Abstract
Background
Extracellular release of high mobility group box 1 (HMGB1) acts as a danger‐associated molecular pattern, thereby “alarming” the immune system and promoting systemic inflammation. We investigated plasma HMGB1 concentrations as a potential diagnostic and prognostic biomarker in critical illness.
Methods
Our study included 218 critically ill patients (145 with sepsis, 73 without sepsis), of whom blood samples were obtained at the time‐point of admission to the medical intensive care unit (ICU).
Results
High mobility group box 1 levels were significantly elevated in critically ill patients (n = 218) compared with healthy controls (n = 66). Elevated HMGB1 plasma levels were independent from the presence of sepsis. Moreover, HMGB1 was not associated with disease severity, organ failure, or mortality in the ICU. We observed a trend toward lower HMGB1 levels in ICU patients with pre‐existing obesity, type 2 diabetes and end‐stage renal disease patients on chronic hemodialysis.
Conclusion
In conclusion, our study did not reveal significant associations between HMGB1 levels at ICU admission and clinical outcomes in critically ill patients. Due to the pathogenic role of HMGB1 in the late phases of experimental sepsis, future studies might assess the potential value of HMGB1 by measuring its plasma concentrations at later time points during the course of critical illness.
Keywords: alarmin, danger‐associated molecular pattern, high mobility group box 1, intensive care unit, organ failure, prognosis, sepsis
1. INTRODUCTION
High mobility group box 1 (HMGB1) protein (OMIM: 163905) was initially identified as a nuclear protein, but it gained tremendous attention as an extracellular molecule acting as a danger‐associated molecular pattern (DAMP). As an intracellular protein, HMGB1 is involved in DNA replication, transcription and repair mechanisms.1, 2, 3 As an extracellular mediator, it plays a key role in activating cascades of inflammatory processes and diverse additional extracellular biological activities.4, 5 Notably, in critical illness, it is therefore assumed that HMGB1 mediates the cellular stress response associated with organ failure, systemic inflammation and infection.6, 7, 8 Thus, HMGB1 is widely considered as the prototypic DAMP or alarmin.9, 10 HMGB1 itself has cytokine, chemokine and growth factor activity, regulating the inflammatory and immunological response as a ligand for toll‐like receptor (TLR) 2/4, the chemokine CXCL12 and for receptor for advanced glycation end‐products (RAGE).11, 12 Tissue macrophages are the main cellular target of HMGB1 and other alarmins.13 HMGB1 is released in response to pathogenic infection and actively secreted for chemotactic and cytokine‐like function, but also supports tissue repair mechanisms, including angiogenesis, fibrosis, and tissue regeneration.1, 5 In experimental models of sepsis, HMGB1 has been described as a late mediator of sepsis, and its blockade in murine sepsis models improved mortality.14, 15 All these characteristics fuelled the hypothesis that circulating HMGB1 could be a valuable diagnostic tool in critically ill patients in terms of systemic inflammation, severity of disease, and mortality.6, 16, 17 Although prior studies have unanimously reported elevated HMGB1 levels in the setting of critical illness, the associations between HMGB1 and clinical outcome are controversial.18, 19, 20 In addition, it remained unclear, whether the presence of critical illness or the presences of severe infection or sepsis are the main determinants of increased HMGB1 plasma levels.19 This prompted us to revisit the role of HMGB1 in a large, well characterized cohort of critically ill patients with and without sepsis at the medical intensive care unit (ICU).
2. MATERIAL AND METHODS
2.1. Study design and patient characteristics
Critically ill patients were included at admission to the medical ICU at the RWTH University Hospital Aachen, Germany. Patients, who were admitted for post‐interventional observational stay or underwent an elective procedure, were excluded.21 The local ethics committee approved our study in accordance to the ethical standards laid down in the Declaration of Helsinki (reference number EK 150/06). The patients were categorized as sepsis and non‐sepsis according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis‐3),22 and were treated following the current guidelines for treatment of sepsis (Surviving Sepsis Campaign).23 As a healthy control group, we analyzed blood donors with normal blood counts, normal values of liver enzymes and a negative serology for viral hepatitis and HIV.24
In order to determine long‐term outcome, we contacted the patients, their relatives and/or the general practitioner in approximately 6 months intervals after discharge from the hospital for 2 years.24
2.2. Measurements of HMGB1 plasma levels
Blood samples were collected at the time of admission (before specific therapeutic measures), centrifuged, and plasma was stored at −80°C. Plasma HMGB1 concentrations were determined using a quantitative sandwich enzyme immunoassay (ELISA), according to manufacturer's instructions (HMGB1 ELISA, #ST51011; IBL International, Hamburg, Germany).
2.3. Statistical analysis
Due to the skewed distribution of the parameters, data are given as median and range, and graphically displayed by box‐and‐whiskers plots. The degree of association between 2 variables was assessed by the Spearman rank correlation test. Comparisons of parameters between 2 different groups were conducted with the Mann‐Whitney U‐test. All values, including outside values as well as far out values, were included. The prognostic value of HMGB1 was explored using 3 groups, consisting of the patients with HMGB1 from the lowest quartile, from the middle 50% and from the highest quartile, by Kaplan Meier curves for ICU as well as for overall survival.24 P‐values less than .05 were considered as statistically significant. All analyses were performed with IBM SPSS Statistics (SPSS; Chicago, IL, USA).
3. RESULTS
3.1. HMGB1 plasma levels are significantly elevated in critically ill patients as compared with healthy controls
High mobility group box 1 plasma levels were significantly elevated in a large cohort of 218 critically ill medical patients (median 10.37 ng/mL, range 0.25‐80 ng/mL; Table 1) at admission to the ICU, as compared with 66 healthy controls (median 2.67 ng/mL, range 0.25‐10.47 ng/mL, P < .001; Figure 1A). Among the critically ill patients, there was no clear association between HMGB1 plasma concentrations and different disease aetiologies leading to ICU admission (data not shown).
Table 1.
Baseline patient characteristics and HMGB1 plasma measurements
| Parameter | All patients | Non‐sepsis | Sepsis | P‐value* |
|---|---|---|---|---|
| Number | 218 | 73 | 145 | |
| Sex (male/female) | 133/85 | 48/25 | 85/60 | .378 |
| Age median (range) (y) | 64 (18‐90) | 61 (18‐85) | 65 (20‐90) | .275 |
| APACHE‐II score median (range) | 18 (2‐43) | 13.5 (2‐33) | 19 (4‐43) | <.001 |
| ICU days median (range) | 7 (1‐137) | 6 (1‐45) | 9 (1‐137) | .004 |
| Death during ICU n (%) | 49 (22.5%) | 9 (12.3%) | 40 (27.6%) | .010 |
| Death during follow‐up (total) n (%) | 89 (40.8%) | 22 (30.1%) | 67 (46.2%) | .028 |
| Mechanical ventilation n (%) | 145 (66.5%) | 46 (63%) | 99 (68.3%) | .451 |
| Ventilation time median (range) [h] | 86 (0‐3628) | 31 (0‐3628) | 117 (0‐2966) | .027 |
| Pre‐existing diabetes n (%) | 63 (28.9%) | 22 (30.1%) | 41 (28.3%) | .874 |
| BMI median (range) (m²/kg) | 25.8 (14‐86) | 25.7 (15.9‐40.5) | 28.9 (14‐86.5) | .539 |
| WBC median (range) (×10³/μL) | 13.1 (0.1‐208) | 12.5 (1.8‐29.6) | 14.1 (0.1‐208) | .017 |
| CRP median (range) (mg/dL) | 100.5 (5‐230) | 17 (5‐230) | 164 (5‐230) | <.001 |
| Procalcitonin median (range) (μg/L) | 0.7 (0.03‐207.5) | 0.2 (0.03‐100) | 2.2 (0.1‐207.5) | <.001 |
| Creatinine median (range) (mg/dL) | 1.3 (0.1‐15) | 1.0 (0.2‐15) | 1.5 (0.1‐10.7) | .013 |
| GFR Cystatin C median (range) (mL/min) | 34.5 (3‐379) | 59 (5‐379) | 22 (3‐218) | .001 |
| AST median (range) (U/L) | 42 (7‐20 332) | 47 (11‐20 332) | 41 (7‐7832) | .165 |
| ALT median (range) (U/L) | 30 (3‐7867) | 37 (7‐7867) | 25 (3‐5890) | .064 |
| Bilirubin median (range) [mg/dL] | 0.7 (0.1‐20.8) | 0.7 (0.1‐20.8) | 0.7 (0.1‐18.9) | .952 |
| INR median (range) | 1.16 (0.92‐13) | 1.17 (0.95‐6.73) | 1.16 (0.92‐13) | .864 |
| HMGB1 day 1 median (range) (ng/mL) | 10.37 (0.20‐80) | 11.21 (0.20‐76.04) | 10.23 (0.20‐80) | .336 |
ALT, alanine aminotransferase; APACHE, Acute Physiology And Chronic Health Evaluation; AST, aspartate aminotransferase; BMI, body mass index; CRP, C‐reactive protein; ICU, intensive care unit; INR, international normalized ration; HMGB1, high mobility group box 1; WBC, white blood cell.
For quantitative variables, median and range (in parenthesis) are given.*P‐values for the comparison of sepsis and non‐sepsis patients are given (U‐test for quantitative variables or chi‐square test for qualitative parameters).
Figure 1.
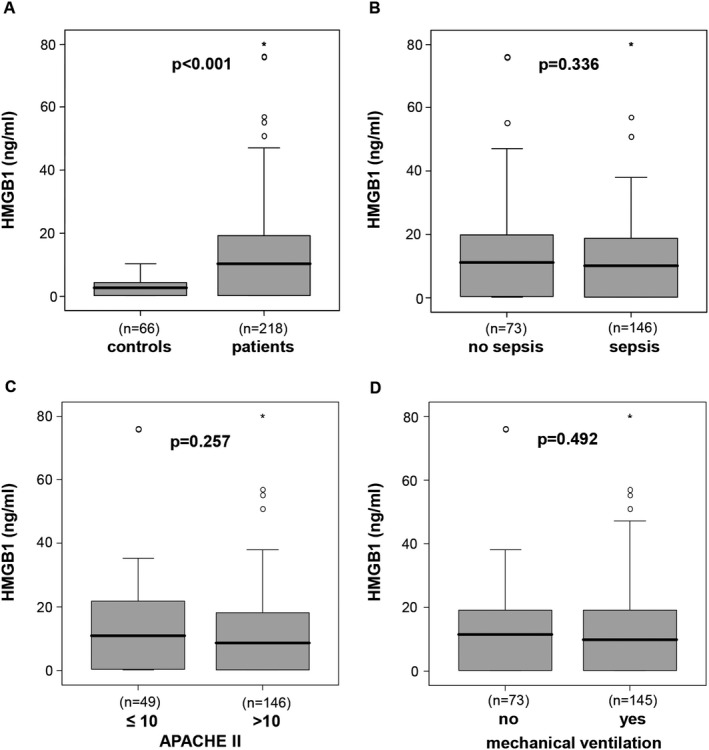
HMGB1 levels in critically ill patients. A, HMGB1 plasma concentrations are significantly elevated in critically ill patients compared with healthy controls. B, HMGB1 levels do not differ between intensive care unit (ICU) patients with or without sepsis. C, High disease severity, as defined by an APACHE‐II score above 10, is not associated with elevated plasma HMGB1. D, The need of mechanical ventilation was not associated with HMGB1 levels at ICU admission. P‐values (U‐test) are given in the figure. HMGB1, high mobility group box 1
3.2. Elevated HMGB1 plasma levels in critically ill patients are independent of the presence of sepsis
Elevated HMGB1 have been previously reported in patients with bacteraemia, sepsis, and septic shock.18, 19, 20 Within the cohort of ICU patients, HMGB1 levels did not differ between patients with sepsis (n = 146, median HMGB1 10.23 ng/mL, range 0.2‐80 ng/mL) and patients without sepsis (n = 73, median 11.21 ng/mL, range 0.2‐76.04 ng/mL; Figure 1B). Typical sites of infection in sepsis were pneumonia, abdominal and urogenital tract, while non‐sepsis causes of critical illness included, among others, cardiopulmonary diseases, acute pancreatitis and decompensated liver cirrhosis (detailed data not shown).
3.3. HMGB1 plasma levels are not associated with disease severity or mechanical ventilation
Circulating HMGB1 has been previously suggested as a biomarker for disease severity in various clinical settings.25 Plasma HMGB1 concentrations were not associated with disease severity, determined by the Acute Physiology And Chronic Health Evaluation‐II (APACHE‐II) score. Patients with a high APACHE‐II score (above 10) did not have higher HMGB1 levels than patients with an APACHE‐II score below or equal to 10 (Figure 1C). In addition, there was also no significant difference in HMGB1 plasma levels between ventilated or non‐ventilated critically ill patients (median 9.9 ng/mL vs median 11.5 ng/mL no ventilation, P = .492) (Figure 1D).
3.4. Association of HMGB1 plasma levels in critically ill patients with metabolic and renal comorbidities
High mobility group box 1 has been associated with metabolic disorders.26, 27 We therefore assessed whether metabolic comorbidities, including pre‐existing obesity or diabetes impacted HMGB1 levels at ICU admission. Interestingly, patients with pre‐existing obesity, defined as a body mass index above 30 kg/m², showed a trend toward lower HMGB1 levels at ICU admission (median 5.93 ng/mL vs median 11.6 ng/mL in non‐obese patients, P = .052) (Figure 2A). Patients with pre‐existing type 2 diabetes have slightly lower HMGB1 levels (median 8.29 ng/mL in diabetics vs median 11.1 ng/mL in non‐diabetics, not significant; Figure 2B). This finding corresponds to the inverse correlation between HMGB1 and blood glucose at ICU admission (Table 2).
Figure 2.
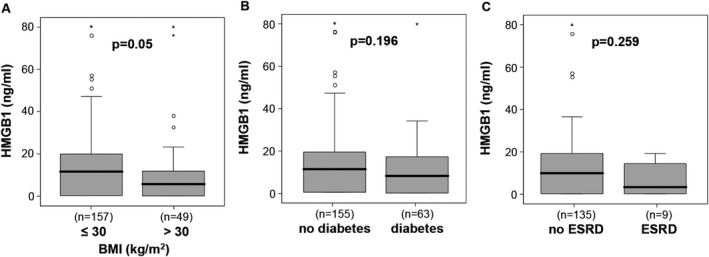
Impact of metabolic comorbidities on HMGB1 levels. HMGB1 plasma concentrations did not differ between ICU patients with or without obesity, as defined by a body‐mass index (BMI) above 30 kg/m2 (A), pre‐existing type 2 diabetes (B) or pre‐existing end‐stage renal disease (ESRD, C). HMGB1, high mobility group box 1; ICU, intensive care unit
Table 2.
Correlations with HMGB1 plasma concentrations at ICU admission day (Spearman rank correlation test, only significant results are shown)
| Parameters | ICU patients | |
|---|---|---|
| r | P | |
| Age | −.238 | <.001 |
| BMI | −.161 | .021 |
| Lactate | .144 | .035 |
| Urea | −.173 | .044 |
| Bilirubin | .176 | .010 |
| Bilirubin conjugated | .213 | .017 |
| Glucose | −.167 | .014 |
| HDL cholesterol | −.324 | .008 |
| Haemoglobin | .164 | .015 |
| Haematocrit | .159 | .018 |
BMI, body mass index; HDL cholesterol, high density lipoprotein cholesterol; HMGB1, high mobility group box 1.
We next investigated the potential association between HMGB1 and renal diseases, based on its involvement in chronic renal disorders.28 A small subgroup of our cohort consisted of end‐stage renal disease patients requiring chronic hemodialysis (n = 9). These patients showed a clear trend toward lower HMGB1 levels at ICU admission (median 3.4 ng/mL in patients with vs median 9.9 ng/mL in patients without chronic renal replacement therapy, P = .259) (Figure 2C). However, in the whole cohort of critically ill patients, we did not observe a correlation between HMGB1 and classical markers of renal failure, such as creatinine or cystatin C. HMGB1, however, correlated inversely with urea, likely reflecting renal function, metabolism, and nutritional status (Table 2).
3.5. High HMGB1 plasma concentrations at ICU admission are not associated with adverse prognosis
In critically patients, who subsequently died during the ICU treatment (n = 49), we did not find significantly altered HMGB1 levels at admission to the ICU, suggesting that HMGB1 is not a prognostic biomarker in critical diseases.16 Nevertheless, we observed a trend toward increased HMGB1 levels in the deceased patients compared to the surviving patients (median 12.89 ng/mL vs median 9.65 ng/mL in ICU survivors) (Figure 3A), while there was no significant difference for the overall mortality either (Figure 3B). By Kaplan‐Meier curve analysis, patients with HMGB1 levels of the highest quartile (>19.2 ng/mL) showed a tendency toward improved ICU (Figure 3C) or overall survival (Figure 3D), but did not reach statistical significance.
Figure 3.
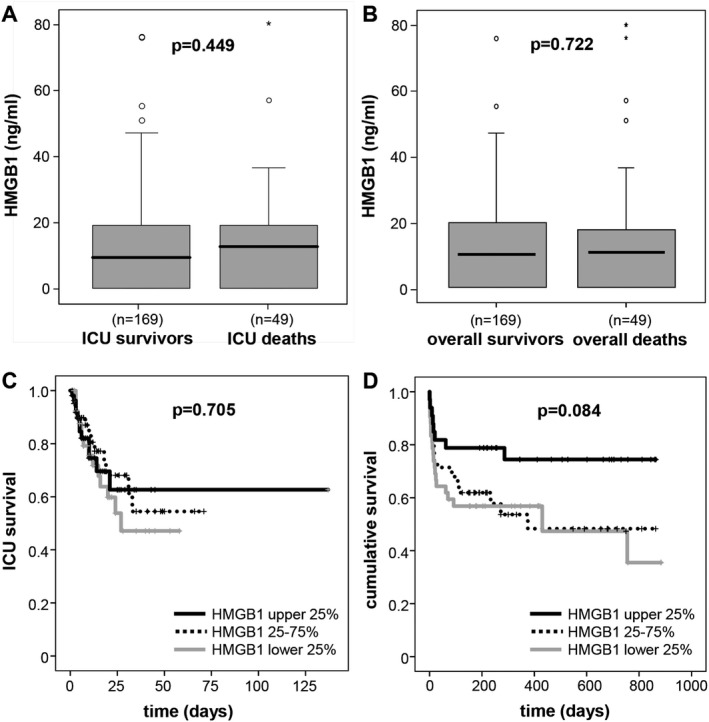
HMGB1 is not a prognostic biomarker for mortality in critically ill patients. A‐B, Patients that died during the course of ICU treatment (A) and/or during follow‐up (B) are characterized by tendency toward higher plasma HMGB1 concentrations already at ICU admission. C‐D, Kaplan‐Meier survival curves of ICU patients are displayed, showing that patients with HMGB1 levels of upper quartile (on admission >19.2 ng/mL; black) had a tendency toward higher ICU (C) as well as overall survival (D) as compared to patients with HMGB1 serum concentrations of lower quartile (on admission <0.2 ng/mL; grey) or middle 50% (dotted line). P‐values are given in the figure. HMGB1, high mobility group box 1; ICU, intensive care unit
4. DISCUSSION
Hypoxic, stressed, injured, or dying cells release DAMPs, or alarmins, to activate the immune system, promote inflammation, but also initiate tissue repair.29 These processes are presumed to be hyper‐activated in critical illness, where cell death, systemic inflammation, tissue hypoperfusion, and infection determine the clinical course in the ICU.30 HMGB1 is a blueprint of DAMPs, because it can be actively released by innate immune cells in response to exogenous bacterial products or endogenous inflammatory stimuli and can be passively released from damaged parenchymal cells.5, 12, 31 Animal models supported the hypothesis that circulating HMGB1 is involved in the pathogenesis of sepsis.7 However, unlike classical inflammatory cytokines (eg, TNF), an increase in HMGB1 is not observed within the first hours after sepsis induction, but was a characteristic of the late phase of sepsis associated with lethality.14, 15, 32, 33, 34 In turn, neutralization of HMGB1 during the late phase of sepsis using specific antibodies prevented rodents from sepsis‐related death.33, 35 Based on its pathogenic role, circulating HMGB1 has been suggested as a biomarker for the assessment of sepsis, disease severity and mortality.6, 16, 17
However, our study with a prospectively enrolled, large and heterogeneous cohort of critically ill medical patients clearly demonstrated that HMGB1 has limited value as a biomarker in the ICU setting. Although we did confirm elevated HMGB1 plasma levels in critically ill patients as compared to healthy controls, in line with previous reports,18, 19, 20 HMGB1 (sampled at ICU admission) was not an indicator of disease severity, organ failure, or mortality. Even more surprising, patients with sepsis did not display different HMGB1 concentrations as compared to ICU patients without sepsis. Earlier studies from Denmark that included 194 and 185 patients had found higher levels of HMGB1 in infectious vs non‐infectious diseases.18, 36 On the contrary, HMGB1 was not able to discriminate sepsis from non‐infectious disease in the setting of an Emergency Department, as identified in a cohort of 631 patients with heterogeneous diseases.37 Similar controversies have been reported regarding the potential association of HMGB1 to disease severity and mortality. HMGB1 was reported to be elevated in patients with severe sepsis and septic shock compared to patients with sepsis,19, 36 but did not differ between survivors or non‐survivors in an observational study that included 247 patients from 24 ICUs in Finland.19 Interestingly, while HMGB1 levels at ICU admission did not predict mortality in a French study on 42 critically ill patients with septic shock, HMGB1 levels at day 3 were able to discriminate between survivors and non‐survivors in a medical ICU setting.20 In a study from the Karolinska institute that analyzed 64 patients (including 33 with septic shock), HMGB1 remained high in patients for 1 week after ICU admission,16 supporting that HMGB1 is a downstream and late mediator of inflammation. Thus, the lack of clinical utility as a biomarker in the early phase of critical disease at ICU admission, as demonstrated by our study, does not preclude its potential value in later phases of the clinical course. Future studies should particularly focus on longitudinal measurements of HMGB1 in the ICU.
In addition, circulating HMGB1 in patient's plasma might not necessarily reflect the full biological activity during critical illness. On the one hand, local concentrations in ischemic or injured tissue might be higher—and immediately bind to TLR2, TLR4 or RAGE on local macrophages.25 On the other hand, a 30‐kDa low molecular weight HMGB1 variant was detected in sepsis patients,5, 14 suggesting that HMGB1 may form large complexes with other serum components that might not be detected by conventional ELISA systems. These unidentified HMGB1‐binding molecules or chemical modifications may affect the biological activities or immunodetection of HMGB1. For instance, a recent study indicated that reactive oxygen species (ROS) may oxidise HMGB1 and consequently abolish HMGB1‐mediated immunostimulatory activities.38, 39 Moreover, exogenous factors might influence HMGB1 concentrations in the circulation. For instance, metformin or statins have been associated with decreased plasma HMGB1.40
Nonetheless, it is important to note the limitations of our study. We conducted a monocentric, observational study with relatively broad inclusion criteria. While this reflects a “real‐world situation” of critically ill patients at a medical ICU, it introduces a substantial heterogeneity regarding patient characteristics, and therefore, resulting in relatively small subgroups, which may be underpowered for further subgroup analyses. Due to the prospective inclusion study design with an uncertain further course in the ICU, a substantial fraction of the patients had a rather low APACHE‐II score, which may have reduced the discriminant role of HMGB1 in our cohort.
5. CONCLUSION
In conclusion, we herein demonstrated significantly elevated HMGB1 plasma concentrations in critically ill patients, corroborating the extracellular properties of HMGB1 as an alarmin signal. However, our study did not reveal a significant association between HMGB1 levels at ICU admission and clinical outcomes in critically ill patients. This overt discrepancy of HMGB1’s important pathogenic role in experimental sepsis and its poor performance as a clinical biomarker for prediction of outcome in critical illness might be related to its function during late stages of critical disease, which cannot be accurately captured at admission to the ICU. Prospective studies should investigate the practical clinical value of longitudinal HMGB1 plasma concentrations during the course of critical illness.
Yagmur E, Buendgens L, Herbers U, et al. High mobility group box 1 as a biomarker in critically ill patients. J Clin Lab Anal. 2018;32:e22584 10.1002/jcla.22584
Funding information
This work was supported by the German Research Foundation (DFG; Ta434/5‐1 and SFB/TRR57) and the Interdisciplinary Center for Clinical Research (IZKF) Aachen.
Tacke and Koch contributed equally to this work.
REFERENCES
- 1. Ding J, Cui X, Liu Q. Emerging role of HMGB1 in lung diseases: friend or foe. J Cell Mol Med. 2017;21:1046‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu B, Wang H, Andersson U, Tracey KJ. Regulation of HMGB1 release by inflammasomes. Protein Cell. 2013;4:163‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ferrari S, Finelli P, Rocchi M, Bianchi ME. The active gene that encodes human high mobility group 1 protein (HMG1) contains introns and maps to chromosome 13. Genomics. 1996;35:367‐371. [DOI] [PubMed] [Google Scholar]
- 4. Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang H, Zhu S, Zhou R, Li W, Sama AE. Therapeutic potential of HMGB1‐targeting agents in sepsis. Expert Rev Mol Med. 2008;10:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gentile LF, Moldawer LL. HMGB1 as a therapeutic target for sepsis: it's all in the timing!. Expert Opin Ther Targets. 2014;18:243‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Ward MF, Sama AE. Targeting HMGB1 in the treatment of sepsis. Expert Opin Ther Targets. 2014;18:257‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51:119‐126. [DOI] [PubMed] [Google Scholar]
- 9. Keyel PA. How is inflammation initiated? Individual influences of IL‐1, IL‐18 and HMGB1. Cytokine. 2014;69:136‐145. [DOI] [PubMed] [Google Scholar]
- 10. Lu B, Wang C, Wang M, et al. Molecular mechanism and therapeutic modulation of high mobility group box 1 release and action: an updated review. Expert Rev Clin Immunol. 2014;10:713‐727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiba S, Baghdadi M, Akiba H, et al. Tumor‐infiltrating DCs suppress nucleic acid‐mediated innate immune responses through interactions between the receptor TIM‐3 and the alarmin HMGB1. Nat Immunol. 2012;13:832‐842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec‐10 selectively repress tissue damage‐induced immune responses. Science. 2009;323:1722‐1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krenkel O, Tacke F. Liver macrophages in tissue homeostasis and disease. Nat Rev Immunol. 2017;17:306‐321. [DOI] [PubMed] [Google Scholar]
- 14. Wang H, Bloom O, Zhang M, et al. HMG‐1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248‐251. [DOI] [PubMed] [Google Scholar]
- 15. Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG‐1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950‐2954. [DOI] [PubMed] [Google Scholar]
- 16. Sundèn‐Cullberg J, Norrby‐Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box‐1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564‐573. [DOI] [PubMed] [Google Scholar]
- 17. Angus DC, Yang L, Kong L, et al. Circulating high‐mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Crit Care Med. 2007;35:1061‐1067. [DOI] [PubMed] [Google Scholar]
- 18. Gaïni S, Pedersen SS, Koldkjaer OG, Pedersen C, Møller HJ. High mobility group box‐1 protein in patients with suspected community‐acquired infections and sepsis: a prospective study. Crit Care. 2007;11:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Karlsson S, Pettilä V, Tenhunen J, Laru‐Sompa R, Hynninen M, Ruokonen E. HMGB1 as a predictor of organ dysfunction and outcome in patients with severe sepsis. Intensive Care Med. 2008;34:1046‐1053. [DOI] [PubMed] [Google Scholar]
- 20. Gibot S, Massin F, Cravoisy A, et al. High‐mobility group box 1 protein plasma concentrations during septic shock. Intensive Care Med. 2007;33:1347‐1353. [DOI] [PubMed] [Google Scholar]
- 21. Koch A, Weiskirchen R, Kunze J, et al. Elevated asymmetric dimethylarginine levels predict short‐ and long‐term mortality risk in critically ill patients. J Crit Care. 2013;28:947‐953. [DOI] [PubMed] [Google Scholar]
- 22. Buendgens L, Yagmur E, Bruensing J, et al. Growth differentiation factor‐15 is a predictor of mortality in critically ill patients with sepsis. Dis Markers. 2017;2017:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304‐377. [DOI] [PubMed] [Google Scholar]
- 24. Koch A, Voigt S, Kruschinski C, et al. Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care. 2011;15:R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Venereau E, De Leo F, Mezzapelle R, Careccia G, Musco G, Bianchi ME. HMGB1 as biomarker and drug target. Pharmacol Res. 2016;111:534‐544. [DOI] [PubMed] [Google Scholar]
- 26. Wang H, Zhu S, Ward MF, Gong J, Sama AE. Hyperglycemia aggravates endotoxin‐induced high mobility group box 1 protein release: yet another reason not to be too sweet. Crit Care Med. 2008;36:2475‐2476. [DOI] [PubMed] [Google Scholar]
- 27. Hagiwara S, Iwasaka H, Hasegawa A, Koga H, Noguchi T. Effects of hyperglycemia and insulin therapy on high mobility group box 1 in endotoxin‐induced acute lung injury in a rat model. Crit Care Med. 2008;36:2407‐2413. [DOI] [PubMed] [Google Scholar]
- 28. Zhou TB. Role of high mobility group box 1 and its signaling pathways in renal diseases. J Recept Signal Transduct Res. 2014;34:348‐350. [DOI] [PubMed] [Google Scholar]
- 29. Timmermanns K, Kox M, Scheffer GJ, Pickkers P. Danger in the intensive care unit: damps in critically ill patients. Shock. 2016;45:108‐116. [DOI] [PubMed] [Google Scholar]
- 30. Hotchkiss RS, Moldawer LL, Opal SM. Sepsis and septic shock. Nat Rev Dis Primers. 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tracey KJ, Fong Y, Hesse DG, et al. Anti‐cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987;330:662‐664. [DOI] [PubMed] [Google Scholar]
- 33. Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high‐mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138‐150. [DOI] [PubMed] [Google Scholar]
- 35. Qin S, Wang H, Yuan R, et al. Role of HMGB1 in apoptosis‐mediated sepsis lethality. J Exp Med. 2006;203:1637‐1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gaïni S, Koldkjaer OG, Møller HJ, Pedersen C, Pedersen SS. A comparison of high‐mobility group box‐1 protein, lipopolysaccharide‐binding protein and procalcitonin in severe community‐acquired infections and bacteremia: a prospective study. Crit Care. 2007;11:R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gámez‐Díaz L, Enriquez LE, Matute JD, et al. Diagnostic accuracy of HMGB‐1, sTREM‐1, and CD64 as markers of sepsis in patients recently admitted to the emergency department. Acad Emerg Med. 2011;18:807‐815. [DOI] [PubMed] [Google Scholar]
- 38. Tang D, Kang R, Zeh HJ 3rd, Lotze MT. High‐mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal. 2011;14:1315‐1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li G, Tang D, Lotze MT. Mènage à Trois in stress: DAMPs, redox and autophagy. Semin Cancer Biol. 2013;23:380‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Souza AW, van der Geest KS, Brouwer E, et al. High mobility group box 1 levels in large vessel vasculitis are not associated with disease activity but are influenced by age and statins. Arthritis Res Ther. 2015;17:158. [DOI] [PMC free article] [PubMed] [Google Scholar]


