The equations are easy to use and only require the values for the analyte regarding I ict level and I ict. As these two parameters are available, it is possible to estimate the true analyte level. However, Ali et al.18 show that there are two levels of interference for PROT, tested at 30 and 74 g/L, which are outside our studied range. For TG, due to the nature of the relationship, with I ict and % of interference, two equations are needed. One equation applies to low levels of I ict with a linear relationship, such as FRUC, HDLc, PROT, P, and UA. However, when I ict is above 25, the relationship is not linear, and a second‐degree equation is needed. In both cases, the interference‐free value (X′) remains easy to estimate. For CREA, the correction is more complicated. CREA from Roche Diagnostics involves an enzymatic method used at the end of reaction, a Trinder reaction. For the same I ict, the lower CREA is, the greater the decrease. Therefore, X′ requires an intermediate step to be calculated. Because the decrease in CREA depends on both CREA level and I ict, a cut‐off level at 105 μmol/L, the upper limit reference value, must be calculated. If (107.73−0.5167 × I ict<105) then the equation for low CREA could be used, and the second linear equation, for high CREA, otherwise. Therefore, the correction would take both normal and pathological CREA values into account. A corrected value for CREA can be used to estimate the glomerular filtrate rate (GFR) more precisely, including CKD‐EPI, with obvious impact on clinical interpretation. For all these parameters (FRUC, HDLc, PROT, Phos, UA, TG, and CREA), the interference‐free value (X′) is close to the “true” value. The Z bias is always less than 5% when TCL is used as an acceptable variation (Figure 2). For CHOLT no simple relationship has been found.19 When icterus is due to cBIL (cholestasis), cholesterolemia generally increases and the X′ value must be calculated with the equation using high cholesterol (>4 mmol/L) spiked samples. In this condition, if I ict ≤40, the Z bias is less than 5%, when TCL is used as acceptable limit. The proposed correction is not suitable if cholesterol is lower than 4 mmol/L or if I ict >40.
The protocol used here is both simple and efficient, requiring only the dilution of cBIL with physiological sera to spike the pool of sera. We chose to spike the sera pool with cBIL because it is totally water‐soluble. Previous studies have often used ucBIL.5, 6, 20 However, in this case, ucBIL needs to be solubilized at high pH due to NaOH. The resulting pH increase could induce:
Spectral change in bilirubin,21, 22, 23 transformation of bilirubin to biliverdin.22, 24
Possible change in analytical conditions for some analytes.
A possible explanation for Bilirubin's interference is the following. CHOLT, CREA, HDLc, TG, and UA involve the same steps for determination in the analyzer. At the last step of the reaction, catalyzed by oxidase or peroxidase, the liberated hydrogen peroxide reacts with compounds to form a quinone imine chromogen (TRINDER reaction). The color intensity of the quinone imine chromogen formed is directly proportional to the analyte concentration in the reaction mixture.
However, Bilirubin interferes in oxidase/peroxidase‐based test systems.6, 19 Proportionally to its concentration, bilirubin reacts with H2O2 formed in the test system, which in turn generates systematically lower results in enzymatic procedures used for the measurement of CHOLT, CREA, HDLc, TG, and UA.
In a strongly acidic solution, the absorption of conjugated bilirubin shifts to the UV wavelengths (340 nm). Therefore, bilirubin interferes in the determination of Phos via the phosphomolybdate method through its reducing effect,25, 26, 27 because of the sulfuric medium. In an alkaline solution, the increase in absorption as a result of oxidation of bilirubin to biliverdin, which shows a broad band at about 650 nm,28, 29 is the main cause of bilirubin interference25 with FRUC and PROT. Furthermore, to some extent, ucBIL in alkaline solution can react with copper to form Bil‐Copper complex, which can contribute to PROT decrease.30, 31, 32, 33
This study presents some limitations. First, the results are based on assays using Roche Diagnostics analyzers for chemistry testing. Further assays realized on other diagnostic analyzers might alter the conclusions. Based on Roche Diagnostics documentation,7 others analytes in biochemistry that could be impacted by icterus are acid phosphatase, creatinine with Jaffé method, lithium and glucose (GOD PAP). None of these analytes are tested in our laboratory with these methods. Second, our results are validated on the studied range (table 1), and extrapolations outside this range should not be made. Third, results are validated for the TCL used in our laboratory. TCL values are calculated in such a way as to always obtain less than 5% of Z outside TCL after correction. Fourth, no overall solution appears for CHOLT when I ict increases. Finally, a totally enzymatic determination of creatinine plasmatic values would probably not involve the interference shown here.
In conclusion, this study shows that only some biochemical laboratory tests are impacted by icterus. For FRUC, HDLc, PROT, Phos, UA, TG, and CREA, we propose a method of estimating the interference‐free value. Only a partial solution was found for CHOLT. Based on I ict and the observed value, a corrected value can be reached, as is done for calcium using protein level. The suggestion to correct laboratory values that have interferences present is generally considered to be ill‐advised this concept has been explored previously for hemolysis34 and, in this case, the consensus is that correction may work for a data series, but would fail for individual patients. However, the methodology used here for Icterus, with significant data (n>300 values), the determination of the interference‐free value which was under TCL, may be used for individual patients. The equation can easily be integrated into the MPL Roche Diagnostic analyzer®. I ict levels are based on analytical performance goals, and equations to recalculate interference‐free values are also proposed. It appears that the impact of the arbitrary 10% threshold differs according to analyte (sodium vs LIP, for example). This suggests that cut‐off levels should be assessed using the most appropriate methods as they evolve (CREA via enzymatic vs Jaffe, for example) and according to analyte. Finally, for the most important parameter, CREA, proper estimation is essential to establish the correct posology regarding GFR. With regard to icterus, CREA is generally underestimated, but our estimating method offers a good interference‐free value suitable for clinicians.
Acknowledgments
The authors wish to thank Elisabeth DE VILLE D'AVRAY for her help in determining dosages and Marjorie Sweetko for proofreading the manuscript.
Nicolay A, Lorec A‐M,Gomez G, Portugal H. Icteric human samples: Icterus index and method of estimating an interference‐free value for 16 biochemical analyses. J Clin Lab Anal. 2018;32:e22229 10.1002/jcla.22229
References
- 1. Monneret D, Mestari F, Atlan G, et al. Hemolysis indexes for biochemical tests and immunoassays on Roche analyzers: determination of allowable interference limits according to different calculation methods. Scand J Clin Lab Invest. 2015;75:162‐169. [DOI] [PubMed] [Google Scholar]
- 2. Agarwal S, Vargas G, Nordstrom C, Tam E, Buffone GJ, Devaraj S. Effect of interference from hemolysis, icterus and lipemia on routine pediatric clinical chemistry assays. Clin Chim Acta. 2015;438:241‐245. [DOI] [PubMed] [Google Scholar]
- 3. Shin DH, Kim J, Uh Y, et al. Development of an integrated reporting system for verifying hemolysis, icterus, and lipemia in clinical chemistry results. Annals Lab Med. 2014;34:307‐312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Salvagno GL, Lippi G, Gelati M, Guidi GC. Hemolysis, lipaemia and icterus in specimens for arterial blood gas analysis. Clin Biochem. 2012;45:372‐373. [DOI] [PubMed] [Google Scholar]
- 5. Ji JZ, Meng QH. Evaluation of the interference of hemoglobin, bilirubin, and lipids on Roche Cobas 6000 assays. Clin Chim Acta. 2011;412:1550‐1553. [DOI] [PubMed] [Google Scholar]
- 6. Clinical Laboratory and Standards Institute . Hemolysis, Icterus, and Lipemia/Turbidity Indices as Indicators of Interference in Clinical Laboratory Analysis. Approved guideline, C56‐A PA, Wayne: CLSI; 2012:52. [Google Scholar]
- 7. Roche global package inserts and application reports . Reagents on Roche/Hitachi Systems: List of Interferences Based on Serum Indices for Serum and Plasma, 26th edn Bazel, Switzerland: Roche Diagnostics; 2014. [Google Scholar]
- 8. Oddoze C, Lombard E, Portugal H. Stability study of 81 analytes in human whole blood, in serum and in plasma. Clin Biochem. 2012;45:464‐469. [DOI] [PubMed] [Google Scholar]
- 9. Plumelle D, Lombard E, Nicolay A, Portugal H. Influence of diet and sample collection time on 77 laboratory tests on healthy adults. Clin Biochem. 2014;47:31‐37. [DOI] [PubMed] [Google Scholar]
- 10. ISO 5725‐6 . Accuracy (trueness and precision) of measurement methods and results. Part 6: use in practice of accuracy values. 1994.
- 11. Ricos C, Alvarez V, Cava F, et al. Desirable quality specifications for total error, imprecision, and bias, derived from biological variation. http://www.Westgard.com/biodatabase1.htm. Accessed December 7, 2017.
- 12. Ricos C, Alvarez V, Cava F, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999;59:491‐500. [DOI] [PubMed] [Google Scholar]
- 13. Szoke D, Braga F, Valente C, Panteghini M. Hemoglobin, bilirubin, and lipid interference on Roche Cobas 6000 assays. Clin Chim Acta. 2012;413:339‐341. [DOI] [PubMed] [Google Scholar]
- 14. Glick MR, Ryder KW, Jackson SA. Graphical comparisons of interferences in clinical chemistry instrumentation. Clin Chem. 1986;32:470‐475. [PubMed] [Google Scholar]
- 15. Nikolac N. Lipemia: causes, interference mechanisms, detection and management. Biochem Medica. 2014;24:57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Z, Guo H, Wang Y, Kong F, Wang R. Interfering effect of bilirubin on the determination of alkaline phosphatase. Int J Clin Exp Med. 2014;7:4244‐4248. [PMC free article] [PubMed] [Google Scholar]
- 17. Nah H, Lee S‐G, Lee K‐S, Won J‐H, Kim HO, Kim J‐H. Evaluation of bilirubin interference and accuracy of six creatinine assays compared with isotope dilution‐liquid chromatography mass spectrometry. Clin Biochem. 2016;49:274‐281. [DOI] [PubMed] [Google Scholar]
- 18. Ali D, Secechetto E, Reigner A, et al. Lipemia and bilirubin influences for twenty‐four biochemiacal parameters measurement. Ann Biol Clin. 2015;73:671‐689. [DOI] [PubMed] [Google Scholar]
- 19. Portugal H, Pauli AM, Sastre B, Pastor J. Enzymatic determination of cholesterol in bile without interference by bilirubin. Ann Biol Clin. 1993;51:119‐124. [PubMed] [Google Scholar]
- 20. Chong YK, Mak CM, Lam HL, Lau MH, Leung DC. Bi‐variate approach to negative interference of bilirubin towards an acetaminophen assay. Clin Biochem. 2015;48:186‐188. [DOI] [PubMed] [Google Scholar]
- 21. Brodersen R. Bilirubin. Solubility and interaction with albumin and phospholipid. J Biol Chem. 1979;254:2364‐2369. [PubMed] [Google Scholar]
- 22. McDonagh AF, Lightner DA. Photooxidation of bilirubin to biliverdin and bilirubin structure. J Chem Educ. 2008;85:199‐U191. [Google Scholar]
- 23. De Matteis F, Lord GA. Desferrioxamine dehydrogenates bilirubin in two stages, leading to a 1:1 red‐coloured adduct. Characterization of the products by high‐performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun Mass Spectrom. 2008;22:4055‐4065. [DOI] [PubMed] [Google Scholar]
- 24. Lightner DA, Holmes DL, McDonagh AF. On the acid dissociation constants of bilirubin and biliverdin – pK(alpha) values from C‐13 NMR spectroscopy. J Biol Chem. 1996;271:2397‐2405. [DOI] [PubMed] [Google Scholar]
- 25. Grafmeyer D, Bondon M, Manchon M, Levillain P. The influence of bilirubin, haemolysis and turbidity on 20 analytical tests performed on automatic analysers. Eur J Clin Chem Clin Biochem. 1995;33:31‐52. [DOI] [PubMed] [Google Scholar]
- 26. Duncanson GO, Worth HGJ. Pseudohypophosphataemia as a result of bilirubin interference. Ann Clin Biochem. 1990;27:253‐257. [DOI] [PubMed] [Google Scholar]
- 27. Vaezi MF, Lacamera RG, Richter JE. Validation studies of Bilitec 2000: an ambulatory duodenogastric reflux monitoring system. Am J Physiol – Gastroint Liver Physiol. 1994;267:G1050‐G1057. [DOI] [PubMed] [Google Scholar]
- 28. Heirwegh KPM, Blanckaert N, Vanhees G. Synthesis, chromatographic purification, and analysis of isomers of biliverdin IX and bilirubin IX. Anal Biochem. 1991;195:273‐278. [DOI] [PubMed] [Google Scholar]
- 29. To T‐L, Fadul MJ, Shu X. Singlet oxygen triplet energy transfer‐based imaging technology for mapping protein–protein proximity in intact cells. Nat Commun. 2014;5:4072. doi: 10.1038/ncomms5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li WH, Shen GR, Soloway RD, et al. Copper bilirubinate and black pigment gallstone. Biospectroscopy. 1995;1:149‐156. [Google Scholar]
- 31. Tiribelli C, Ostrow JD. New concepts in bilirubin and jaundice: Report of the Third International Bilirubin Workshop, April 6‐8, 1995, Trieste. Italy. Hepatology. 1996;24:1296‐1311. [DOI] [PubMed] [Google Scholar]
- 32. Adhikari S, Joshi R, Gopinathan C. Bilirubin as an anti precipitant against copper mediated denaturation of bovine serum albumin: formation of copper‐bilirubin complex. Biochim Biophys Acta‐Gen Subj. 1998;1380:109‐114. [DOI] [PubMed] [Google Scholar]
- 33. Chromy V, Svachova L, Novosad L, et al. Albumin‐based or albumin‐linked calibrators cause a positive bias in serum proteins assayed by the biuret method. Clin Chem Lab Med. 2009;47:91‐101. [DOI] [PubMed] [Google Scholar]
- 34. Mansour MMH, Azzazy HME, Kazmierczak SC. Correction factors for estimating potassium concentrations in samples with in vitro hemolysis A detriment to patient safety. Arch Pathol Lab Med. 2009;133:960‐966. [DOI] [PubMed] [Google Scholar]


