Abstract
Background
The survival rate of septic patients mainly depends on a rapid and reliable diagnosis. A rapid, broad range, specific and sensitive quantitative diagnostic test is the urgent need. Thus, we developed a TaqMan‐Based Multiplex real‐time PCR assays to identify bloodstream pathogens within a few hours.
Methods
Primers and TaqMan probes were designed to be complementary to conserved regions in the 16S rDNA gene of different kinds of bacteria. To evaluate accurately, sensitively, and specifically, the known bacteria samples (Standard strains, whole blood samples) are determined by TaqMan‐Based Multiplex real‐time PCR. In addition, 30 blood samples taken from patients with clinical symptoms of sepsis were tested by TaqMan‐Based Multiplex real‐time PCR and blood culture.
Results
The mean frequency of positive for Multiplex real‐time PCR was 96% at a concentration of 100 CFU/mL, and it was 100% at a concentration greater than 1000 CFU/mL. All the known blood samples and Standard strains were detected positively by TaqMan‐Based Multiplex PCR, no PCR products were detected when DNAs from other bacterium were used in the multiplex assay. Among the 30 patients with clinical symptoms of sepsis, 18 patients were confirmed positive by Multiplex real‐time PCR and seven patients were confirmed positive by blood culture.
Conclusion
TaqMan‐Based Multiplex real‐time PCR assay with highly sensitivity, specificity and broad detection range, is a rapid and accurate method in the detection of bacterial pathogens of sepsis and should have a promising usage in the diagnosis of sepsis.
Keywords: bacterial pathogen, bloodstream infection, multiplex real‐time PCR, sepsis
1. INTRODUCTION
Sepsis is a leading cause of morbidity and mortality worldwide in hospitalized patient. The survival rate of septic patients mainly depends on a rapid and reliable diagnosis, since in the case of severe sepsis there is an average 7.6% decrease in survival rate per hour from the onset of hypotension without effective antimicrobial treatment.1, 2 While the early identification of a pathogen increases the chance of targeting the correct etiologic agent and may avoid misuse of antibiotics. So far blood culture (BC) is regarded as the gold standard to detect blood pathogens, however, blood cultures do not always provide high diagnostic accuracy for infection in a timely manner, routinely taking several days before the result is positive availably and is often negative.3 Furthermore, the common practice of antibiotic therapy prior to pathogen identification coupled with inconsistent laboratory support makes etiological diagnosis extremely difficult. Therefore, a fast and accurate diagnostic method is highly desirable. Recently, several molecular methods4, 5 for diagnosis of bloodstream infections were developed, and they are also being used as an adjunct to traditional methods for faster and accurate results. Methods based on PCR techniques come to the forefront, which enables precise and rapid detection of microbial genetic markers. Unfortunately, identification of microbes directly in blood encounters numerous obstacles associated with their very small number in the sample, the presence of inhibitors disrupting DNA amplification and the need to obtain nucleic acid isolates of very good quality.6, 7, 8 The mentioned difficulties were the reason why, so far, there have been very few commercially available diagnostic kits for molecular diagnosis of sepsis. Thus, it is necessary to develop a broad range and rapid detective system for sepsis. In this study, we describe a TaqMan‐Based Multiplex real‐time PCR detection system, which allowed for rapid detection of 10 most frequent bacterial pathogens from blood samples,9, 10, 11, 12, 13, 14, 15 this system may provide more rapid, broad range, and accurate diagnosis of sepsis.
2. MATERIALS AND METHODS
2.1. Bacterial spectrum of detective system determination
Data from clinical laboratory of TongDe Hospital of ZheJiang Province, from 2013 to 2015, a total of 1257 positive blood bacterial culture samples were identified, the 11 most frequent bacterial pathogens were as follows: Pseudomonas aeruginosa, Staphyloccocus aureus, Acinetobacter baumannii, Klebsiella pneumonia, Escherichia coli, Coagulase‐negative Staphylococci, Enterococcus faecium, Enterococcus faecalis, Enterobacter cloacae, Stenotrophomonas maltophilia, Pseudomonas cepacia. The percentage of the pathogens were up to 95.4% of all positive samples. Furthermore, based on a large number of references,9, 10, 11, 12, 13, 14, 15 we found that the 11 pathogens accounted for most of the proportion of sepsis. Escherichia coli is a very frequent pathogen in sepsis, but most of Taq DNA polymerase reagent has residual sequence of E. coli DNA. To avoid cross‐reaction with residual gene segment, E. coli was excluded from the study. Finally, the rest 10 most frequent bacterial pathogens were identified as experiment pathogens, they were very similar to 10 most common bacterial species found in the SENTRY Antimicrobial Surveillance Program.9 To exclude the possibility of cross‐reaction, five types of pathogens were taken in study as negative controls. The negative controls were as follows: Enterobacter aerogenes (ATCC 13048), Klebsiella oxytoca (ATCC 700324), Serratia marcescens (ATCC 13880), Streptococcus pyogenes (ATCC 12344), Candida albicans (ATCC 10231).
2.2. Design of primers and probes
Primers were designed to be complementary to conserved regions in the 16S rDNA gene of different kinds of bacteria. This was based on a computer alignment of 16S rDNA gene sequences of different kinds of bacteria available from GenBank. Multiple sequence alignment use a computer software ClustalX2 (Conway Institute UCD, Dublin, Ireland). Primers and probes were designed using the online tool‐ primer 3 (version 4.0.0, Whitehead Institute for Biomedical Research, Cambridge, MA, USA). The primers and probes used for Multiplex real‐time PCR in this study were designed to simultaneously detect the following 10 pathogens: P. aeruginosa (ATCC 27853), S. aureus (ATCC25923), A. baumannii (ATCC 19606), K. pneumonia (ATCC 13883), Coagulase‐negative Staphylococci (Staphylococcus epidermidis ATCC 51625; Staphylococcus haemolyticus ATCC 29970; Saprophytic staphylococcus ATCC BAA 750; Staphylococcus hominis, Staphylococcus capitis), E. faecium (ATCC 700221), E. faecalis (ATCC 29212), E. cloacae (ATCC 13047), S. maltophilia (ATCC 51331), P. cepacia (ATCC 25416). Different annealing temperatures (56°C, 57°C, 59°C, 60°C, 61°C) were compared in this study. The multiplex PCR has a higher specificity and amplification efficiency at an annealing temperature of 60°C. An annealing temperature of 60°C was selected to complete the study. The sequences were selected and aligned using the software Bioedit 7.2.1 (Tom Hall Ibis Biosciences, Carlsbad, CA, USA). These designed primers and probes were synthesized by Shanghai Generay Biotech Co Ltd (Shanghai, China). Standard strains including the 10 target pathogens and five negative controls were provided by clinical laboratory of TongDe Hospital of ZheJiang Province. Staphylococcus hominis and Staphylococcus capitis was provided by clinical laboratory of TongDe Hospital of ZheJiang Province, too. Sequences of these primers, target genes, and other related information are presented in Tables 1 and 2.
Table 1.
Bacteria species and their GenBank accession numbers used in this study
| Species | Probe sequence | Size, bp | Accession number |
|---|---|---|---|
| Pseudomonas aeruginosa | TTGACGTTACCAACAGAATAAGCACCG | 109 | CP000438 |
| Staphyloccocus aureus | AAGAACATATGTGTAAGTAACTGTGCACA | 104 | KC428665 |
| Acinetobacter baumannii | CTACTTTAGTTAATACCTAGAGATAGTGGAC | 109 | NC_011595 |
| Klebsiella pneumonia | TTAATAACCTYRKCGATTGACGTTACCC | 106 | NR_103916 |
| Coagulase‐negative Staphylococci | AACAAAYGTGTAAGTAACTRTGCACGTC | 109 | CP000029 |
| Enterococcus faecium | CAAGGATGAGAGTAACTGTTCATCCCT | 105 | NC_017022 |
| Enterococcus faecalis | CGTTAGTAACTGAACGTCCCCTGAC | 105 | AY246953 |
| Enterobacter cloacae | AACCTTATCGCCTTCCTCCCCGC | 108 | KC990813 |
| Stenotrophomonas maltophilia | CCCTTTTGTTGGGAAAGAAATCCAGC | 108 | NC_011071 |
| Pseudomonas cepacia | CCGGAAAGAAATCCTTGGTCCTAATATG | 108 | AY741362 |
Table 2.
Sequences of primers used in the TaqMan‐Based multiplex real‐time PCR
| Primers | Sequence |
|---|---|
| Forward 1 | TCGGMTCGTAAAACTCTGTT |
| Forward 2 | GCCTTCGGGTTGTAAAGY |
| Forward 3 | GCCTTATGGTTGTAAAGCAC |
| Forward 4 | GGTCTTCGGATTGTAAAGC |
| Reverse | CTGCTGGCACGAAGTTAGC |
2.3. DNA extraction
DNA was extracted from standard strains or blood samples. Briefly, we first added 200 μL of standard strains or blood samples, 200 μL of Lysis Buffer and 30 μL of magnetic bead in the tube bacteria cultures, and the mixture was heated for 5 minutes at 68°C. Subsequently, 200 μL of absolute alcohol was added and the solution was put in Magnetic frame. After the supernatant was removed, the precipitate was added to 500 μL of washing solution 1 (added absolute alcohol before use), and removed supernatant again. It was then added to 500 μL of washing solution 2, after supernatant was removed, 500 μL of washing solution 3 was added. Finally, removed the supernatant, and the precipitate was allowed to stand for 2 minutes, then, 80 μL of elution buffer was added, the DNA template was prepared. With this method, the DNA extraction was completed in approximately 10 minutes.
2.4. Multiplex real‐time PCR procedure
The reaction mixture contained 1 U of Faststart Taq polymerase, 3 μL of Mg2+ (25 mmol/L MgCl2), 2 μL of dNTP (2.5 mmol/L each), 2.5 μL of 10×PCR buffer (with Mg2+), 0.75 μL primers mixtures, 0.3 μL (10 μmol/L) probe each, and 5 μL of DNA sample. The final volume of the reaction mixture was adjusted to 25 μL. The multiplex real‐time PCR conditions were as follows: initial denaturation at 95°C for 10 minutes, followed by denaturation at 95°C for 5 seconds, and then 40 cycles consisting of 60°C for 31 seconds. The abridged general view of Multiplex real‐time PCR is showed in Figure 1.
Figure 1.
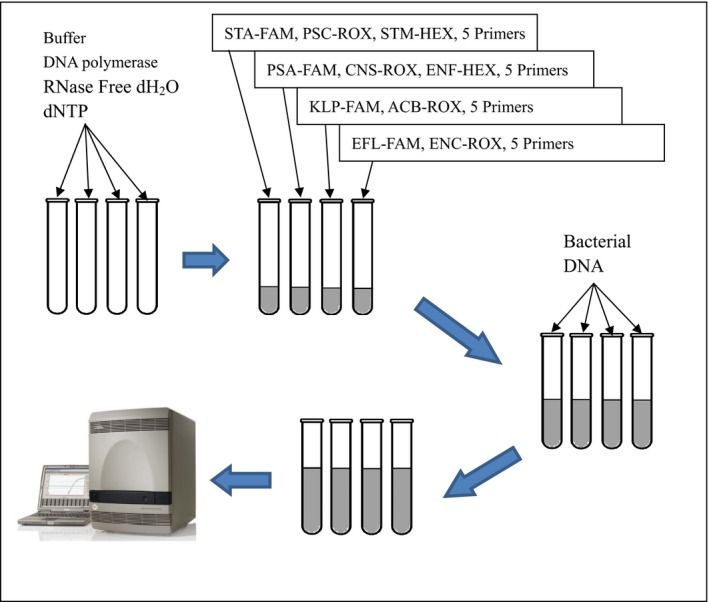
The abridged general view of Multiplex real‐time PCR. There are 2‐3 probes labeled with different Fluorescence labels (FAM\ROX\HEX) in one tube, and every tube represents a test channel of the Multiplex real‐time PCR system
2.5. Verification of Multiplex real‐time PCR
2.5.1. Verification with known blood samples
To validate the specificity, the known blood samples were identified by TaqMan‐Based Multiplex real‐time PCR. Double whole blood samples were collected for 10 experimental pathogens, 20 patients were included in this study. The 20 whole blood samples were gathered at the same time with blood bacterial culture which were collected for blood routine tests and were stored at 4°C in the laboratory, and we found out them for Multiplex real‐time PCR after bacteria cultures were designated positive. The average age of patients was 46.5 years old (ranging from 20 to 75); 12(60%) were female and 8(40%) were male. Underlying diseases were intestinal infection (9), pulmonary infection (6), biliary tract infection (2), urinary tract infection (2), liver abscess (1).
2.5.2. Sensitivity test
To determine the detection range, we prepared a 10‐fold dilution series from 105 colony‐forming units (CFU)/mL to 101 CFU/mL for 10 standard strains, each concentration was tested 10 times. CT values of ≤35 cycles were recorded as positive results.
2.5.3. Specificity test
To validate the specificity of TaqMan‐Based Multiplex real‐time PCR, DNA was extracted from 10 standard strains, then was added to a negative control sample, and subjected to the multiplex PCR in parallel with the negative control sample. The experiment was repeated twice. Furthermore, the specificity was confirmed by PCR product sequencing, DNA sequencing was carried out in Sangon Biotech (Shanghai) Co Ltd (Shanghai, China).
2.5.4. Comparative trial of Multiplex real‐time PCR and blood culture in small clinical samples with suspected sepsis
A total of 30 blood samples taken from patients with clinical symptoms of sepsis were tested by TaqMan‐Based Multiplex real‐time PCR and blood culture. Sepsis was defined as an infection plus two or more of the following systemic inflammatory response syndrome criteria: temperature >38 or <36°C; heart rate >90/min; respiratory rate >20 breaths/min (or carbon dioxide partial pressure ‐PaCO2 <32 mm Hg); white blood cell count >12 000 cells/μL or <4000 cells/μL (or >10% band forms). All the bacterial strains were cultured and identified by clinical laboratory of TongDe Hospital of ZheJiang Province.
3. RESULTS
3.1. Result of blood samples
A total of 20 whole blood samples were gathered after bacteria cultures were designated positively. Double samples were collected for all species. All the blood samples were detected positively by TaqMan‐Based Multiplex PCR.
3.2. Sensitivity
A 10‐fold dilution series from 105 to 101 CFU/mL were used to determine the detection range. Different bacteria showed various sensitivity. The positive rate of each bacteria at different concentrations were showed in Table 3. The mean frequency of positive for Multiplex real‐time PCR was 96% at a concentration of 1×102 CFU/mL, and it was 100% at a concentration >1×103 CFU/mL (Figure 2).
Table 3.
The positive rate of each bacteria at different concentrations
| Concentration bacterial | 1×101 CFU/mL | 1×102 CFU/mL | 1×103 CFU/mL | 1×104 CFU/mL | 1×105 CFU/mL |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa | 40% | 100% | 100% | 100% | 100% |
| Staphyloccocus aureus | 30% | 90% | 100% | 100% | 100% |
| Acinetobacter baumannii | 40% | 100% | 100% | 100% | 100% |
| Klebsiella pneumonia | 30% | 100% | 100% | 100% | 100% |
| Coagulase‐negative Staphylococci | 20% | 90% | 100% | 100% | 100% |
| Enterococcus faecium | 40% | 100% | 100% | 100% | 100% |
| Enterococcus faecalis | 50% | 100% | 100% | 100% | 100% |
| Stenotrophomonas maltophilia | 20% | 80% | 100% | 100% | 100% |
| Pseudomonas cepacia | 30% | 100% | 100% | 100% | 100% |
| Enterobacter cloacae | 30% | 100% | 100% | 100% | 100% |
Figure 2.
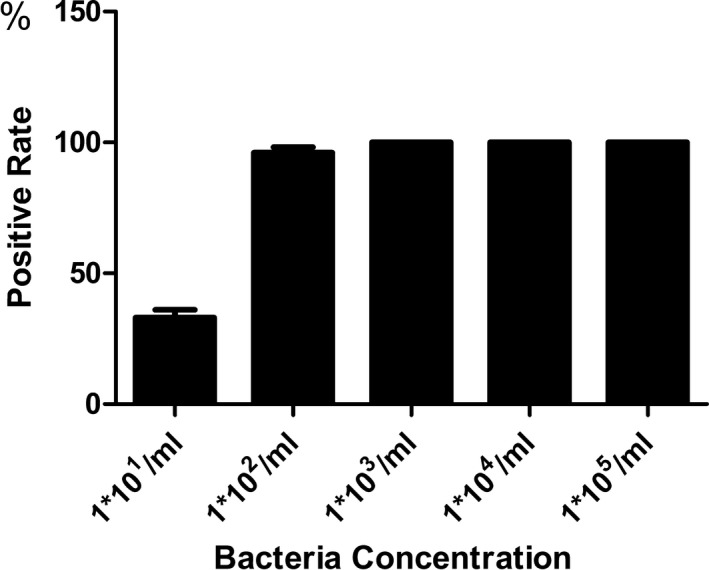
The mean positive rate for Multiplex real‐time PCR at various concentration. The mean frequency of positive for Multiplex real‐time PCR was 96% at a concentration of 1×102 CFU/mL, and it was 100% at a concentration greater than 1×103 CFU/mL
3.3. Specificity
Standard strains including the 10 target pathogens and five negative controls were detected in this study. All of the 10 experimental samples were positive when detected by the TaqMan‐Based Multiplex PCR. Meanwhile no PCR products were detected when DNAs from other bacterium were used in the multiplex assay. This revealed that TaqMan‐Based Multiplex real‐time PCR did not cross‐react with other bacterium. Furthermore, sequencing data confirmed the amplification of expected DNA sequences from the experimental samples. The results are presented in Figure 3.
Figure 3.
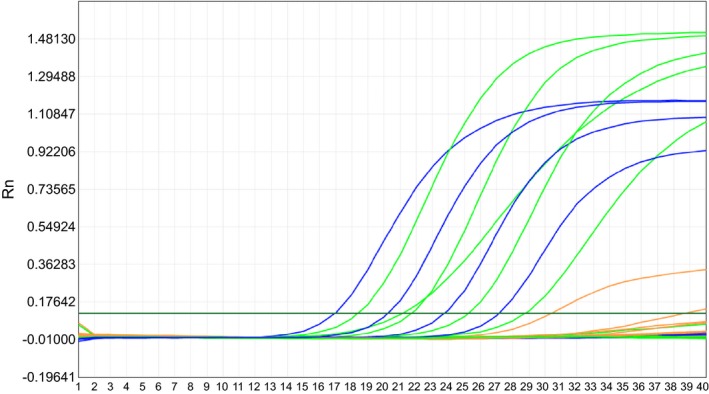
The result for all experimental samples. The 10 types experimental standard strains were showed positive results when detected by the TaqMan‐Based Multiplex real‐time PCR. CT values of ≤35 cycles were recorded as positive results. No PCR products were detected when DNAs from other five bacteriums were used in the Multiplex real‐time PCR assay. CT values of ≤35 cycles were recorded as positive results
3.4. Result of small clinical sample with suspected sepsis
Among the 30 patients with suspected sepsis, 18 patients (coagulase‐negative Staphylococci, n=6; S. aureus, n=4; K. pneumonia, n=4; E. faecium, n=2; P. aeruginosa, n=2;) were confirmed positive by Multiplex real‐time PCR, seven patients were confirmed positive by blood culture (coagulase‐negative Staphylococci, n=3; S. aureus, n=1; E. faecium, n=2; P. aeruginosa, n=1), the seven positive patients in the blood culture group were showing positive results in the Multiplex real‐time PCR, too. Blood culture was taken as the gold standard for evaluation of the results. For Multiplex real‐time PCR the performance test showed: sensitivity 100%; specificity 52.2%; NPV 100%; PPV 38.9% and accuracy of 63.3% when compared with the blood culture. The 12 blood samples which were confirmed positive by Multiplex real‐time PCR and confirmed negative by blood culture were amplified by Multiplex real‐time PCR and subjected to DNA sequencing to confirm the results. Sequencing data confirmed the amplification of expected DNA sequences from the bacteria.
4. DISCUSSION
Sepsis is a serious disease with high mortality in hospitalized patient. Rapid and modern diagnosis of sepsis requires application of methods that enable the detection of microorganisms directly from the patient's blood sample within a few hours, which will increase the chances of the patient’s survival.16
Conventional method, blood culture, has some disadvantages with regard to the desired rapidity and sensitivity. Furthermore, it is influenced by various factors and can reduce the chance of a positive result. Recently published guidelines recommend antibiotic administration within 1 hour for patients suspected of having septic shock or severe sepsis.17 During severe infection, empirical antibiotic therapy is inappropriate in roughly one‐third of cases, and this substantially increases mortality and hospital length of stay.18 In the setting of infection, any tool enabling prompt and accurate documentation of pathogen might theoretically reduce mortality and may serve to improve hospital resource use.18 Thus, a rapid, sensitive, specific, and broad range identification method is required. Chang et al.19 reveal that multiplex real‐time PCR allows speeding up the diagnosis of sepsis with a sensitivity up to 80% and a specificity up to 95% in cases of bacteremia. The TaqMan‐Based Multiplex real‐time PCR assay is an effective method for the detection of microbial infection and has a high reproduction quality. It has been widely used in gene expression research and microbial detection. The 16S rDNA gene sequence is composed of both conserved and variable regions. The conserved regions are highly conserved in all bacteria, different bacteria could be distinguished by the 16S rDNA analysis using special primers. In this study, we introduced a fluorescent‐labeled TaqMan probe into the PCR procedure, which was designed to be complementary to conserved regions in the 16S rDNA gene of 10 different kinds of bacteria. All the results reveal that TaqMan‐Based Multiplex real‐time PCR is a rapid, broad range, specific, and sensitive method.
Firstly, the time required for TaqMan‐Based Multiplex real‐time PCR assay was more rapid compared to bacterial culture. Usually it took no more than 2 hours to complete the whole experiment, which included only 0.5 hour of sample preparation and 1 hour for DNA amplification because thermal cycling was much faster and amplicon detection was performed in real time. It allowed the rapid quantification of bacteria without the need for post‐PCR processing. Meanwhile, it enable the detection of microorganisms directly from the patient's blood sample accurately within 2 hours.
In this study, a TaqMan‐Based Multiplex real‐time PCR assay was designed for detection and identification of 10 pathogens. All of the experimental samples are positive (both standard strains and whole blood samples) when detected by the TaqMan‐Based Multiplex PCR without false negative, thus we hold the opinion that it is an accurate method with broad detection range.
The sensitivity of the TaqMan‐Based Multiplex real‐time PCR was 96% at a concentration of 1×102 CFU/mL, and it was 100% at a concentration >1×103 CFU/mL. All of the experimental samples (P. aeruginosa, S. aureus, A. baumannii, K. pneumonia, coagulase‐negative Staphylococci, E. faecium, E. faecalis, E. cloacae, S. maltophilia, P. cepacia) are positive when detected by the TaqMan‐Based Multiplex real‐time PCR. At the same time negative control of five bacterial strainds emonstrated negative results in TaqMan‐Based Multiplex real‐time PCR. No fluorescence was detected and no cross‐reaction was found in negative control. Furthermore, among the 30 suspected sepsis patients, bacteria were identified in 18 samples by Multiplex real‐time PCR, and seven samples were identified by both methods. The 12 blood samples which were confirmed positive by Multiplex real‐time PCR and confirmed negative by blood culture were amplified by Multiplex real‐time PCR and subjected to DNA sequencing to confirm the results, sequencing data confirmed the amplification of expected DNA sequences from the bacteria. We have the opinion that bacterial culture might be negative in some patients due to low bacterial count or empirical antibiotics therapy before collection of blood. However, TaqMan‐Based Multiplex real‐time PCR could report a positive result because both dead and viable microorganisms that could be detected. This system may have high sensitivity and specificity.
4.1. Limitations
In the clinical trials, due to small sample size of patients with clinical symptoms of sepsis in this study, differences between Multiplex real‐time PCR and blood culture were not significant, the further research is needed to do. In addition, there is an issue that cannot be ignored, most of Taq DNA polymerase was expressed by E. coli, thus, the cross‐reaction between residual gene segment and probe for E. coli is difficult to avoid. Even though E. coli is a very frequent pathogen in sepsis, it was excluded from the study under existing condition. We believe that further studies are needed to address this issue.
4.2. Summary
This study suggests that TaqMan‐Based Multiplex real‐time PCR assay with high sensitivity, specificity, and broad detection range, is a rapid and accurate method in the detection of bacterial pathogens of sepsis and should have a promising usage in the diagnosis of sepsis.
ACKNOWLEDGMENTS
This work was supported by grant from the Application Research Fund of Public Welfare Technology of Zhejiang Province. No. 2013C33197. This study adhered to the tenets of the Declaration of Helsinki. Ethical approval was given by the ethics committee of Tongde Hospital of Zhejiang Province, with the following reference number: [2016]016.
Liu C‐F, Shi X‐P, Chen Y, Jin Y, Zhang B. Rapid diagnosis of sepsis with TaqMan‐Based multiplex real‐time PCR. J Clin Lab Anal. 2018;32:e22256 10.1002/jcla.22256
REFERENCES
- 1. Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589‐1596. [DOI] [PubMed] [Google Scholar]
- 2. Degoricija V, Sharma M, Legac A, et al. Survival analysis of 314 episodes of sepsis in medical intensive care unit in university hospital: impact of intensive care unit performance and antimicrobial therapy. Croat Med J. 2006;47:385‐397. [PMC free article] [PubMed] [Google Scholar]
- 3. Book M, Lehmann LE, Zhang XH, et al. Monitoring infection: from blood culture to polymerase chain reaction (PCR). Best Pract Res Clin Anaesthesiol. 2013;27:279‐288. [DOI] [PubMed] [Google Scholar]
- 4. Lehmann LE, Alvarez J, Hunfeld KP, et al. Potential clinical utility of polymerase chain reaction in microbiological testing for sepsis. Crit Care Med. 2009;37:3085‐3090. [DOI] [PubMed] [Google Scholar]
- 5. Ecker DJ, Sampath R, Li H, et al. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn. 2010;10:399‐415. [DOI] [PubMed] [Google Scholar]
- 6. Gosiewski T, Szała L, Pietrzyk A, et al. Comparison of methods for isolation of bacterial and fungal DNA from human blood. Curr Microbiol. 2014;68:149‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Loonen AJM, Wolffs PFG, Bruggeman CA, et al. Developments for improved diagnosis of bacterial bloodstream infections. Eur J Clin Microbiol Infect Dis. 2014;33:1687‐1702. [DOI] [PubMed] [Google Scholar]
- 8. Schrader C, Schielke A, Ellerbroek L, et al. PCR inhibitors – occurrence, properties and removal. J Appl Microbiol. 2012;113:1014‐1026. [DOI] [PubMed] [Google Scholar]
- 9. Biedenbach DJ, Moet GJ, Jones RN. Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY Antimicrobial Surveillance Program (1997‐2002). Diagn Microbiol Infect Dis. 2004;50:59‐69. [DOI] [PubMed] [Google Scholar]
- 10. Waters D, Jawad I, Ahmad A, et al. A etiology of community‐acquired neonatal sepsis in low and middle income countries. J Glob Health. 2011;1:154‐170. [PMC free article] [PubMed] [Google Scholar]
- 11. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309‐317. [DOI] [PubMed] [Google Scholar]
- 12. Hamer DH, Darmstadt GL, Carlin JB, et al. Etiology of bacteremia in young infants in six countries. Pediatr Infect Dis J. 2015;34:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buetti N, Marschall J, Atkinson A, et al. National bloodstream infection surveillance in Switzerland 2008‐2014: different patterns and trends for university and community hospitals. Infect Control Hosp Epidemiol. 2016;37:1060‐1067. [DOI] [PubMed] [Google Scholar]
- 14. Pien BC, Sundaram P, Raoof N, et al. The clinical and prognostic importance of positive blood cultures in adults. Am J Med. 2010;123:819‐828. [DOI] [PubMed] [Google Scholar]
- 15. Wilson J, Elgohari S, Livermore DM, et al. Trends among pathogens reported as causing bacteraemia in England, 2004‐2008. Clin Microbiol Infect. 2011;17:451‐458. [DOI] [PubMed] [Google Scholar]
- 16. Jamal W, Tamaray G, Pazhoor A, et al. Comparative evaluation of BacT/ALERT 3D and BACTEC systems for the recovery of pathogens causing bloodstream infections. Med Princ Pract. 2006;15:223‐227. [DOI] [PubMed] [Google Scholar]
- 17. Dellinger RP, Levy MM, Rhodes A, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580‐637. [DOI] [PubMed] [Google Scholar]
- 18. Shorr AF, Micek ST, Welch EC, et al. Inappropriate antibiotic therapy in Gram‐negative sepsis increases hospital length of stay. Crit Care Med. 2011;39:46‐51. [DOI] [PubMed] [Google Scholar]
- 19. Chang SS, Hsieh WH, Liu TS, et al. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis – a systemic review and meta‐analysis. PLoS ONE. 2013;8:e62323. [DOI] [PMC free article] [PubMed] [Google Scholar]


