Abstract
Objective
Our study was to explore the roles between serum soluble suppression of tumorigenicity 2 (sST2) and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) while evaluating ventricular function to properly diagnose chronic heart failure (CHF).
Methods
In total, 197 CHF patients were recruited and classified into ventricular function's II, III, and IV groups, and 106 healthy people into normal control group. To detect concentrations of Sst2 and NT‐proBNP, ELISA and electro‐chemiluminescence immuno assay were implemented. An automatic biochemical analyzer was used to determine the levels of the following: blood urea nitrogen (BUN), creatinine (Cr), alanine aminotransferase (ALT), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), and uric acid (UA). A receiver operating characteristic (ROC) curve was adopted to detect the diagnostic value sST2 and NT‐ProBNP in CHF and the logistic regression analysis involving the risk factors of CHF.
Results
Serum sST2 and NT‐proBNP concentrations were increased significantly in the ventricular function's II, III, and IV groups in a manner dependent on concentration as opposed to the manner the normal control group occupied. The area under the curve (AUC) of sST2, found NT‐proBNP and sST2+NT‐proBNP to be 0.942 (95% CI: 0.917‐0.966), 0.920 (95% CI: 0.891‐0.948), and 0.968 (95% CI: 0.953‐0.984), respectively. sST2, NT‐proBNP, UA, and Cr were verified as important risk factors of CHF.
Conclusion
Serum sST2 and NT‐ProBNP could act as diagnostic indicators for CHF.
Keywords: chronic heart failure, N‐terminal pro‐brain natriuretic peptide, soluble suppression of tumorigenicity 2, ventricular function
1. INTRODUCTION
Heart failure (HF) is a pivotal public health issue which influences over 5.7 million American people and takes $37.2 billion every year and its morbidity reaches epidemic proportions.1 Moreover, it is evaluated that 45% of women and 59% of men die in 5 years after a HF diagnosis.2 Chronic HF (CHF) is a long‐term product of heart failure, is usually managed by treating symptoms and patients suffering from deteriorating symptoms and signs may need hospitalization or more frequent doctor visits.3 The most common causes of HF are as follow: coronary artery disease, high blood pressure, atrial fibrillation, previous myocardial infarction (heart attack), valvular heart disease, cardiomyopathy, infection, and excessive alcohol consumption.4, 5, 6 The prevalence and occurrence of HF progressively increases with age, with the most affected age group being over 50 years.3 In developed countries, 1%‐2% of the adult population suffers from HF, increasing to ≥10% for those older than 70 years.7 The National Institute for Health and Care Excellence (NICE) recommends measurement of brain natriuretic peptide combined with an ultrasound of the heart in the occurrence of symptom development. Up until now no elite diagnostic criterion has been established for HF.
Suppression of tumorigenicity 2 (ST2), namely an interleukin (IL)‐33 related receptor, is expressed by hematopoietic and epithelial cells regulating Th2‐type responses.8 ST2 is localized to the cell surface (ST2L), which can also be produced in its soluble form (soluble ST2) to function as a decoy receptor.9, 10 It is a result of cardiomyocyte stress along with fibrosis, which can offer incremental value to natriuretic peptides for risk stratification of patients suffering from various cardiovascular diseases.11 Brain natriuretic peptide (BNP) is mainly released from ventricular myocytes as a direct response to myocardial stretch and overloaded intracardiac pressure.12 When released into circulation, the BNP splits into active C‐terminal BNP and inactive N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) fragments.13 Previously mentioned above, NT‐proBNP concentration is elevated in the event of a cardiac impairment. NT‐proBNP also exhibits longer biological half‐life, measuring of which aids in the emergency diagnosis of HF.14, 15 Interestingly enough, growing evidence supports the idea that sST2 and NT‐proBNP play important roles as significant diagnostic indicators for CHF.13, 16 However, Hughes et al.17 demonstrated that sST2 may not be useful in predicting the incidence of cardiovascular events or HF. At the same time, NT‐proBNP has been studied in CHF patients with other predictors, and no data support the role of sST2 and NT‐proBNP combination in evaluating ventricular function and in turn diagnosing CHF.18 Due to the presence of this conflicting data, we studied the diagnostic value of serum sST2 and NT‐proBNP in adherence to CHF.
2. MATERIALS AND METHODS
2.1. Study subjects
One hundred and ninety‐seven CHF patients were selected randomly from the First Rehabilitation Hospital of Shanghai who were admitted between August 2015 and August 2016 to undergo this study. Of those 197, 130 were males and 67 were females having aged 62.31±11.56 years. According to New York Heart Association Functional Classification (NYHA‐FC), there were 62 patients in class II, 70 patients in class III, and 65 patients in class IV of ventricular function. The CHF diagnostic criteria used were in accordance with the European Society of Cardiology (ESC) Guidelines having specified the prevention, diagnosis, and treatment of both acute and chronic HF.19 The principle diagnostic indicators included the following: paroxysmal nocturnal dyspnea, cardiac dilatation, rales (vein), and jugular venous distention. Secondary diagnostic indicators included: liver enlargement, edema, pleural effusion, and nocturnal cough. A patient was correctly diagnosed with CHF if they showed signs of two principle diagnostic indicators or a principle diagnostic indicator along with two secondary diagnostic indicators. The patients were excluded from the study if they were diagnosed with any of the following: acute myocardial infarction, severe liver diseases, fibrous dysplasia, and systemic diseases including diabetes mellitus, malignant tumor, hepatitis, or autoimmune diseases such as human immunodeficiency virus (HIV). One hundred and six healthy individuals (70 males and 36 females) aged 60.31±10.32 years were also included in this study serving as the normal control group during the same period. For these healthy individuals, routine laboratory blood and urine tests, liver and kidney function tests, color Doppler ultrasounds of heart, and chest X‐rays were all found at normal levels. The Ethics Committee of the First Rehabilitation Hospital of Shanghai approved this protocol. In addition, to further this study, written informed consents were obtained from each participating subject.
2.2. New York Heart Association Functional Classification (NYHA)
In accordance with NYHA, all patients were classified into the ventricular function class II, III, and IV.20 Patients in NYHA class I showed no symptoms and no limitations during ordinary physical activities, eg, no palpitation, no angina pectoris, or shortness of breath when walking or climbing stairs. While patients included in Class II showed mild symptoms (mild shortness of breath and/or angina) and slight limitations during ordinary activities, the patients were found to be comfortable at rest with limited exertion. Patients who belonged to Class III showed marked limitations in activities due to heightened results of their symptoms, even during less‐than‐ordinary activities such as walking short distances (20‐100 m). The patients in Class III were only found to be comfortable while at rest; whereas the patients included in Class IV exhibited severe limitations in ordinary physical activities. Any physical activity that brought discomfort and showed symptoms occurred at rest also.
2.3. Sample collection
All subjects were fasting and 20 mL of venous blood was extracted from each subject. Each sample was divided evenly and put into two tubes. One tube with blood coagulation accelerator added was centrifuged (2054 g) at 4°C for 15 minutes with serum obtained, whereas another tube was added with anticoagulant agent. All samples needed to be preserved in a −80°C refrigerator without repeated thawing if they were not detected in time.
2.4. Detection methods
To detect concentration of sST2, an enzyme‐linked immunosorbent assay (ELISA) Kit (Shanghai Research Institute for Enzyme‐linked Biology, Shanghai, China) was implemented. A standard curve for the sST2 concentration was plotted with the sST2 concentration as the X‐axis and optical density (OD) as the Y‐axis. The corresponding sST2 concentration was found according to the OD value in the standard curve, multiplying with the dilution ratio for the real concentration of the sST2 in serum samples.
The concentration of NT‐proBNP was determined using the electro‐chemiluminescence immunoassay analyzer (Cobas E411, F. Hoffmann‐La Roche CO, Ltd, Basel, Switzerland) and the detection kit, namely the Human N end forebrain natriuretic peptide assay kit provided by Roche Diagnostics CO, Ltd (Shanghai, China). The blood sample was added into the electro‐chemiluminescence immunoassay analyzer according to the instructions of the kit with the real concentration of the NT‐proBNP in samples being detected.
An automatic biochemical analyzer (Automatic biochemical analyzer 7180, Hitachi Ltd, Tokyo, Japan) was used to determine the levels of blood urea nitrogen (BUN), creatinine(Cr), alanine aminotransferase (ALT), triglyceride(TG), high‐density lipoprotein cholesterol (HDL‐C), low‐density lipoprotein cholesterol (LDL‐C), and uric acid (UA).
2.5. Statistical analysis
Data were analyzed using the statistical package for the social sciences (SPSS) version 21.0 (SPSS Inc., Chicago, IL, USA). Measurement data were displayed as mean±standard deviation and conformed to the test of normality. That data skewed distribution was presented using the median and interquartile (Q=P75‐P25) and those in accordance with the normal distribution were examined by the Kolmogorov‐Smirnov test and Shapiro‐Wilk test. The measurement data were tested by the t test, and among multiple groups, the comparisons were detected by the analysis of variance, whereas the count data were presented by the frequency with χ2 for comparisons. Spearman's correlation coefficient was used to determine the relationship between concentrations of sST2 and NT‐ProBNP, with their indexes relating to ventricular function. A receiver operating characteristic (ROC) curve was plotted to assess CHF diagnostic value of sST2 and NT‐ProBNP. Binary logistic regression analysis was conducted for the risk factors of CHF. A significant level of P<.05 was used to evaluate the significant differences in the statistics.
3. RESULTS
3.1. Baseline characteristics of subjects
No significant differences in gender, age, weight, heart rate, body mass index (BMI), BUN, ALT, and TG between the CHF patients belonged to different classes of ventricular function and the control subjects. However, the levels of UA, Cr, HDL‐C, and LDL‐C exhibited significant differences (P<.05, Table 1).
Table 1.
Comparison of baseline characteristics of subjects among the ventricular function class II, III, and IV groups and normal control group
| Characteristic | Class II group (n=62) | Class III group (n=70) | Class IV group (n=65) | Normal control group (n=106) | P |
|---|---|---|---|---|---|
| Gender (M/F) | 41/21 | 46/24 | 43/22 | 70/46 | .804 |
| Age (y) | 59.76±14.97 | 61.54±11.34 | 64.31±10.70 | 60.31±10.32 | .108 |
| Weight (kg) | 64.52±13.46 | 65.00±14.55 | 71.01±17.98 | 68.29±15.75 | .056 |
| HR (/min) | 79.34±9.66 | 82.03±11.57 | 80.49±10.88 | 78.56±9.31 | .150 |
| BMI (kg/m2) | 24.95±5.92 | 24.93±5.84 | 27.66±7.53 | 29.10±8.24 | .096 |
| BUN (mmol/L) | 6.67±2.01 | 6.95±2.25 | 7.01±2.34 | 6.58±1.98 | .513 |
| ALT (U/L) | 25.99±6.21 | 27.12±7.53 | 29.01±8.15 | 26.49±6.17 | .066 |
| UA (μmol/L) | 352.65±27.18a | 389.12±30.52a, b | 411.25±32.69a, b, c | 301.21±20.64 | <.001 |
| TG (mmol/L) | 1.61±0.56 | 1.57±0.32 | 1.72±0.63 | 1.53±0.49 | .118 |
| Cr (μmol/L) | 86.30±9.87a | 109.32±12.98a, b | 132.57±16.08a, b, c | 69.77±8.54 | <.001 |
| HDL‐C (mmol/L) | 1.98±0.46a | 1.79±0.43a | 1.35±0.25a, b, c | 2.31±0.57 | <.001 |
| LDL‐C (mmol/L) | 2.75±0.41a | 3.12±0.61a, b | 3.65±0.78a, b, c | 2.50±0.35 | <.001 |
M, male; F, female; HR, heart rate; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; ALT, alanine aminotransferase, UA, uric acid; TG, triglyceride; HDL‐C, high‐density lipoprotein cholesterol, LDL‐C, low‐density lipoprotein cholesterol.
P<.05, compared with the normal control group.
P<.05, compared with the Class II group.
P<.05, compared with the Class III group.
3.2. Comparisons of serum ST2 and NT‐ProBNP levels among four groups
The sST2 and NT‐ProBNP concentrations were higher in the ventricular function II, III, and IV than concentrations in the normal control group (P<.05). Severity‐dependent manners showed in the concentrations of sST2 and NT‐ProBNP (P<.05, Figure 1).
Figure 1.
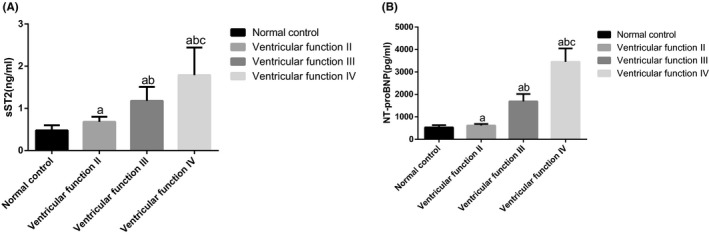
Comparisons of sST2 and NT‐ProBNP concentrations among four groups. Note: (A) Comparisons of sST2 concentrations among four groups; (B) Comparisons of NT‐ProBNP concentrations among four groups; a P<.05, compared with the control group; b P<.05, compared with the ventricular function II group; c P<.05, compared with the ventricular function III group; sST2, serum soluble suppression of tumorigenicity 2; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide
3.3. Correlations of serum sST2 and NT‐ProBNP levels with NYHA and clinical features among four groups
Spearman's correlation analysis was adopted to detect the correlations lgsST2 and lgNT‐proBNP had with ventricular function (NYHA) classification and clinical feature. The result showed that there were no remarkable correlation between lgsST2 and lgNT‐proBNP with gender, age, weight, heart rate, BMI, BUN, ALT, and TG between the normal control and CHF groups (P>.05), both having positive correlations with UA, Cr, and LDL‐C but negative correlation with HDL‐C. The lgsST2 and lgNT‐proBNP correlated positively with each other, especially among CHF patients. They are also in positive correlation with the ventricular function classification (Tables 2 and 3).
Table 2.
Correlation analyses for the concentrations of sST2 and NT‐ProBNP with baseline characteristics of normal controls
| Characteristic | sST2 | NT‐proBNP | ||
|---|---|---|---|---|
| r | P | r | P | |
| Gender (M/F) | .178 | .069 | .038 | .698 |
| Age (y) | −.054 | .589 | .05 | .612 |
| Weight (kg) | .434 | .659 | −.014 | .887 |
| HR (/min) | −.019 | .844 | .122 | .214 |
| BMI (kg/m2) | −.037 | .703 | .057 | .563 |
| BUN (mmol/L) | −.023 | .816 | −.084 | .391 |
| ALT (U/L) | −.813 | .407 | −.03 | .762 |
| UA (μmol/L) | .497 | <.001 | .207 | .033 |
| TG (mmol/L) | −.019 | .844 | 0 | .998 |
| Cr (μmol/L) | .36 | <.001 | .319 | <.001 |
| HDL‐C (mmol/L) | −.326 | <.001 | −.293 | .002 |
| LDL‐C (mmol/L) | .364 | <.001 | .397 | <.001 |
| lgNT‐proBNP (pg/mL) | .733 | <.001 | — | — |
| lgsST2 (ng/mL) | — | — | .733 | <.001 |
M, male; F, female; HR, heart rate; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; ALT, alanine aminotransferase, UA, uric acid; TG, triglyceride; HDL‐C, high‐density lipoprotein cholesterol, LDL‐C, low‐density lipoprotein cholesterol; sST2, serum soluble suppression of tumorigenicity 2; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide; CHF, chronic heart failure.
Table 3.
Correlation analyses for the concentrations of sST2 and NT‐ProBNP with baseline characteristics of CHF patients
| Characteristic | sST2 | NT‐proBNP | ||
|---|---|---|---|---|
| r | P | r | P | |
| Gender (M/F) | −.090 | .210 | .009 | .905 |
| Age (y) | .095 | .184 | .133 | .063 |
| Weight (kg) | .110 | .125 | .120 | .094 |
| HR (/min) | .135 | .058 | .042 | .553 |
| BMI (kg/m2) | .089 | .212 | .129 | .070 |
| BUN (mmol/L) | .043 | .548 | .049 | .494 |
| ALT (U/L) | .077 | .285 | .138 | .053 |
| UA (μmol/L) | .463 | <.001 | .591 | <.001 |
| TG (mmol/L) | .021 | .765 | .060 | .401 |
| Cr (μmol/L) | .624 | <.001 | .815 | <.001 |
| HDL‐C (mmol/L) | −.321 | <.001 | −.499 | <.001 |
| LDL‐C (mmol/L) | .418 | <.001 | .499 | <.001 |
| lgNT‐proBNP (pg/mL) | .706 | <.001 | — | — |
| lgsST2 (ng/mL) | — | — | .706 | <.001 |
| Ventricular function (NYHA) | .736 | <.001 | .966 | <.001 |
M, male; F, female; HR, heart rate; BMI, body mass index; BUN, blood urea nitrogen; Cr, creatinine; ALT, alanine aminotransferase, UA, uric acid; TG, triglyceride; HDL‐C, high‐density lipoprotein cholesterol, LDL‐C, low‐density lipoprotein cholesterol; sST2, serum soluble suppression of tumorigenicity 2; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide; CHF, chronic heart failure; NYHA, New York Heart Association Functional Classification.
3.4. Relevance of serum sST2 and NT‐ProBNP levels as diagnostic indicators of CHF
The ROC curve indicated that the area under the curve of sST2 was found/calculated to be 0.942 (95% CI: 0.917‐0.966) and the AUC of NT‐proBNP was found/calculated to be 0.920 (95% CI: 0.891‐0.948). The AUC of the sST2+NT‐proBNP curve was found to be 0.968 (95% CI: 0.953‐0.984) and the combination of sST2 and NT‐proBNP had a sensitivity of 85.3% and specificity of 97.2%. These results suggested that sST2 combined with NT‐ProBNP may be a better diagnostic indicator for CHF (Figure 2 and Table 4). Association between CHF and sST2, NT‐proBNP, UA, Cr, HDL‐C, and LDL‐C was examined with binary logistic regression analysis, demonstrating that sST2 (OR=1.148, 95% CI: 1.052‐1.253), NT‐proBNP (OR=1.012, 95% CI: 1.000‐1.023), UA (OR=1.084, 95% CI: 1.036‐1.133), and Cr (OR=1.187, 95% CI: 1.068‐1.320) were the risk factors for CHF, whereas the HDL‐C and LDL‐C had no relation with the occurrence of CHF (P>.05).
Figure 2.
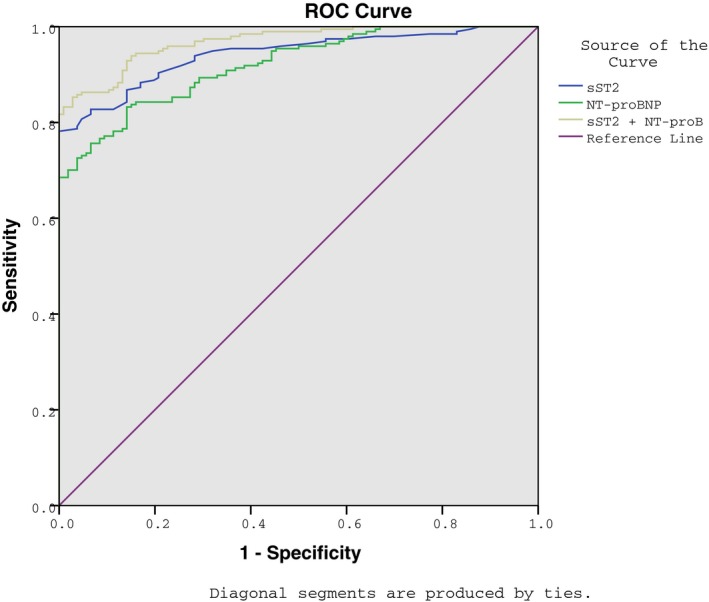
The diagnostic value of sST2 and NT‐ProBNP in CHF, estimated using an ROC curve. Note: sST2, serum soluble suppression of tumorigenicity 2; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide; CHF, chronic heart failure; ROC, receiver operating characteristic
Table 4.
The diagnostic value of sST2 and NT‐ProBNP in CHF, estimated using the ROC curve
| Variable | AUC | SE | P | 95%CI | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|
| sST2 | 0.942 | 0.012 | <.001 | 0.917‐0.966 | 78.2 | 100 |
| NT‐proBNP | 0.920 | 0.014 | <.001 | 0.891‐0.948 | 83.2 | 85.8 |
| sST2+NT‐proBNP | 0.968 | 0.008 | <.001 | 0.953‐0.984 | 85.3 | 97.2 |
sST2, serum soluble suppression of tumorigenicity 2; NT‐proBNP, amino‐terminal pro‐brain natriuretic peptide; CHF, chronic heart failure; ROC, receiver operating characteristic; AUC, area under the curve; SE, standard error; CI, confidence interval.
4. DISCUSSION
Chronic heart failure is a leading public health problem and its occurrence is rapidly increasing.21 Although recent decades have witnessed great achievements in it treatment, the mortality of HF patients continues to increase.22This study investigated the significance of sST2 and NT‐ProBNP and their combination in evaluating ventricular function, hence diagnosing CHF. The findings indicated that serum sST2 and NT‐ProBNP can act as indicators in evaluating ventricular function, therefore successfully diagnosing CHF.
Initially, our study demonstrated that the levels of UA, Cr, and LDL‐C were increased but the level of HDL‐C was decreased with the severity of ventricular dysfunction. Zhang et al.23 also reported that serum UA level correlated with the severity of ventricular dysfunction and could be adapted as an independent predictor of death in patients with CHF. Vizzardi et al.24 advocated that serum Cr levels exhibited an independent prognostic value for cardiovascular diseases. HDL‐C levels were found to supply cardiovascular protection by facilitating reverse cholesterol transport from macrophages.25 Rohatgi et al.26 demonstrated that a low HDL‐C level is one of the risk factors for the occurrence and progression of atherosclerotic cardiovascular disease. Finally, Imano et al.27 demonstrated that a high LDL‐C level was positively related with the risk of CHF. However, by logistic regression analysis among UA, Cr, LDL‐C, and HDL‐C, only UA and Cr were found to be risk factors of CHF. An explanation for this could be provided by the fact that CHF resulted from a wide spectrum of cardiovascular diseases.4 In addition, a relatively small sample size may contribute in the weak association between LDL‐C and HDL‐C levels and ventricular dysfunction in CHF patients. To be more precise, a performance of a large cohort study would be necessary.
Essentially, our findings suggested that the sST2 and NT‐ProBNP concentrations increased significantly in the CHF patients than those in the healthy control subject groups, showing severity‐dependent manners. The sST2 concentration plays an important role in inflammation and fibrosis, both of which are promoted in CHF, leading to adverse ventricular remodeling.28 As shown in a study reported by Weinberg et al.29 patients with severe chronic NYHA class III to IV HF, the alternations of sST2 levels were an indicator in predicting subsequent death or transplantation. Usually requiring prolonged volume and pressure‐overload induced ventricular wall expansion, the release of BNP was primarily regulated at the transcriptional level.12 Although BNP and NT‐ProBNP were equally released, the NT‐ProBNP with higher molecular weight and longer half‐life had higher serum levels than BNP and might have been a more sensitive indicator of cardiac function.30 Consistent with our study, Mir et al.31 reported that NT‐ProBNP concentration was significantly increased in patients with congestive HF, possibly reflecting the degree of cardiac dysfunction. Januzzi et al.30 found that the diagnostic threshold of NT‐ProBNP was 443 mg/L which only above could be used to diagnose HF. Consequently, the concentrations of sST2 and NT‐ProBNP were associated with the severity of CHF, in turn reflecting ventricular function. In addition, the diagnostic value of sST2, NT‐ProBNP, and the presence of their combination in CHF was detected and it was also revealed that the combination of sST2 and NT‐ProBNP was a better diagnostic indicator than sST2 and NT‐ProBNP individually for CHF. Consistently, Bayes‐Genis et al.21 attempts to explore the combined usefulness of ST2 and NT‐proBNP to promote the prediction of mortality in CHF. It is believed that our study coupled with that of Bayes‐Genis et al. will highlight the combined usefulness of sST2 and NT‐proBNP in clinical practice for diagnosing CHF.
Nevertheless, the two investigated indicators were analyzed in frozen samples. The risk of this method of analyzation being that the absolute levels of sST2 and NT‐ProBNP could be influenced by measurement deviation from frozen instead of fresh samples. Besides, CHF is a multifactorial disease and a larger cohort study would be further required to eliminate bias. In spite of all these limitations, our study provides evidence that sST2, NT‐proBNP, UA, and Cr as risk factors of CHF and serum sST2 and NT‐ProBNP can act as indicators in evaluating ventricular function, hence diagnosing CHF. If our results are validated, the incorporation of these two biomarkers into clinical practice for the prediction of the incidence and prevalence of CHF could be accomplished quicker.
ACKNOWLEDGMENTS
This study was supported by grants from Science and Technology Commission of Yangpu District, Shanghai, Health and Family Planning Commission Research Projects of Yangpu District, Shanghai (No. YP15M02). We would like to acknowledge the helpful comments on this study received from our reviewers.
Jin X‐L, Huang N, Shang H, et al. Diagnosis of chronic heart failure by the soluble suppression of tumorigenicity 2 and N‐terminal pro‐brain natriuretic peptide. J Clin Lab Anal. 2018;32:e22295 10.1002/jcla.22295
Xiao‐Ling Jin and Ning Huang are regarded as co‐first authors.
REFERENCES
- 1. Dennison CR, McEntee ML, Samuel L, Johnson BJ, Rotman S, Kielty AandRussell SD. Adequate health literacy is associated with higher heart failure knowledge and self‐care confidence in hospitalized patients. J Cardiovasc Nurs. 2011;26:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. WRITING GROUP MEMBERS , Lloyd‐Jones D, Adams RJ, et al. Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46. [DOI] [PubMed] [Google Scholar]
- 3. Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lewis EF, Solomon SD, Jablonski KA, et al. Predictors of heart failure in patients with stable coronary artery disease: a PEACE study. Circ Heart Fail. 2009;2:209‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. National Clinical Guideline Centre . Chronic Heart Failure: National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care: Partial Update. London: Royal College of Physicians; 2010. [PubMed] [Google Scholar]
- 6. McMurray JJ, Pfeffer MA. Heart failure. Lancet. 2005;365:1877‐1889. [DOI] [PubMed] [Google Scholar]
- 7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev Esp Cardiol. 2016;69:1167. [DOI] [PubMed] [Google Scholar]
- 8. Watanabe M, Takizawa H, Tamura M, et al. Soluble ST2 as a prognostic marker in community‐acquired pneumonia. J Infect. 2015;70:474‐482. [DOI] [PubMed] [Google Scholar]
- 9. Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL‐33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Investig. 2007;117:1538‐1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayakawa H, Hayakawa M, Kume A, Tominaga S. Soluble ST2 blocks interleukin‐33 signaling in allergic airway inflammation. J Biol Chem. 2007;282:26369‐26380. [DOI] [PubMed] [Google Scholar]
- 11. Bayes‐Genis A, Zhang Y, Ky B. ST2 and patient prognosis in chronic heart failure. Am J Cardiol. 2015;115(7 Suppl):64B‐69B. [DOI] [PubMed] [Google Scholar]
- 12. Oyama MA, Fox PR, Rush JE, Rozanski EA, Lesser M. Clinical utility of serum N‐terminal pro‐B‐type natriuretic peptide concentration for identifying cardiac disease in dogs and assessing disease severity. J Am Vet Med Assoc. 2008;232:1496‐1503. [DOI] [PubMed] [Google Scholar]
- 13. Fox PR, Oyama MA, Reynolds C, et al. Utility of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) to distinguish between congestive heart failure and non‐cardiac causes of acute dyspnea in cats. J Vet Cardiol. 2009;11(Suppl 1):S51‐S61. [DOI] [PubMed] [Google Scholar]
- 14. Fan J, Jouni H, Khaleghi M, Bailey KR, Kullo IJ. Serum N‐terminal pro‐B‐type natriuretic peptide levels are associated with functional capacity in patients with peripheral arterial disease. Angiology. 2012;63:435‐442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luterek K, Szymusik I, Bartkowiak R, Koltowski L, Filipiak KJ, Wielgos M. N‐terminal pro‐B‐type natriuretic peptide: a potential marker of fetal heart failure in hemolytic disease. Neuro Endocrinol Lett. 2011;32:657‐662. [PubMed] [Google Scholar]
- 16. Miller WL, Saenger AK, Grill DE, Slusser JP, Bayes‐Genis A, Jaffe AS. Prognostic value of serial measurements of soluble suppression of tumorigenicity 2 and galectin‐3 in ambulatory patients with chronic heart failure. J Cardiac Fail. 2016;22:249‐255. [DOI] [PubMed] [Google Scholar]
- 17. Hughes MF, Appelbaum S, Havulinna AS, et al. ST2 may not be a useful predictor for incident cardiovascular events, heart failure and mortality. Heart. 2014;100:1715‐1721. [DOI] [PubMed] [Google Scholar]
- 18. Taniguchi R, Sato Y, Yamada T, et al. Combined measurements of cardiac troponin T and N‐terminal pro‐brain natriuretic peptide in patients with heart failure. Circ J. 2004;68:1160‐1164. [DOI] [PubMed] [Google Scholar]
- 19. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 20. Fisher JD. New York Heart Association Classification. Arch Intern Med. 1972;129:836. [PubMed] [Google Scholar]
- 21. Bayes‐Genis A, de Antonio M, Galan A, et al. Combined use of high‐sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur J Heart Fail. 2012;14:32‐38. [DOI] [PubMed] [Google Scholar]
- 22. van den Berge JC, Akkerhuis MK, Constantinescu AA, Kors JA, van Domburg RT, Deckers JW. Temporal trends in long‐term mortality of patients with acute heart failure: data from 1985‐2008. Int J Cardiol. 2016;224:456‐460. [DOI] [PubMed] [Google Scholar]
- 23. Zhang CY, Ma LL, Wang LX. Relationship between serum uric acid levels and ventricular function in patients with idiopathic pulmonary hypertension. Exp Clin Cardiol. 2013;18:e37‐e39. [PMC free article] [PubMed] [Google Scholar]
- 24. Vizzardi E, Nodari S, Caretta G, et al. Effects of spironolactone on long‐term mortality and morbidity in patients with heart failure and mild or no symptoms. Am J Med Sci. 2014;347:271‐276. [DOI] [PubMed] [Google Scholar]
- 25. Khera AV, Cuchel M, de la Llera‐Moya M, et al. Cholesterol efflux capacity, high‐density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rohatgi A. High‐density lipoprotein function measurement in human studies: focus on cholesterol efflux capacity. Prog Cardiovasc Dis. 2015;58:32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Imano H, Noda H, Kitamura A, et al. Low‐density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS). Prev Med. 2011;52:381‐386. [DOI] [PubMed] [Google Scholar]
- 28. Dieplinger B, Egger M, Haltmayer M, et al. Increased soluble ST2 predicts long‐term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin Chem. 2014;60:530‐540. [DOI] [PubMed] [Google Scholar]
- 29. Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721‐726. [DOI] [PubMed] [Google Scholar]
- 30. Januzzi JL, van Kimmenade R, Lainchbury J, et al. NT‐proBNP testing for diagnosis and short‐term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT‐proBNP Study. Eur Heart J. 2006;27:330‐337. [DOI] [PubMed] [Google Scholar]
- 31. Mir TS, Marohn S, Laer S, Eiselt M, Grollmus O, Weil J. Plasma concentrations of N‐terminal pro‐brain natriuretic peptide in control children from the neonatal to adolescent period and in children with congestive heart failure. Pediatrics. 2002;110:e76. [DOI] [PubMed] [Google Scholar]


