Abstract
Background
In this study, the significance of fibrinogen concentration assessed by a combination of Clauss and prothrombin time (PT)‐derived methods for screening for congenital dysfibrinogenemia were investigated, and the screening efficiency of fibrinogen PT‐derived/Clauss ratio on congenital dysfibrinogenemia was analyzed.
Methods
We compared fibrinogen concentrations determined by the Clauss, PT‐derived, and enzyme‐linked immunosorbent assay (ELISA) methods in 73 patients with congenital dysfibrinogenemia and 81 normal controls. Receiver operating characteristic (ROC) curves were utilized to evaluate the efficacy of fibrinogen PT‐derived/Clauss ratio in screening for congenital dysfibrinogenemia.
Results
Fibrinogen concentrations determined by the Clauss method were dramatically lower than by the PT‐derived method and ELISA, and correlated poorly with the latter two methods in patients with congenital dysfibrinogenemia. Fibrinogen concentrations in normal controls were slightly lower according to the Clauss method than to the PT‐derived method and ELISA; however, each method yielded results within the normal range and the correlation was good. The area under the ROC curve of fibrinogen PT‐derived/Clauss ratio for diagnosis of congenital dysfibrinogenemia was 1 with a standard error of 0, 95% confidence interval of 0.976‐1.00, and optimal critical diagnosis point of 1.43. When fibrinogen PT‐derived/Clauss ratio was >1.43, the sensitivity and specificity for diagnosis of congenital dysfibrinogenemia were both 100%.
Conclusions
The combined use of Clauss and PT‐derived methods for determining fibrinogen concentrations improves the efficiency of screening for congenital dysfibrinogenemia, as the fibrinogen PT‐derived/Clauss ratio has high sensitivity and specificity in diagnosis of congenital dysfibrinogenemia. This ratio could serve an important screening tool for this disease.
Keywords: congenital dysfibrinogenemia, diagnosis, fibrinogen Clauss method, fibrinogen PT‐derived/Clauss ratio, PT‐derived method
Abbreviations
- PT
prothrombin time
- ROC
receiver operating characteristic
- ELISA
enzyme‐linked immunosorbent assay
1. INTRODUCTION
Fibrinogen, a glycoprotein with coagulation function, is synthesized by the liver. It is converted into soluble fibrin by thrombin and finally converted into a stable insoluble fibrin clot after cross‐linking with Factor XIIIa and Ca2+.1, 2 Fibrinogen, a ligand for platelet glycoprotein IIb/IIIa, is involved in platelet aggregation.3 Congenital fibrinogen deficiencies include quantitative deficiencies (afibrinogenemia and hypofibrinogenemia) and qualitative deficiencies (dysfibrinogenemia and hypodysfibrinogenemia) of fibrinogen.4 Congenital dysfibrinogenemia, a hereditary disease that causes dysfunctional fibrinogen, is caused by mutation in one of the fibrinogen genes. There is significant heterogeneity in the clinical manifestations of this disease, most patients being asymptomatic. Approximately 25% of patients experience bleeding, 20% have thrombosis, and some have both a bleeding tendency and thrombosis.5 Most asymptomatic patients are identified by routine coagulation tests, the diagnostic findings being an obviously prolonged thrombin time with normal or slightly prolonged prothrombin time (PT) and activated partial thromboplastin time. The PT‐derived method shows normal fibrinogen concentrations, whereas the Clauss method shows remarkably decreased fibrinogen activity.6, 7, 8
Quantitation of fibrinogen concentrations is an important screening tool for coagulation disorders, with screening methods comprising physical, chemical, immunological, and coagulable protein methods. The Clauss and PT‐derived methods are both coagulable protein methods. Many recent studies have focused on the Clauss and PT‐derived methods for quantitating fibrinogen concentrations, mainly in the general population or in patients receiving anticoagulant therapy.9, 10, 11 In this study, we measured plasma fibrinogen concentrations in 73 patients with congenital dysfibrinogenemia and 81 normal controls by the Clauss, PT‐derived, and immunological methods, compared their results. Receiver operating characteristic (ROC) curves were used to evaluate the value of fibrinogen PT‐derived/Clauss ratio for screening for congenital dysfibrinogenemia.
2. MATERIALS AND METHODS
This study was approved by the Ethics Committee of our institution and informed consent was obtained from all participants.
2.1. Plasma of normal controls
Plasma was collected from 81 healthy controls who were undergoing health checks. They comprised 31 male and 50 female persons aged 7‐81 years (mean 39.5 years). Their results of coagulation were normal. The PT‐derived method resulted in fibrinogen concentrations of 2‐5 g/L. Liver, kidney, heart, and lung function were normal. The controls included no menstruating or pregnant women.
2.2. Plasma of patients with congenital dysfibrinogenemia
Dysfibrinogenemia is caused by qualitative abnormalities in the fibrinogen molecule that leads to abnormal fibrinogen function. In the study, plasma was collected from 73 patients with congenital dysfibrinogenemia attending the First Affiliated Hospital of Guangxi Medical University of China from November 2014 to December 2016. This condition had been diagnosed based on clinical manifestations, coagulation assays, gene analysis, and family surveys. This group comprised 21 male and 52 female persons aged 2‐90 years (mean 41 years).
2.3. Specimen preparation
Plasma was obtained from venous blood drawn in the morning from fasting participants and anticoagulated by adding 0.109 mol/L sodium citrate in a 1:9 ratio. All specimens were centrifuged at 1006.2 × g for 10 minutes to produce platelet‐poor plasma, which was assessed within 2 hours.
2.4. Analysis of plasma specimens
Coagulation of sodium citrate‐anticoagulated whole blood was detected with an ACL TOP‐700 Automated Coagulation Analyzer (Instrumentation Laboratory, Milan, Italy) and the required reagents. Fibrinogen concentrations were determined by both the Clauss and PT‐derived methods. Antigenic fibrinogen concentrations were analyzed with a fibrinogen enzyme‐linked immunosorbent assay (ELISA) kit (Huamei Biological Engineering Co., Ltd, Hubei, China) using a ELISA reader Ceres 900 (Bio‐tek Instruments, Winooski, VT, USA).
2.5. Statistical analysis
Data were analyzed using SPSS 22.0 (SPSS, Chicago, IL, USA) software and are expressed as the mean ± standard deviation. Paired data were compared by the Student's paired t test. The means of two independent groups were compared by the independent samples t test. The relationships between two variables were analyzed using the Pearson correlation analysis. ROC curves were analyzed with Medcalc.v9.2.0 (MedCalc Software bvba, Ostend, Belgium) software; the accuracy of fibrinogen PT‐derived/Clauss ratio in the diagnosis of congenital dysfibrinogenemia was evaluated by the area under the ROC curve; and the optimal critical point of diagnosis was obtained by analyzing every point in the ROC curve to determine sensitivity and specificity. A value of P < .05 was considered to denote statistical significance.
3. RESULTS
3.1. Comparison of fibrinogen concentrations quantitated by the Clauss, PT‐derived, and ELISA methods
According to the Clauss method, fibrinogen concentrations were significantly lower in patients with congenital dysfibrinogenemia than in normal controls, whereas the fibrinogen PT‐derived/Clauss ratio showed them to be significantly higher. Intragroup comparison of the Clauss and PT‐derived methods showed that fibrinogen concentrations were significantly lower in patients with congenital dysfibrinogenemia when measured by the Clauss method. However, in normal controls, fibrinogen concentrations were slightly lower when measured by the Clauss method. Results of the Clauss and PT‐derived methods were both in the normal range. All the above differences were statistically significant (P < .05; Table 1).
Table 1.
Comparison of plasma fibrinogen concentrations in patients with congenital dysfibrinogenemia and normal controls according to the Clauss and PT‐derived methods
| Group | n | PT‐derived method (g/l) | Clauss method (g/l) | t | P | Fibrinogen PT‐derived/Clauss ratio |
|---|---|---|---|---|---|---|
| Congenital dysfibrinogenemia | 73 | 3.70 ± 0.88 | 0.62 ± 0.19 | 32.948 | <.001 | 6.24 ± 1.43 |
| Normal control | 81 | 3.95 ± 0.66 | 3.56 ± 0.70 | 11.262 | <.001 | 1.11 ± 0.11 |
| t | 1.943 | 36.399 | −30.495 | |||
| P | .054 | <.001 | <.001 |
PT, Prothrombin time.
The mean fibrinogen antigen measured by ELISA in normal controls was 3.89 ± 0.57 g/L and showed slightly higher values than measured by the Clauss method, nevertheless, no significant difference was found when comparing to the PT‐derived method. In patients with congenital dysfibrinogenemia, the mean fibrinogen antigen measured by ELISA was 3.64 ± 0.68 g/L, similar to the mean fibrinogen concentration measured by the PT‐derived method, and functional fibrinogen values measured by Clauss were markedly lower.
3.2. Analysis of correlation between fibrinogen concentrations quantitated by the Clauss, PT‐derived, and ELISA methods
There was a good correlation between fibrinogen concentrations determined by the Clauss and PT‐derived methods in normal controls (r = .900, P < .001; Figure 1A). However, there was a poor correlation in fibrinogen concentrations measured by each method in patients with congenital dysfibrinogenemia (r = .522, P < .001; Figure 1B).
Figure 1.
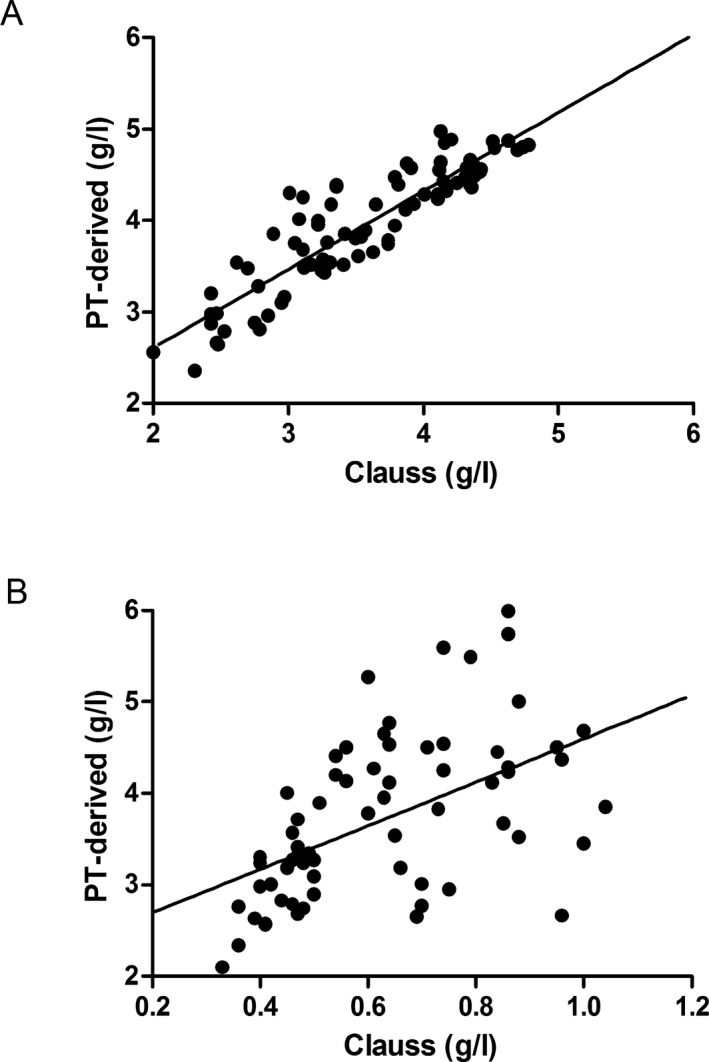
Scatterplot of fibrinogen concentrations measured by the Clauss and PT‐derived methods in normal controls (A) and in patients with congenital dysfibrinogenemia (B)
Fibrinogen concentrations determined by the Clauss method correlated well with ELISA in normal controls (r = .871, P < .001; Figure 2A). However, there was a poor correlation between these two methods in patients with congenital dysfibrinogenemia (r = .454, P < .001; Figure 2B).
Figure 2.
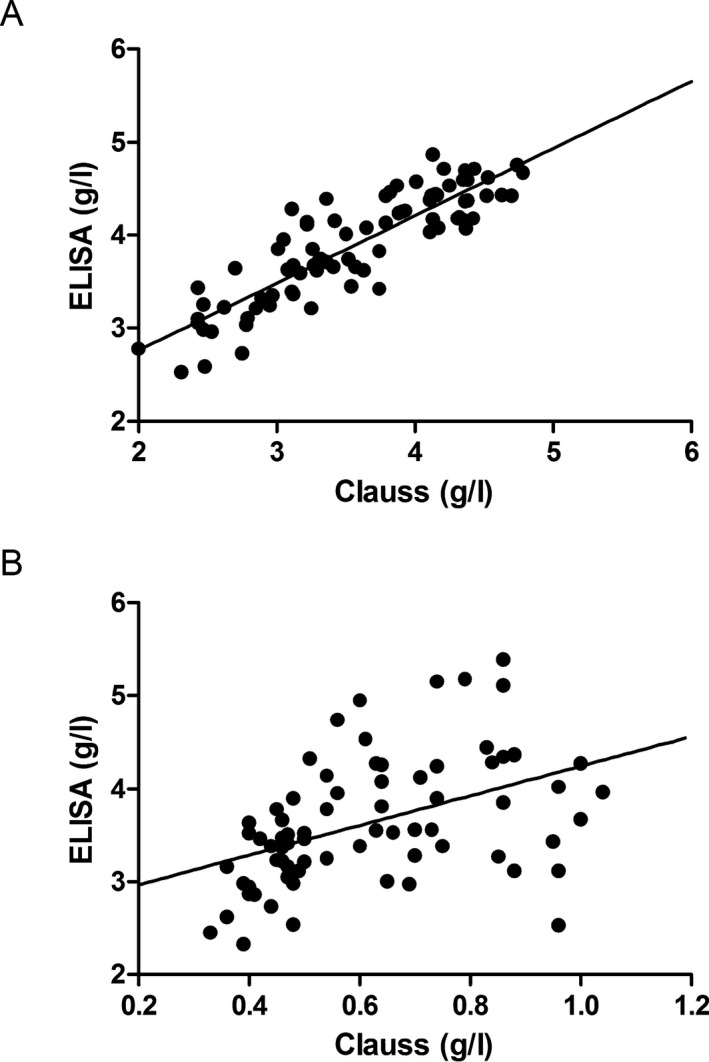
Scatterplot of fibrinogen concentrations measured by the Clauss method and ELISA in normal controls (A) and in patients with congenital dysfibrinogenemia (B)
Fibrinogen concentrations determined by the PT‐derived method had a good correlation with ELISA in both normal controls (r = .923, P < .001; Figure 3A) and in patients with congenital dysfibrinogenemia (r = .898, P < .001; Figure 3B).
Figure 3.
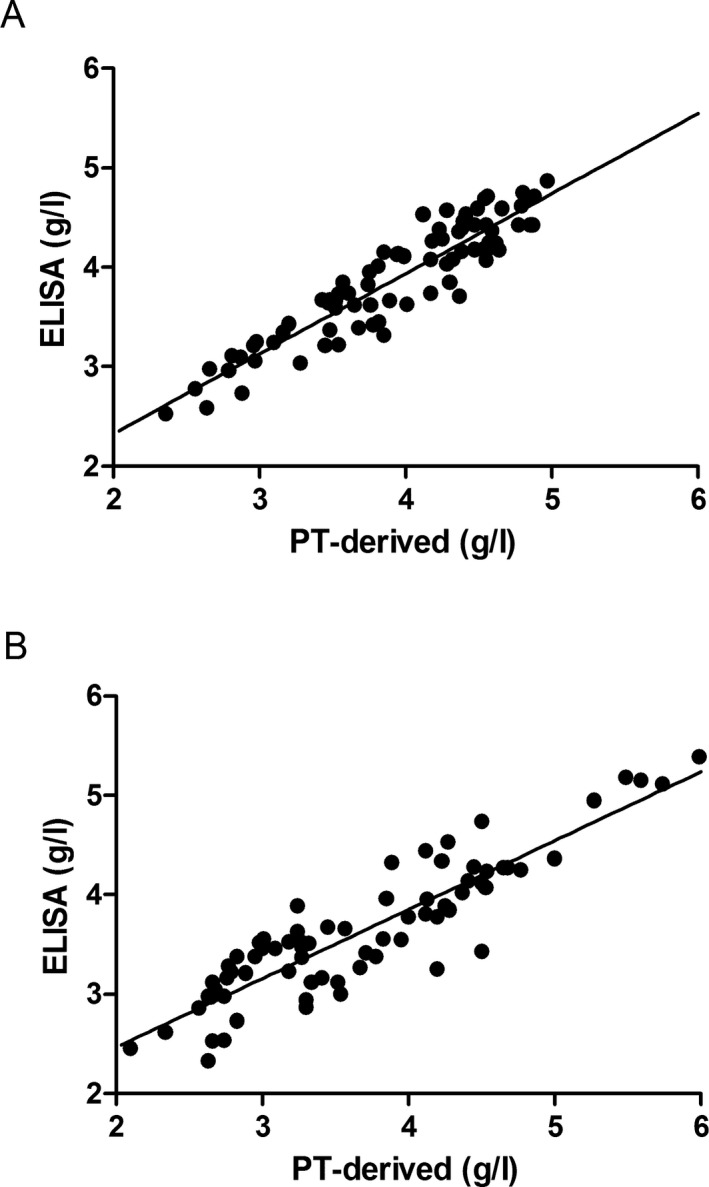
Scatterplot of fibrinogen concentrations measured by the PT‐derived method and ELISA in normal controls (A) and in patients with congenital dysfibrinogenemia (B)
3.3. ROC curves for the diagnosis of congenital dysfibrinogenemia using the fibrinogen PT‐derived/Clauss ratio
The area under the ROC curve was 1 with a standard error of 0, 95% confidence interval of 0.976‐1.000, and optimal critical diagnostic point of 1.43. When the fibrinogen PT‐derived/Clauss ratio was >1.43, the sensitivity and specificity for diagnosis of congenital dysfibrinogenemia were both 100% (Figure 4).
Figure 4.
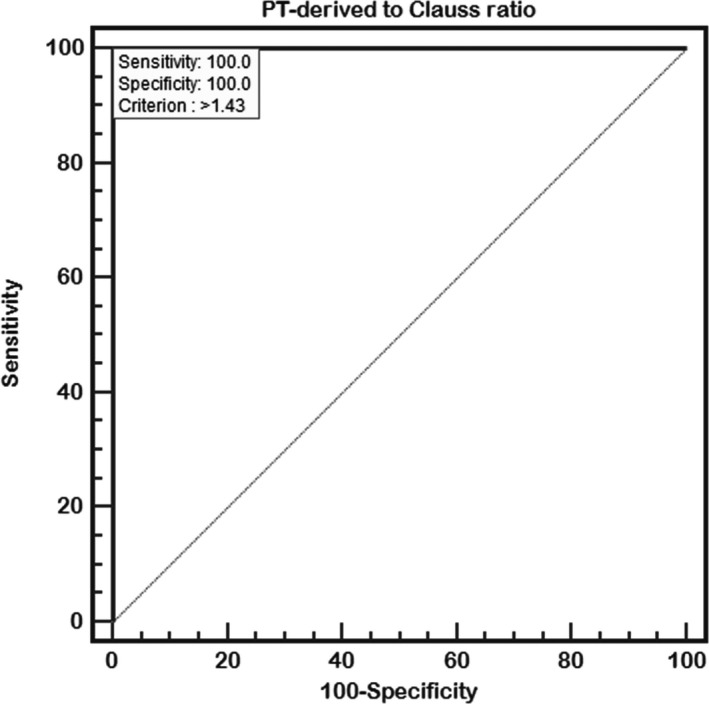
Receiver operating characteristic (ROC) curves for the diagnosis of congenital dysfibrinogenemia using the fibrinogen PT‐derived/Clauss ratio
4. DISCUSSION
The Clauss method is a routine means of testing recommended by the National Committee for Clinical Laboratory Standards. The procedure is as follows. Excessive thrombin is added to plasma (final concentration 50‐100 NIH/mL) and tested, after which the time required for the plasma to coagulate is determined, this time being negatively associated with fibrinogen concentrations. Fibrinogen concentrations are calculated from standard curves of diluted standard plasma fibrinogen.12, 13 This method is simple, reliable and can measure low concentrations of fibrinogen; however, it has certain shortcomings. When fibrinogen degradation products, high concentrations of anticoagulants (eg, heparin) or abnormal polymerization of fibrin induced by fibrinogen defects are present in the plasma, fibrinogen concentrations measured by the Clauss method can be low.14 Therefore, interference from any of these factors should be excluded when this method is used to determine fibrinogen concentrations. Thus, specimens for determination of fibrinogen concentrations by the Clauss method should not be collected within 4 hours of receiving heparin anticoagulant or thrombolytic therapy.
In contrast, the PT‐derived method is an indirect mean of determining fibrinogen concentrations. The principle of this method is as follows: during determination of PT, fibrinogen is converted into fibrin; the turbidity of plasma is proportional to fibrinogen concentrations, which are calculated by an endpoint or rate method.9, 15 This method is not only rapid, simple, and economical, but also has some limitations. The PT‐derived method can be used to calculate fibrinogen concentrations based on changes in plasma turbidity. Therefore, jaundice, hemolysis, lipidemia, and other factors that interfere with transparency can influence the results of the PT‐derived method. A previous study16 has shown that different thromboplastin reagents affect basal light scatter on analyzers to varying degrees, resulting in differing results. The results of the PT‐derived method vary greatly between different analyzers, particularly in patients with disseminated intravascular coagulation, hyperfibrinogenemia, or receiving anticoagulant or thrombolytic therapy.16
Compared with the PT‐derived method, fibrinogen concentrations measured by the Clauss method in 81 normal controls were slightly lower; however, all results were within the normal range, and the correlation was good. A similar result has been described by Chitolie et al15 In our study, there were statistically significant differences between the Clauss and PT‐derived methods regardless of the same standard material was used, it may be caused by methodology. Fibrinogen concentrations measured by the Clauss method in 73 patients with congenital dysfibrinogenemia were dramatically lower, which is consistent with Llamas et al and Miesbach et al's results.9, 17 Llamas et al9 used the Clauss and PT‐derived methods to determine the fibrinogen concentrations in 23 patients with suspected dysfibrinogenemia and definitively diagnosed this condition in six of them. They reported that results of the Clauss method were markedly lower than those of the PT‐derived method in these six patients. Using different instruments and reagents, Miesbach et al17 determined fibrinogen concentrations in 27 patients with dysfibrinogenemia and found that, regardless of what reagents or instruments they utilized, the fibrinogen concentrations were definitely lower according to the Clauss method than according to the PT‐derived method. During abnormal coagulation of defective fibrinogen molecules in patients with congenital dysfibrinogenemia, fibrinopeptide A/B release disorder or fibrin monomer polymerization abnormalities can occur. Thus, when the Clauss method is used to determine fibrinogen concentrations, plasma coagulation time is prolonged, resulting in reduced fibrinogen concentrations. If only the Clauss method is used in patients with congenital dysfibrinogenemia, the condition can easily be misdiagnosed as hypofibrinogenemia, whereas if only the PT‐derived method or immunoturbidimetry is utilized, fibrinogen concentrations appear normal or slightly high, easily resulting in missed diagnoses.18 Thus, both the Clauss and PT‐derived methods should be employed to determine fibrinogen concentrations when attempting to diagnose congenital dysfibrinogenemia. But a few fibrinogen variants, such as fibrin Longmont, should be noted. Functional fibrinogen concentrations were determined by an electromechanical Clauss assay and antigen levels analyzed by rocket immunoelectrophoresis were both within the normal range.19 Further study of fibrin Longmont demonstrated that protofibril formation was normal, nevertheless, the lateral aggregation of protofibrils was impaired and then resulting in abnormal fibrin polymerization.20 As far as we know, the clot formation can be determined by mechanical or optical techniques. Clot detection by mechanical endpoint techniques depends on the tensile strength of the clot formation; optical systems depend on the change in turbidity resulting from the formation of fibrin fibers clot.12 In such variant, the normal formation of protofibril showed similar rate of protofibril growth as well as the final protofibril size to normal fibrinogen.21 So, it may be the explanation of fibrin Longmont showed normal functional and immunologic fibrinogen levels.
Immunological assays provide accuracy results of fibrinogen concentrations; however, sources of errors must be taken into account, for example, intact and various types of degraded fibrinogen molecules can be identified by specific monoclonal antibodies that falsely cause overestimation of fibrinogen antigen levels.22 In the present study, fibrinogen concentrations measured by ELISA showed similar results to the PT‐derived method and correlated well both in normal controls and in patients with congenital dysfibrinogenemia. This suggested that PT‐derived method provide diagnostic values similar to the ELISA method.
Normal fibrinogen antigen and significantly decreased fibrinogen activity are important features of coagulation in patients with dysfibrinogenemia.23, 24 Peyvandi25 reported that the fibrinogen antigen/activity ratio is usually higher than 2:1 in patients with dysfibrinogenemia; however, the sensitivity and specificity for diagnosis of this disease are unknown. The PT‐derived method rapidly provides fibrinogen concentrations with no extra cost, and fibrinogen concentrations measured by the PT‐derived method were corresponding to antigen levels in this study. So, fibrinogen PT‐derived/Clauss ratio was calculated for each participant. The ratio of 81 normal controls was 1.11 ± 0.11, whereas in 73 patients with congenital dysfibrinogenemia, the fibrinogen PT‐derived/Clauss ratio being 6.24 ± 1.43. ROC curve analysis demonstrated that the area under the ROC curve was 1 with a standard error of 0 and 95% confidence interval of 0.976‐1.00, indicating that diagnosis of congenital dysfibrinogenemia based on the fibrinogen PT‐derived/Clauss ratio is extremely accurate. When the fibrinogen PT‐derived/Clauss ratio was >1.43, the sensitivity and specificity for diagnosis of congenital dysfibrinogenemia were both 100%. We therefore concluded that the fibrinogen PT‐derived/Clauss ratio is an important screening tool for congenital dysfibrinogenemia.
This study compared fibrinogen concentrations measured by the Clauss, PT‐derived and ELISA methods in normal controls and in patients with congenital dysfibrinogenemia, evaluated the accuracy of the fibrinogen PT‐derived/Clauss ratio for diagnosis of congenital dysfibrinogenemia using ROC curves for the first time to the best of our knowledge, and calculate the sensitivity and specificity when the ratio is at the optimal critical point. Nevertheless, this study also has some limitations including the relatively small number of cases, which may have introduced some bias.
In summary, the clinical manifestations of congenital dysfibrinogenemia are heterogeneous and most patients are asymptomatic. Laboratory examination is the key to diagnosing congenital dysfibrinogenemia. If the Clauss or PT‐derived methods used alone to measure fibrinogen concentrations, congenital dysfibrinogenemia may easily be misdiagnosed or missed. Thus, it is necessary to determine fibrinogen concentrations by both the Clauss and PT‐derived methods. ROC curves revealed that diagnosing congenital dysfibrinogenemia using the fibrinogen PT‐derived/Clauss ratio is extremely accurate; thus, the ratio could serve as a critical screening tool for diagnosing congenital dysfibrinogenemia.
AUTHOR CONTRIBUTIONS
Liqun Xiang and Meiling Luo disposed and analyzed the study data, and wrote the initial draft of the paper. Jie Yan designed the study and revised the article. Lin Liao, Weijie Zhou and Xuelian Deng collected the data. Donghong Deng and Peng Cheng participate in statistical analysis. Faquan Lin revised the article. All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
ACKNOWLEDGMENTS
We thank the Department of Hematology and Clinical Laboratory, First Affiliated Hospital of Guangxi Medical University, China. We thank all of the participants in this study.
Xiang L, Luo M, Yan J, et al. Combined use of Clauss and prothrombin time‐derived methods for determining fibrinogen concentrations: Screening for congenital dysfibrinogenemia. J Clin Lab Anal. 2018;32:e22322 10.1002/jcla.22322
Funding information
This research was supported by the National Natural Science Foundation of China [Grant number 81560342]
Liqun Xiang and Meiling Lu contributed equally to this work and are co‐first authors.
REFERENCES
- 1. Franzblau EB, Punzalan RC, Friedman KD, Roy A, Bilen O, Flood VH. Use of purified fibrinogen concentrate for dysfibrinogenemia and importance of laboratory fibrinogen activity measurement. Pediatr Blood Cancer. 2013;60:500‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mukai S, Ikeda M, Takezawa Y, Sugano M, Honda T, Okumura N. Differences in the function and secretion of congenital aberrant fibrinogenemia between heterozygous gammaD320G (Okayama II) and gammaDeltaN319‐DeltaD320 (Otsu I). Thromb Res. 2015;136:1318‐1324. [DOI] [PubMed] [Google Scholar]
- 3. Podolnikova NP, Gorkun OV, Loreth RM, Yee VC, Lord ST, Ugarova TP. A cluster of basic amino acid residues in the gamma370‐381 sequence of fibrinogen comprises a binding site for platelet integrin alpha(IIb)beta3 (glycoprotein IIb/IIIa). Biochemistry. 2005;44:16920‐16930. [DOI] [PubMed] [Google Scholar]
- 4. Asselta R, Robusto M, Plate M, Santoro C, Peyvandi F, Duga S. Molecular characterization of 7 patients affected by dys‐ or hypo‐dysfibrinogenemia: identification of a novel mutation in the fibrinogen Bbeta chain causing a gain of glycosylation. Thromb Res. 2015;136:168‐174. [DOI] [PubMed] [Google Scholar]
- 5. Yan J, Luo M, Cheng P, et al. A novel mutation in the fibrinogen Aalpha chain (Gly13Arg, fibrinogen Nanning) causes congenital dysfibrinogenemia associated with defective peptide A release. Int J Hematol. 2017;105:506‐514. [DOI] [PubMed] [Google Scholar]
- 6. Pietrys D, Balwierz W, Iwaniec T, Vorjohann S, Neerman‐Arbez M, Undas A. Two different fibrinogen gene mutations associated with bleeding in the same family (A alphaGly13Glu and gammaGly16Ser) and their impact on fibrin clot properties: fibrinogen Krakow II and Krakow III. Thromb Haemost. 2011;106:558‐560. [DOI] [PubMed] [Google Scholar]
- 7. Casini A, De Maistre E, Casini‐Stuppi V, Fontana P, Neerman‐Arbez M, de Moerloose P. Fibrinogen geneva II: a new congenitally abnormal fibrinogen alpha chain (Gly17Asp) with a review of similar mutations resulting in abnormal knob A. Blood Coagul Fibrinolysis. 2014;25:280‐282. [DOI] [PubMed] [Google Scholar]
- 8. Shapiro SE, Phillips E, Manning RA, et al. Clinical phenotype, laboratory features and genotype of 35 patients with heritable dysfibrinogenaemia. Br J Haematol. 2013;160:220‐227. [DOI] [PubMed] [Google Scholar]
- 9. Llamas P, Santos AB, Outeirino J, Soto C, Tomas JF. Diagnostic utility of comparing fibrinogen Clauss and prothrombin time derived method. Thromb Res. 2004;114:73‐74. [DOI] [PubMed] [Google Scholar]
- 10. Sobas F, Hanss M, Ffrench P, Trzeciak MC, Dechavanne M, Negrier C. Human plasma fibrinogen measurement derived from activated partial thromboplastin time clot formation. Blood Coagul Fibrinolysis. 2002;13:61‐68. [DOI] [PubMed] [Google Scholar]
- 11. Rumley A, Woodward M, Hoffmeister A, Koenig W, Lowe GD. Comparison of plasma fibrinogen by Clauss, prothrombin time‐derived, and immunonephelometric assays in a general population: implications for risk stratification by thirds of fibrinogen. Blood Coagul Fibrinolysis. 2003;14:197‐201. [DOI] [PubMed] [Google Scholar]
- 12. Mackie IJ, Kitchen S, Machin SJ, Lowe GD. Guidelines on fibrinogen assays. Br J Haematol. 2003;121:396‐404. [DOI] [PubMed] [Google Scholar]
- 13. Lippi G, Franchini M, Favaloro EJ. Diagnostics of inherited bleeding disorders of secondary hemostasis: an easy guide for routine clinical laboratories. Semin Thromb Hemost. 2016;42:471‐477. [DOI] [PubMed] [Google Scholar]
- 14. Seifried E, Oethinger M, Tanswell P, Hoegee‐de Nobel H, Nieuwenhuizen W. Studies on the functionality of fibrinogen during rt‐PA therapy: results of three different methods of fibrinogen determination. Blood Coagul Fibrinolysis. 1992;3:81‐87. [DOI] [PubMed] [Google Scholar]
- 15. Chitolie A, Mackie IJ, Grant D, Hamilton JL, Machin SM. Inaccuracy of the ‘derived’ fibrinogen measurement. Blood Coagul Fibrinolysis. 1994;5:955‐957. [DOI] [PubMed] [Google Scholar]
- 16. Mackie J, Lawrie AS, Kitchen S, et al. A performance evaluation of commercial fibrinogen reference preparations and assays for Clauss and PT‐derived fibrinogen. Thromb Haemost. 2002;87:997‐1005. [PubMed] [Google Scholar]
- 17. Miesbach W, Schenk J, Alesci S, Lindhoff‐Last E. Comparison of the fibrinogen Clauss assay and the fibrinogen PT derived method in patients with dysfibrinogenemia. Thromb Res. 2010;126:e428‐e433. [DOI] [PubMed] [Google Scholar]
- 18. Yan J, Deng D, Luo M, et al. Dysfibrinogenemia in a patient undergoing artificial abortion after misdiagnosis and review of the literature. Clin Chim Acta. 2015;447:86‐89. [DOI] [PubMed] [Google Scholar]
- 19. Lefkowitz JB, DeBoom T, Weller A, Clarke S, Lavrinets D. Fibrinogen longmont: a dysfibrinogenemia that causes prolonged clot‐based test results only when using an optical detection method. Am J Hematol. 2000;63:149‐155. [DOI] [PubMed] [Google Scholar]
- 20. Lounes KC, Lefkowitz JB, Coates AI, Hantgan RR, Henschen‐Edman A, Lord ST. Fibrinogen longmont. A heterozygous abnormal fibrinogen with B beta Arg‐166 to Cys substitution associated with defective fibrin polymerization. Ann N Y Acad Sci. 2001;936:129‐132. [PubMed] [Google Scholar]
- 21. Lounes KC, Lefkowitz JB, Henschenedman AH, Coates AI, Hantgan RR, Lord ST. The impaired polymerization of fibrinogen Longmont (Bbeta166Arg–>Cys) is not improved by removal of disulfide‐linked dimers from a mixture of dimers and cysteine‐linked monomers. Blood. 2001;98:661‐666. [DOI] [PubMed] [Google Scholar]
- 22. Undas A. How to assess fibrinogen levels and fibrin clot properties in clinical practice?. Semin Thromb Hemost 2016;42:381‐388. [DOI] [PubMed] [Google Scholar]
- 23. Cunningham MT, Brandt JT, Laposata M, Olson JD. Laboratory diagnosis of dysfibrinogenemia. Arch Pathol Lab Med. 2002;126:499‐505. [DOI] [PubMed] [Google Scholar]
- 24. Luo M, Deng D, Xiang L, et al. Three cases of congenital dysfibrinogenemia in unrelated Chinese families: heterozygous missense mutation in fibrinogen alpha chain Argl6His. Medicine (Baltimore) 2016;95:e4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Peyvandi F. Epidemiology and treatment of congenital fibrinogen deficiency. Thromb Res. 2012;130(Suppl 2):S7‐S11. [DOI] [PubMed] [Google Scholar]


