Abstract
Background
Our aim was to examine the performance of IMMULITE 2000 assay for specific IgE (sIgE) by comparing it with ImmunoCAP technology in light of a clinical background.
Methods
Measurements of sIgE were done in a selected patient group (N = 569; varied sample size for each allergen) and in a random sample group (N = 100; 8 allergens). sIgE results were correlated with skin‐prick test results (selected patients) and medical history (nonselected patients).
Results
We have detected fair to excellent correlation and agreement between the results of both assays, despite their methodological differences, both in selected and nonselected patient group (ρc = 0.431–0.976; ρc = 0.390–0.972, respectively). Associations of sIgE levels with skin‐prick test (SPT) levels and medical history have shown significant correlation for both assays for majority of tested allergens, where applicable (D. pteronyssinus, cat dander, egg white, milk, peanut, orchard grass, Alternaria tenuis, and common ragweed in selected patients; birch, cat dander, common ragweed, D. pteronyssinus, and orchard grass in nonselected; P < 0.05 for all).
Conclusions
Laboratory testing for sIgE can be successfully accomplished by IMMULITE 2000 immunoanalyzer at a diagnostic accuracy relative to SPT, comparable to the results acquired by CAP technology but not fully comparable on the level of an individual patient.
Keywords: specific IgE, in vitro tests, IMMULITE 2000, ImmunoCAP
Introduction
Atopic diseases such as asthma, allergic rhinitis, and atopic dermatitis—all share common pathogenic pathways and are characterized by an increase in circulating allergen‐specific IgE (sIgE) antibodies 1. Developing countries still record a significant growth in incidence and prevalence of allergic disorders, which makes an early diagnosis even more important in preventing further disease progression 2.
In vitro laboratory tests for sIgE antibodies together with skin‐prick test (SPT) are routinely used in the diagnosis of allergic disorders initiated through a type I hypersensitivity mechanism 3. However, the presence of sIgE is not 100% specific for the development of symptoms upon allergen exposure, but its presence can be viewed as a significant risk factor. Results of such tests need to be correctly interpreted because they are important in establishing a diagnosis of an allergic disease and in depicting a proper treatment (e.g., allergen‐specific immunotherapy). They also serve as a biomarker of a predisposition for allergic disease and must be observed within the context of patient's clinical background 4.
The first assay for routine sIgE detection in serum was radioisotope‐based radioallergosorbent test (RAST), which was introduced into market as Phadebas RAST assay (Pharmacia Diagnostics, Uppsala, Sweden) soon after IgE discovery in 1967 5. Phadebas RAST assay, together with subsequently modified ones, used radioiodinated polyclonal antihuman IgE as signal detection antibody and had issues with time consumption (two overnight incubations), detection limit, and results categorization 6. Through years, assay technologies evolved and method called Pharmacia CAP Systems based on ImmunoCAP technology has been developed. ImmunoCAP is an in vitro test using a 3‐dimensional cellulose solid allergen phase 7. It is a quantitative test developed by Pharmacia Diagnostics from the original RAST technology 8. It has been a standard and a method of choice, due to its analytical and clinical performances, widespread use, and satisfactory consistency in comparison with SPT results 9, 10. Altogether, it has shown improvements in quality and allergen composition which varied from natural proteins to recombinant proteins, greater speed, higher binding capacity and use of nonisotopic labels 11, 12. However, this system has its limitations among which sample management, turnaround time, personnel requirements, and solid‐phase allergen immobilization are the most prominent ones 13.
The new sIgE assay systems have been developed with improvements in technology, procedure automation, shorter turnaround times, high throughput and use of chemiluminescence 12, 14. The sIgE assay studied here is IMMULITE 2000 Immunoassay System (Siemens, Zagreb, Croatia). Its characteristics are: a broader working range, ability to report results quantitatively and categorized, use of liquid allergens, automation features which reduce labor requirements, total assay time, and possibility of error 15.
Aim of this study was to evaluate the performance of IMMULITE 2000 assay by comparing its results with results obtained with the established ImmunoCAP technology in light of a clinical background. We compared their performance and agreement both in selected and nonselected patient groups. In addition, the laboratory results given from these two assays were compared with SPT results (selected patient group) and clinical expression (nonselected patient group) to examine the clinical data reliability.
Materials and Methods
Study population
We performed analysis in two patient groups. First studied group consisted of selected patients, for which the inclusion criteria were clinical history consistent with IgE‐mediated allergy to aeroallergens and insect venom or food allergy which were considered in this study with a positive SPT to allergen extracts. The exclusion criteria were use of medication that could influence the SPT results and the presence of other comorbidities such as infections, immunological disorders, and autoimmune diseases. Serum analysis was done in accordance with clinical indications during the usual diagnostic process and using an excess of serum for additional (IMMULITE 2000) specific and total IgE measurements for which patients and/or guardians had signed an informed consent.
The other group consisted of nonselected patients. Serum samples from a total of 100 randomly selected patients were obtained from banked excess serum on which patients and/or guardians had given written consent for additional sIgE antibody testing. All of the serum samples were originally collected for clinical evaluation from Children's Hospital Srebrnjak (CHS) between April 2013 and March 2015.
The study was approved by the Hospital Ethics Committee and was done in accordance with the Declaration of Helsinki 16.
Serologic analyses
n selected patients' group (N = 569, 371 male, mean age 8.8 [SD 5.3] years), serum samples were tested for total IgE, Dermatophagoides pteronyssinus (d1), cat dander (e1), egg white (f1), milk (f2), peanut (f13), orchard grass (g3), honey bee venom (i1), yellow jacket venom (i3), Alternaria tenuis (m6), birch (t3), common ragweed (w1), and two recombinant allergens: rApi m1‐Apis mellifera and rVes v5‐Vespula vulgaris. Sample size for each of the tested parameters varied (Table 1).
Table 1.
Agreement Between Immulite 2000 and UniCAP 100 Systems in Selected Group of Patients
| Tested parameter | N | Agreement | Lin's concordance test values (95% Cl) |
|---|---|---|---|
| Total IgE | 121 | Almost perfect | 0.976 (0.970–0.980) |
| Aeroallergens | |||
| Alternaria tenuis | 76 | Substantial | 0.651 (0.548–0.735) |
| Birch | 75 | Substantial | 0.610 (0.498–0.702) |
| Cat dander | 71 | Substantial | 0.761 (0.668–0.831) |
| Common ragweed | 74 | Almost perfect | 0.931 (0.893–0.956) |
| D. pteronyssinus | 66 | Almost perfect | 0.918 (0.872–0.948) |
| Orchard Grass | 74 | Substantial | 0.720 (0.638–0.786) |
| Food allergens | |||
| Egg white | 77 | Substantial | 0.654 (0.559–0.731) |
| Milk | 68 | Almost perfect | 0.927 (0.889–0.952) |
| Peanut | 79 | Almost perfect | 0.895 (0.845–0.930) |
| Insect venom | |||
| Honey bee venom | 40 | Almost perfect | 0.951 (0.910–0.974) |
| Yellow jacket venom | 36 | Almost perfect | 0.828 (0.711–0.900) |
| rApi m 1 ‐ Apis mellifera | 33 | Almost perfect | 0.859 (0.747–0.923) |
| rVes v 5 ‐ Vespula vulgaris | 28 | Moderate | 0.431 (0.331–0.521) |
0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement.
In nonselected group of patients (N = 100, 54 male, mean age 8.5 [SD 3.9] years), the testing was performed for five aeroallergens and three food allergens in all serum samples. Chosen allergens were representative for our laboratory workload and geographical region. Aeroallergen group was composed of Dermatophagoides pteronyssinus (d1), cat dander (e1), common ragweed (w1), birch (t3), and orchard grass (g3). The allergens in the food group were: egg white (f1), milk (f2), and peanut (f13).
Blood samples were drawn up to 4 ml in serum separation vacuum tubes and sent to the laboratory. Upon centrifugation at 3000 g for 10 min, a serum sample from every subject for the sIgE measurement was kept at −20°C (storage under 24 hr) or at −80°C (long‐term storage), until the analysis on each of two platforms was performed, on the same day. IgE antibodies specific for listed allergens were quantified by the IMMULITE 2000 (Siemens Medical Solutions Diagnostics, Tarrytown, New York) and UniCAP 100 FEIA (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer's assay protocols. Serum volume requirements differ among assays; hence, 50 μl per allergen is needed for IMMULITE 2000 and 40 μl per allergen for UniCAP.
Method used
We measured the concentration of circulating sIgE by two methods: UniCAP 100 fluorescence enzyme immunoassay (CAP FEIA) system (Thermo Fisher) and IMMULITE 2000 chemiluminescent immunoassay system (Siemens). The allergens were obtained from the manufacturers and all testing was performed according to their instructions.
The UniCAP 100, which is based on ImmunoCAP technology, is a semiautomated procedure that requires manual allergen distribution. The allergen of interest is covalently coupled to a solid‐phase cellulose capsule consisting of a flexible, activated hydrophilic polymer carrier, which has a large surface and high binding capacity, which ensures the coupling of a maximum allergen amount 5, 17. The secondary IgE antibodies are bound to the enzyme β‐galactosidase 3. This enzyme recognizes allergen‐bound patient IgE and transforms methylumbelliferyl‐β‐D‐galactoside into a fluorescent product. The intensity of fluorescence produced in described manner depends on the concentration of the product and it directly correlates with the IgE bound to the allergen 3, 12. The calibrator is IgE bound to anti‐IgE caps using a six point quantitative curve. Calibration range is between <0.35 kUa/l and >100 kUA/l (UA = allergen‐specific unit), but results can also be reported using a six classes system 8.
The IMMULITE 2000 system is a fully automated immunoanalyzer which uses chemiluminescent detection system for sIgE quantification. Allergens used in IMMULITE system are bound covalently to soluble biotinylated polylysine polymers in a fluid phase that binds to a streptavidin‐biotin complex 15. The secondary anti‐IgE antibody is conjugated to alkaline phosphatase which recognizes patient‐bound IgE. A chemiluminiscent signal emission is initiated by substrate addition 12. The correlation between signal intensity and amount of allergen is determined from a seven concentration points' curve 3.
IMMULITE and UniCAP differ methodologically but use similar units of measurements relative to the total IgE standard (WHO IgE reference standard 75/502), which is a calibration method accepted as a gold standard 15. 1 IU has been shown to be equal to 2.42 ng of IgE protein 18. The levels for specific IgE are automatically extrapolated from a dose–response curve of total IgE according to a reference IgE standard.
The main differences between these two assays arise from the technology which is used and from the source and quality of allergenic extract.
Skin‐prick tests and clinical diagnosis
Skin‐prick test was carried out using commercially available allergen extracts (Stallergenes, Antony, France) for Alternaria tenuis, Birch, Cat dander, Common ragweed, D. pteronyssinus, Orchard Grass, Egg white, Milk, and Peanut. Test was performed and interpreted according to the established criteria 19. A drop of approximately 0.02 ml of allergen extracts and control solutions (histamine for positive control and saline for negative control) were injected with standardized polymethacrylate points (Stallergenes). Test reactions were read after 15 min, and the wheal diameters were measured. Only the reaction wheal showing a mean diameter of ≥3 mm bigger than the negative control was considered positive 20.
Clinical diagnosis of an allergic disease in a nonselected group was established before banking the serum by an experienced allergy specialist and was checked after the serum analysis from using the data from the patient's hospital digital medical record.
Statistical analyses
Categorical results were presented as absolute and relative (%) numbers. Continuous variables were presented as mean and standard deviation (SD), if they had a normal distribution. In cases where the distribution was not normal variables were presented as a median and interquartile range (IQR). The results of sIgE measurement with IMMULITE 2000 and UniCAP 100 were analyzed in order to determine intermethod agreement using Lin's concordance coefficient and Sign test for paired samples to test for differences. Spearman rank order correlation coefficient was calculated to assess the association between specific IgE and the SPT results in the selected sample. Binomial logistic regression analysis was performed to analyze the agreement between the level of sIgE (log values) and the positivity of clinical signs and/or symptoms connected with the exposure to specific allergen in the nonselected sample. The significance of all tests was assessed at the level of P < 0.05. Statistical analyses were done using STATISTICA version 12 (StatSoft, Inc., Tulsa, OK).
Results
Selected group of patients
Selected group of patients varied in size per each tested parameter. The agreement and Lin's concordance coefficient values for sIgE detection with IMMULITE 2000 and UniCAP 100 systems for the selected group of patients are displayed in Table 1 and Figure 1. The intermethod comparison has shown almost perfect agreement for total IgE and these allergens: D. pteronyssinus, milk, peanut, honey bee venom, yellow jacket venom, rApi m 1 (Apis mellifera), and common ragweed. Allergens such as cat dander, egg white, orchard grass, and birch have shown substantial agreement, while moderate agreement was detected for rVes v 5 (Vespula vulgaris).
Figure 1.
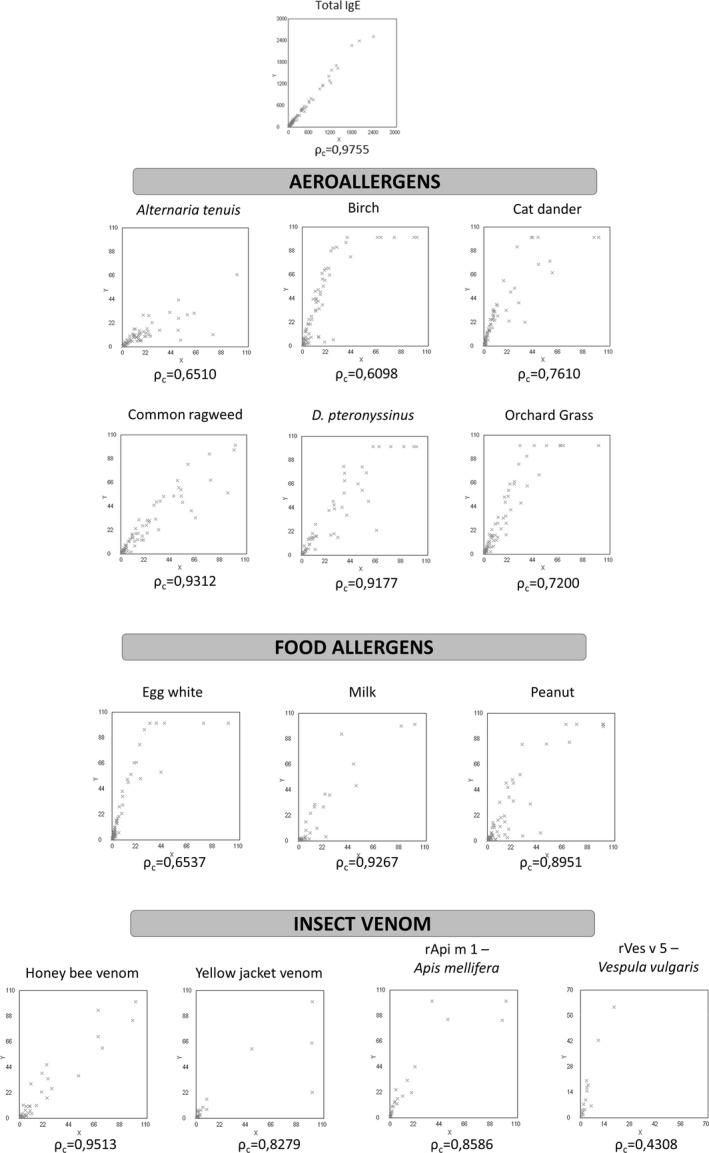
Scatterplots of the intermethod comparison between Unicap 100 FEIA and Immulite 2000 CLEIA systems in selected patients for listed parameters (x‐axis = UniCAP 100; y‐axis = IMMULITE 2000).
The IMMULITE values were significantly higher for total IgE (P < 0.0001), D. pteronyssinus (P = 0.0042), cat dander (P < 0.0001), egg white (P < 0.0001), milk (P < 0.0001), orchard grass (P < 0.0001), recombinant allergens for Apis mellifera (P < 0.0001) and Vespula vulgaris (P = 0.0013), birch (P < 0.0001), and common ragweed (P = 0.0251); and comparable for other tested allergens (Table 2).
Table 2.
Comparison Between Methods for the Values of Total IgE and sIgE Associations With the Results of Skin‐Prick Test (Where Applicable) in Selected Group of Patients (N = 569)
| Tested parameter | Assay system | Comparing two methods | Correlation with SPT | |||
|---|---|---|---|---|---|---|
| Median (IQR) | Z | P‐value | Spearman R | P‐value | ||
| Total IgE | CAP | 94.9 (37.7–231.0) | 8.000 | <0.0001 | N/A | N/A |
| IML | 113.0 (42.7–277.0) | N/A | N/A | |||
| Aeroallergens | ||||||
| Alternaria tenuis | CAP | 9.90 (2.68–18.80) | 4.301 | <0.0001 | 0.432 | 0.0014 |
| IML | 6.82 (2.73–11.90) | 0.322 | 0.0199 | |||
| Birch | CAP | 7.96 (2.28–18.10) | 5.929 | <0.0001 | 0.203 | 0.1027 |
| IML | 19.70 (3.90–54.80) | 0.335 | 0.0060 | |||
| Cat dander | CAP | 4.53 (1.58–12.00) | 7.464 | <0.0001 | 0.249 | 0.0456 |
| IML | 15.10 (5.12–32.50) | 0.373 | 0.0022 | |||
| Common ragweed | CAP | 10.16 (2.65–30.40) | 2.239 | 0.0251 | 0.337 | 0.0038 |
| IML | 12.60 (2.30–31.70) | 0.390 | 0.0007 | |||
| D. pteronyssinus | CAP | 12.20 (2.25–52.60) | 2.864 | 0.0042 | 0.347 | 0.0047 |
| IML | 17.2 (3.17–68.50) | 0.357 | 0.0035 | |||
| Orchard grass | CAP | 6.66 (1.89–19.10) | 6.554 | <0.0001 | 0.343 | 0.0040 |
| IML | 13.40 (3.55–46.90) | 0.404 | 0.0006 | |||
| Food allergens | ||||||
| Egg white | CAP | 1.94 (0.92–5.76) | 7.685 | <0.0001 | 0.496 | <0.0001 |
| IML | 5.36 (2.60–19.50) | 0.508 | <0.0001 | |||
| Milk | CAP | 1.44 (0.59–6.08) | 4.308 | <0.0001 | 0.626 | <0.0001 |
| IML | 0.55 (0.25–3.30) | 0.759 | <0.0001 | |||
| Peanut | CAP | 3.98 (1.29–17.50) | 0.574 | 0.5663 | 0.521 | <0.0001 |
| IML | 3.79 (0.54–21.20) | 0.671 | <0.0001 | |||
| Insect venom | ||||||
| Honey bee venom | CAP | 5.74 (1.63–21.25) | 0.961 | 0.3367 | N/A | N/A |
| IML | 5.01 (1.41–27.15) | N/A | N/A | |||
| Yellow jacket venom | CAP | 1.24 (0.45–3.22) | 1.014 | 0.3105 | N/A | N/A |
| IML | 1.12 (0.16–5.85) | N/A | N/A | |||
| rApi m 1 ‐ Apis mellifera | CAP | 1.11 (0.23–6.47) | 5.127 | <0.0001 | N/A | N/A |
| IML | 4.30 (0.62–18.00) | N/A | N/A | |||
| rVes v 5 ‐ Vespula vulgaris | CAP | 0.66 (0.09–2.87) | 3.213 | 0.0013 | N/A | N/A |
| IML | 1.61 (0.08–8.30) | N/A | N/A | |||
CAP, UniCAP 100 FEIA; IML, IMMULITE 2000 CLEIA.
Associations of sIgE values with SPT mean wheal values (where applicable) are shown in Table 2. Statistically significant correlations were found for both assays for D. pteronyssinus, cat dander, egg white, milk, peanut, orchard grass, Alternaria tenuis, and common ragweed (P < 0.05 for all, Spearman rank correlation). Correlation coefficients were, in general, higher (not significantly) for IMMULITE except for Alternaria tenuis.
Nonselected group of patients
In the nonselected group, samples of a total of 100 patients were analyzed. Ninety‐nine patients had skin‐prick test results, of which 45 (45.4%) had a positive one for different allergens. Forty‐nine patients were positive for atopy and 36 had a positive family history for atopy.
Table 3 (Fig. 2) shows the agreement and Lin's concordance coefficient values for sIgE detection with IMMULITE 2000 and UniCAP 100 systems for the nonselected group of patients. The intermethod comparison showed the almost perfect agreement for D. pteronyssinus, orchard grass, birch, and common ragweed. Cat dander, egg white, and peanut showed moderate agreement, while only milk had fair agreement.
Table 3.
Agreement Between Immulite 2000 and UniCAP 100 Systems in Nonselected Group of Patients
| Tested allergen | N | Agreement | Lin's concordance test values (95% Cl) |
|---|---|---|---|
| Aeroallergens | |||
| Birch | 100 | Almost perfect | 0.952 (0.931–0.966) |
| Cat dander | 100 | Moderate | 0.570 (0.539–0.599) |
| Common ragweed | 100 | Almost perfect | 0.972 (0.962–0.980) |
| D. pteronyssinus | 100 | Almost perfect | 0.964 (0.947–0.976) |
| Orchard grass | 100 | Almost perfect | 0.894 (0.860–0.920) |
| Food allergens | |||
| Egg white | 100 | Moderate | 0.457 (0.399–0.512) |
| Milk | 100 | Fair | 0.390 (0.325–0.450) |
| Peanut | 100 | Moderate | 0.475 (0.319–0.606) |
0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement.
Figure 2.
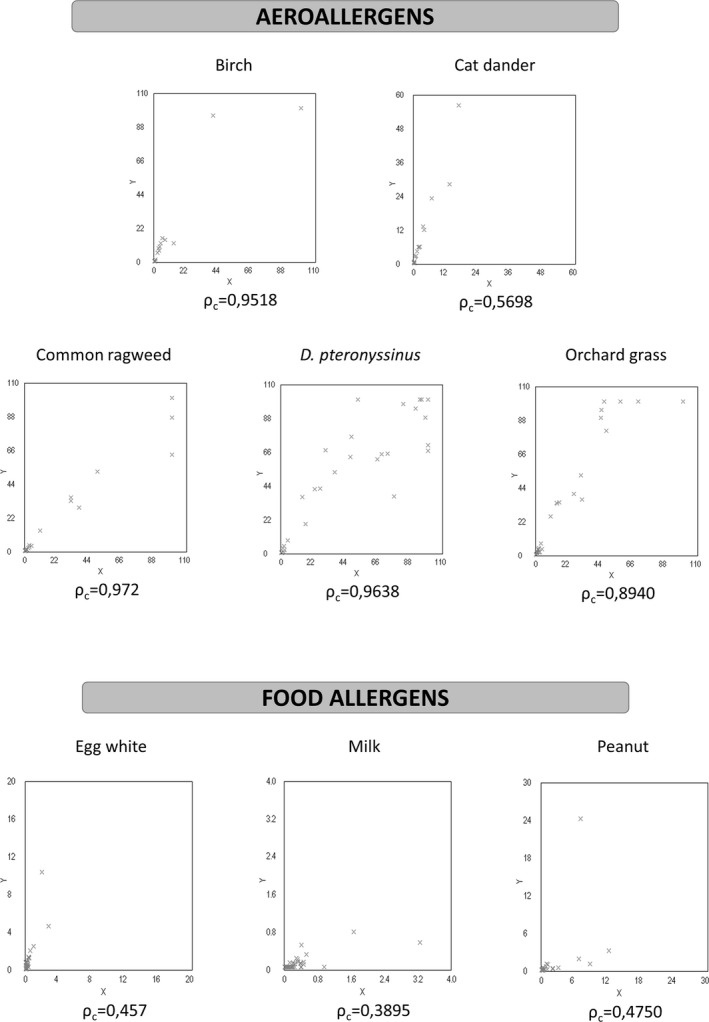
Scatterplots of intermethod comparison between Unicap 100 FEIA and Immulite 2000 CLEIA systems in nonselected patients for listed allergens (x‐axis = UniCAP 100; y‐axis = IMMULITE 2000).
The IMMULITE values were significantly higher for cat dander (P < 0.0001), egg white (P < 0.0001), peanut (P < 0.0001), orchard grass (P < 0.0001), and birch (P < 0.0001); and comparable for milk and ragweed (Table 4). When associating the results of sIgE with clinical features (Table 4), the odds ratios for positive allergy diagnosis with the rise in sIgE to egg white, peanut, and birch were higher for IMMULITE but not significantly different and comparable for other allergens.
Table 4.
Comparison between methods for the values of sIgE and the odds for positive allergy diagnosis with the rise in sIgE in nonselected group of patients (N = 100)
| Allergens | Assay system | Comparing two methods | Association with clinical features | ||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | Z | P‐value | OR | 95% Cl | P‐value | ||
| Aeroallergens | |||||||
| Birch | CAP | 0.02 (0.01–0.12) | 6.777 | <0.0001 | 5.43 | 2.34–12.57 | <0.0001 |
| IML | 0.05 (0.05–0.05) | 7.13 | 2.87–17.74 | <0.0001 | |||
| Cat dander | CAP | 0.01 (0.01–0.06) | 8.471 | <0.0001 | 5.30 | 1.42–19.76 | 0.01199 |
| IML | 0.05 (0.05–0.05) | 5.56 | 1.51–20.40 | 0.0089 | |||
| Common ragweed | CAP | 0.05 (0.04–0.23) | 1.333 | 0.1824 | 10.83 | 3.54–33.12 | <0.0001 |
| IML | 0.05 (0.05–0.05) | 11.45 | 3.68–35.60 | <0.0001 | |||
| D. pteronyssinus | CAP | 0.06 (0.02–28.35) | 4.954 | <0.0001 | 21.37 | 4.51–101.40 | 0.0001 |
| IML | 0.05 (0.05–41.80) | 20.62 | 4.59–92.56 | <0.0001 | |||
| Orchard grass | CAP | 0.03 (0.01–0.89) | 7.182 | <0.0001 | 5.60 | 2.80–11.17 | <0.0001 |
| IML | 0.05 (0.05–1.62) | 5.58 | 2.88–10.80 | <0.0001 | |||
| Food allergens | |||||||
| Egg white | CAP | 0.03 (0.01–0.12) | 8.182 | <0.0001 | 417.22 | 2.36–73644 | 0.0206 |
| IML | 0.05 (0.05–0.25) | 1047.93 | 0.82–1333980 | 0.0536 | |||
| Milk | CAP | 0.05 (0.02–0.18) | 0.203 | 0.8391 | No positive clinical features | ||
| IML | 0.05 (0.05–0.05) | ||||||
| Peanut | CAP | 0.03 (0.02–0.11) | 4.014 | <0.0001 | 4.22 | 0.90–19.82 | 0.0643 |
| IML | 0.05 (0.05–0.05) | 12.79 | 1.64–99.61 | 0.0137 | |||
OR (95% CI) for the positive diagnosis of allergy with each log point of sIgE.
CAP, UniCAP 100 FEIA; IML, IMMULITE 2000 3 g CLEIA; SD, standard deviation; OR, odds ratio; 95% Cl, 95% confidence interval.
Discussion
Allergen detection is critical in diagnosis and management of allergic disorders. General approaches currently used in practice are skin‐prick test (in vivo) and serum assay for specific IgE detection (in vitro). These two methods have their advantages and disadvantages. The SPT is more sensitive but time consuming and the skin reactions can be influenced by medication and dermatologic conditions 20, 21. In vitro test is less sensitive because sIgE does not have to be present in circulation, but more specific due to the fact that medications and dermatologic condition do not influence results.
As measurement of sIgE is important in allergy diagnosis and patient management, we compared sIgE levels measured by two different diagnostic platforms: UniCAP 100 and IMMULITE 2000. Both methods use WHO IgE reference standard 75/502 for calibration and should be mutually comparable because 1 kU of total IgE measured by IMMULITE and 1kU measured by UniCAP are equal to 1 International Unit (IU) of total IgE 22. One of the major differences between UniCAP and IMMULITE is the origin and quality of allergenic extracts which are being used.
We examined these two assays using serum samples from two groups of patients: selected patients chosen due to their positive allergy background and nonselected randomly chosen patients from a biobank. In addition, we have correlated our data with patients' SPT results (selected patients) and clinical histories (nonselected patients) to examine the magnitude and the cause of differences.
We have detected fair to excellent correlation and agreement between these two assays, despite their methodological differences for most of the tested allergens, but also found inter‐ and intra‐assay discordance for some allergens, which is consistent with previous studies. As this discordance was different for different allergens, it does not seem to occur due to the systemic differences between these two assays but was more likely to be connected with diversity and quality of allergenic extracts used 12, 23, 24, 25.
Our study has demonstrated good agreement of the IMMULITE method with CAP technology, which has been known as a “gold standard” for the routine sIgE detection. The agreement of the results obtained by IMMULITE with the results obtained in vivo by SPT was higher than those given by CAP although these differences were not statistically significant. The same was the case when the agreement with clinical features of allergy was tested. Specific IgE results given by IMMULITE 2000 appear to show better correlation with SPT results than sIgE results measured with UniCAP 100, which has been already reported 12. Complete concordance between sIgE in vitro test results and SPT cannot be expected due to the fact that in vitro tests measure circulating sIgE, while SPT is in vivo test which measures cutaneous mast cells' reactivity to tested allergen extracts. IMMULITE 2000 has shown a tendency to report results of sIgE at higher concentrations for several allergens than UniCAP 100, which has been more expressed in the higher range of results but for some of the allergens the situation was the opposite. Those differences can result from technical differences between these two methods, but can also be the consequence of the difference in allergen sources, and both.
Finally, IMMULITE 2000 overcomes limitations of UniCAP 100 using liquid phase allergens, enzyme‐enhanced chemiluminescent signal detection, use of zero calibrator, lowest calibrator being as low as 0.0 kU/l (lowest calibrator for UniCAP 100 is 0.35 kU/l), a detection limit of 0.1 kU/l, a functional sensitivity of 0.2 kU/l, and some efficiency features such as shorter turnaround time and higher automation level 15, 26.
Expanding the nonselected patients group should be taken into consideration in future studies. This would ensure a more precise assessment of odds for a positive diagnosis of allergy connected with a positive value of specific IgE and allow a more detailed comparison between the tested assays. Based on our results, we can only make limited conclusions because this study was not designed to identify the causes of different IgE antibody levels measured by the IMMULITE and UniCAP. Additional studies should be undertaken to provide better insight in intermethod discrepancies.
Our conclusion is that laboratory testing for sIgE can be successfully accomplished by an IMMULITE 2000 immunoanalyzer, at a diagnostic accuracy relative to clinical SPT. Obtained results are comparable to the results acquired by widespread CAP technology but because of some differences both methods are not fully comparable on the level of an individual patient. It should be noted that none of these methods of allergy detection represents an ideal solution. Results of such tests should always be interpreted considering patient's medical history and physical findings.
Acknowledgments
The authors thank the Siemens Healthcare Diagnostics for providing the material for sIgE determination free of costs. The authors state that Siemens Healthcare Diagnostics did not have any impact on the research design, research conduct, interpretation of the results, or drafting of the manuscript.
References
- 1. Stone K. Atopic diseases of childhood. Curr Opin Pediatr 2002;14:634–646. [DOI] [PubMed] [Google Scholar]
- 2. Asher M, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006;368:733–743. [DOI] [PubMed] [Google Scholar]
- 3. Goikoetxea M, Sanz M, García B, et al. Recommendations for the use of in vitro methods to detect specific immunoglobulin E: Are they comparable? J Investig Allergol Clin Immunol 2013;23:448–454. [PubMed] [Google Scholar]
- 4. Hamilton R, Mudd K, White M, et al. Extension of food allergen specific IgE ranges from the ImmunoCAP to the IMMULITE systems. Ann Allergy Asthma Immunol 2011;107:139–144. [DOI] [PubMed] [Google Scholar]
- 5. Johansson S, Bennich H. Immunological studies of an atypical (myeloma) immunoglobulin. Immunology 1967;13:381–394. [PMC free article] [PubMed] [Google Scholar]
- 6. Williams P, Dolen W, Koepke J, et al. Immunoassay of specific IgE: Use of a single point calibration curve in the modified radioallergosorbent test. Ann Allergy 1992;69:48–52. [PubMed] [Google Scholar]
- 7. Liang K, Su M, Jiang R. Comparison of the skin test and ImmunoCAP system in the evaluation of mold allergy. J Chin Med Assoc 2006;69:3–6. [DOI] [PubMed] [Google Scholar]
- 8. Glovsky M. Measuring allergen‐specific IgE. Where have we been and where are we going? Methods Mol Biol 2007;378:205–219. [DOI] [PubMed] [Google Scholar]
- 9. Dolen W. IgE antibody in the serum‐detection and diagnostic significance. Allergy 2003;58:717–723. [DOI] [PubMed] [Google Scholar]
- 10. Plebani M, Bernardi D, Basso D, et al. Measurement of specific immunoglobulin E: Intermethod comparison and standardization. Clin Chem 1998;44:1974–1979. [PubMed] [Google Scholar]
- 11. Sastre J. Molecular diagnosis in allergy. Clin Exp Allergy 2010;40:1142–1160. [DOI] [PubMed] [Google Scholar]
- 12. Ollert M, Weissenbacher S, Rakoski J, et al. Allergen‐specific IgE measured by a continuous random‐access immunoanalyzer: Interassay comparison and agreement with skin testing. Clin Chem 2005;51:1241–1249. [DOI] [PubMed] [Google Scholar]
- 13. Lee Y, Sohn J, Lee J, et al. Allergen‐specific IgE measurement with the IMMULITE 2000 system: Intermethod comparison of detection performance for allergen‐specific IgE antibodies from Korean allergic patients. Clin Chim Acta 2009;401:25–32. [DOI] [PubMed] [Google Scholar]
- 14. Li T, Chuang T, Tse S, et al. Development of a third generation allergen‐specific IgE assay on the continuous random access IMMULITE 2000 analyzer. Clin Chem 2003;49:A20. [PubMed] [Google Scholar]
- 15. Li T, Chuang T, Tse S, et al. Development and validation of a third generation allergen‐specific IgE assay on the continuous random access IMMULITE 2000 analyzer. Ann Clin Lab Sci 2004;34:67–74. [PubMed] [Google Scholar]
- 16. World Medical Association . World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194. [DOI] [PubMed] [Google Scholar]
- 17. Yman L. 2001. The Immunoassay Handbook, 2nd edn London: Nature Publishing Group. [Google Scholar]
- 18. Bazaral M, Hamburger R. Standardization and stability of immunoglobulin E (IgE). J Allergy Clin Immunol 1972;49:189–191. [DOI] [PubMed] [Google Scholar]
- 19. Maccario J, Oryszczyn M, Charpin D, et al. Methodologic aspects of the quantification of skin prick test responses: The EGEA study. J Allergy Clin Immunol 2003;111:750–756. [DOI] [PubMed] [Google Scholar]
- 20. Krouse J, Mabry R. Skin testing for inhalant allergy 2003: Current strategies. Otolaryngol Head Neck Surg 2003;129:33–49. [DOI] [PubMed] [Google Scholar]
- 21. Gendo K, Larson E. Evidence‐based diagnostic strategies for evaluating suspected allergic rhinitis. Ann Intern Med 2004;140:278–289. [DOI] [PubMed] [Google Scholar]
- 22. Wood R, Segall N, Ahlstedt S, et al. Accuracy of IgE antibody laboratory results. Ann Allergy Asthma Immunol 2007;99:34–41. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Godbold J, Sampson H. Correlation of serum allergy (IgE) tests performed by different assay systems. J Allergy Clin Immunol 2008;121:1219–1224. [DOI] [PubMed] [Google Scholar]
- 24. Li T, Fu P, Zic V. Performance validation of a third‐generation allergen‐specific IgE assay in the clinical laboratory: Interlaboratory and intermethod comparison. Clin Chim Acta 2005;361:199–205. [DOI] [PubMed] [Google Scholar]
- 25. Villalta D, Da Re M, Conte M, et al. Allergen component specific IgE measurement with the Immulite™ 2000 System: Diagnostic accuracy and intermethod comparison. J Clin Lab Anal 2014;29:135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams P, Barnes J, Szeinbach S, Sullivan T. Analytical precision and accuracy of commercial immuno‐assays for specific IgE: Establishing a standard. J Allergy Clin Immunol 2000;105:1221–1230. [DOI] [PubMed] [Google Scholar]


