Abstract
Background
High admission cholesterol has been associated with better outcome after acute ischaemic stroke (AIS), but a paradox not completely illustrated. The purpose of this study was to investigate the effect of the total cholesterol to high‐density lipoprotein cholesterol (TC/HDL‐C) on short‐term survival after AIS.
Methods
Consecutive patients admitted in 2013 and 2015 were enrolled in the present study. The logistic regression analysis was conducted to evaluate predictors of 3‐month outcomes. The primary endpoint was death. Secondary endpoint was good (modified Rankin Scale score 0‐2 or equal to prestrike modified Rankin Scale score) at 3 months.
Results
Of 871 patients enrolled in the final analysis, 94 (10.8%) individuals died during 3 months of observation. The serum TC and TC/HDL‐C levels at admission were significantly associated with stroke outcomes at 3 months, and the HDL‐C level was only correlated with the good outcomes at 3 months. Mortality risk was markedly decreased for patients with high TC/HDL‐C ratio (odds ratio: 0.23, 95% confidence interval [CI]: 0.10‐0.50 for Q4:Q1; P‐trend <.001) after adjustment. The effect of TC/HDL‐C ratio on the probability of good outcomes was still obvious (odds ratio: 2.18, 95% CI: 1.40‐3.39 for Q4:Q1; P‐trend=.029). According to the receiver operating characteristic analyses, the best discriminating factor was a TG/HDL‐C ≥3.37 (area under the ROC curve [AUC]=0.643, sensitivity 61.3%, specificity 61.7%) as well as the TC/HDL‐C ≥4.09 for good outcomes (AUC: 0.587, sensitivity 63.9%, specificity 79.7%).
Conclusions
High TC/HDL‐C ratio may be associated with increased short‐term survival and better outcomes after AIS.
Keywords: cholesterol, HDL‐C, stroke, survival, TC
Abbreviations
- AIS
acute ischaemic stroke
- AUC
area under the ROC curve
- CI
confidence interval
- LDL‐C
low‐density lipoprotein cholesterol
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- OR
odds ratio
- ROC
receiver operating characteristic
- TC/HDL‐C
total cholesterol to high‐density lipoprotein cholesterol
1. Introduction
Ischaemic stroke remains a leading cause of mortality and results in a long‐term disability in adults worldwide.1 Stroke severity is currently the strongest predictor of functional outcome and mortality.2 Furthermore, several predictors of long‐term outcome have been widely investigated in ischaemic stroke, such as age, hypertension, diabetes mellitus, cardiac disease, and cardioembolic etiology.2 Identification of these modified predictors of long‐term outcome facilitates the better selection of appropriate therapeutic strategy to improve clinical outcome for ischaemic stroke. However, despite available diagnosis and treatment strategies, stroke mortality remains not to be changed dramatically over the past decades.3 Therefore, for better stratification regarding clinical outcome of ischaemic stroke, a cheap and easily measurable marker is required.
A few investigations regarding the influence of serum lipid levels on clinical outcome after acute ischaemic stroke (AIS) have been reported with contradictory results. High total cholesterol (TC) levels were demonstrated to be correlated with less severe stroke and lower mortality of ischaemic stroke,4, 5, 6, 7 but other studies indicated a negative association between lower TC and low‐density lipoprotein cholesterol (LDL‐C) levels and worse outcome.8, 9 Furthermore, limited studies among Chinese population highlighted the function of dyslipidaemia as a predictor of AIS.6, 7, 10 In the present study, using data from a total of 871 stroke patients in China, we assessed the correlation of quartile lipid levels including TC, high‐density lipoprotein cholesterol (HDL‐C), LDL‐C, Non‐HDL‐C, and TC/HDL‐C with AIS outcome at 3 months.
2. Materials and Methods
2.1. Subjects
This study was approved by the Ethics Committee of Nanjing Medical University and based on the Declaration of Helsinki. A retrospective, hospital‐based, follow‐up study was conducted. All consecutive subjects with first‐ever AIS who were admitted to the Emergency Ward or Stroke Unit in the First Affiliated Hospital with Nanjing Medical University and the Jiangyin People's Hospital within 36 h of onset between January 2013 and December 2015 were recruited to the current study. All patients were diagnosed according to the World Health Organization criteria and further identified by neuroimaging technology.11 Routine examinations were used to assess individuals, such as standard 12‐lead electrocardiograms, CT brain scans, and blood samples tests.12, 13 Patients were then admitted to the neurology ward with continuous management for at least 72 hours post‐stroke or stabilization. Extracranial/transcranial Doppler ultrasound and echocardiography were conducted from days 2 to 7 after admission if necessary. Other diagnostic procedures including arteriography, chest radiograph, electroencephalogram, and laboratory tests were conducted/repeated as indicated until discharge for the individuals.14 All subjects were followed up for 3 months after AIS and lipid levels on admission were available.
2.2. Data collection
The detailed information on AIS including age, gender, smoking, baseline National Institutes of Health Stroke Scale (NIHSS) at admission, medical history, stroke etiology,15 laboratory parameters at admission, and stroke outcome at 3 months were collected using a standardized questionnaire. All subjects were classified into four groups according to the lipid level quartile at admission.
2.3. Outcome definition
Stroke outcome was evaluated at 3 months after AIS according to the modified Rankin Scale (mRS). All subjects were evaluated at 3 months via telephone, mail, or outpatient department visit. The corresponding outcomes were evaluated after 3 months1 good outcome (mRS of 0‐2 or equal to the prestroke mRS);2 death.
2.4. Statistical analysis
Continuous data were compared between groups by ANOVA or Mann–Whitney U test. Non‐continuous data were compared between groups by the χ2 test. The statistical significance was set at P<.05. Clinical information and values were presented as the frequencies and percentages or means and standard deviations as appropriate. Non‐HDL‐C values were calculated by subtracting the HDL‐C from the TC. The TC/HDL‐C was obtained by dividing TC by the HDL‐C. The main statistical analysis used a backward stepwise logistic regression model to evaluate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for stroke outcomes according to lipid level quartile at admission. A distinction between two multivariable models were made as follows: model “a” adjusted for age and gender; model “b” with additional adjustment for smoking, NIHSS, medical history, stoke etiology, and laboratory characteristics (WBC, platelet, glucose, and creatinine). The trend test by modeling the medians of each category as continuous variables was assessed with the Wald test. Receiver operating characteristic (ROC) curves were used to evaluate the distinguished capacities of the areas under the curve (AUCs) and 95% CIs for TC, HDL‐C, LDL‐C, non‐HDL‐C, and TC/HDL‐C by R 3.0.3 Software (Institute for Statistics and Mathematics, Austria). P<.05 was considered statistically significant. Data statistical analyses were performed by SPSS 20.0 Software (IBM, San Jose, CA, USA).
3. Results
3.1. General features
A total of 871 patients with AIS were included with the mean age of 68.36±12.60 in the final analysis, and there were markedly more men (58.4%) than women. Baseline characteristics of AIS patients are summarized in Table 1. During a 3‐month follow‐up period, 246 (28.2%) patients with AIS presented with good outcomes, and 94 (10.8%) patients died. The serum TC, non‐HDL‐C, and TC/HDL‐C level at admission were significantly associated with stroke outcomes at 3 months, and the HDL‐C level was only correlated with the good outcomes at 3 months (P<.05).
Table 1.
Baseline characteristics of patients according to clinical outcomes
| Clinical outcomes | ||||||
|---|---|---|---|---|---|---|
| Good | Non‐good | P‐value | Survival | Death | P‐valuea | |
| Clinical information | ||||||
| Age | 65.93±12.17 | 69.32±12.65 | <.001 | 68.05±12.56 | 70.95±12.75 | .035 |
| Gender (male/female) | 147/99 | 362/263 | .621 | 447/330 | 62/32 | .117 |
| Smoking (yes/no) | 64/182 | 156/469 | .747 | 212/685 | 27/67 | .273 |
| Baseline NIHSS | 8 (2‐11) | 8 (3‐13) | .074 | 8 (2‐11) | 8 (4‐13) | .011 |
| Medical history | ||||||
| Hypertension | 137 (55.69) | 388 (62.08) | .083 | 463 (59.59) | 62 (65.96) | .233 |
| Diabetes mellitus | 62 (25.20) | 124 (19.84) | .082 | 173 (22.27) | 27 (28.72) | .160 |
| Atrial fibrillation | 54 (21.95) | 138 (22.08) | .967 | 176 (22.65) | 16 (17.02) | .213 |
| TIA | 6 (2.44) | 18 (2.88) | .720 | 22 (2.83) | 2 (2.13) | .694 |
| Stroke etiology | ||||||
| Atherosclerotic | 204 (82.93) | 504 (80.64) | .568 | 630 (81.08) | 78 (82.98) | .870 |
| Cardioembolic | 31 (12.60) | 96 (15.36) | 115 (14.80) | 12 (12.77) | ||
| Cryptogenic | 11 (4.47) | 25 (4.00) | 32 (4.12) | 4 (4.26) | ||
| Laboratory characteristics | ||||||
| WBC (109/L) | 7.37 (5.79‐9.34) | 7.74 (6.12‐9.61) | .083 | 7.62 (6.01‐9.54) | 7.93 (6.28‐9.63) | .236 |
| Platelet (109/L) | 185 (144‐217) | 181 (149‐216) | .563 | 180 (147‐216) | 191 (153‐220) | .223 |
| Glucose (mmol/L) | 6.37 (5.32‐8.01) | 6.39 (5.28‐8.11) | .742 | 6.37 (5.28‐8.07) | 6.75 (5.36‐8.15) | .171 |
| Creatinine (mmol/L) | 75 (65‐88) | 75 (62‐87) | .234 | 75 (64‐89) | 71 (60‐81) | .011 |
| TC (mmol/L) | 4.21 (3.92‐4.78) | 4.37 (4.07‐5.01) | <.001 | 4.35 (4.04‐5.00) | 4.15 (3.80‐4.69) | <.001 |
| HDL‐C (mmol/L) | 1.26 (1.12‐1.51) | 1.22 (1.10‐1.45) | .044 | 1.23 (1.10‐1.45) | 1.27 (1.13‐1.52) | .186 |
| LDL‐C (mmol/L) | 2.83 (2.40‐3.34) | 2.90 (2.51‐3.47) | .076 | 2.90 (2.51‐3.45) | 2.76 (2.37‐3.39) | .064 |
| Non‐HDL‐C (mmol/L) | 2.90 (2.64‐3.43) | 3.13 (2.74‐3.81) | <.001 | 3.10 (2.73‐3.78) | 2.81 (2.58‐3.15) | <.001 |
| TC/HDL‐C | 3.41 (2.88‐3.88) | 3.59 (3.16‐4.32) | <.001 | 3.54 (3.15‐4.31) | 3.24 (2.79‐3.76) | <.001 |
NIHSS, NIH Stroke Scale; TIA, transient ischaemic attack; WBC, white blood cell; TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC/HDL‐C, TC to HDL‐C ratio
Good outcome is defined as mRS of 0‐2 or equal to prestroke mRS.
Values are medians (interquartile range) or frequencies and percentages if necessary. Statistically significant results are in italic font.
Chi‐squared test or Mann–Whitney U test as appropriate.
3.2. Adjusted association of lipid level and TC/HDL‐C with outcomes
After adjustment with age and gender, patients who developed non‐good or who died had lower TC and TC/HDL‐C, and AIS patients with good outcomes or survival had higher TC and TC/HDL‐C. The non‐HDL‐C level was not independently associated with any of the two outcome measures. Furthermore, further adjustment indicated similar results of TC, non‐HDL‐C, and TC/HDL‐C. The results of the logistic regression analyses are detailed in Tables 2 and 3.
Table 2.
Adjusted odds ratios for good outcome according to lipid levels
| Main parameters | Lipid levels | |||||||
| Quartile1 | Quartile2 | P‐value | Quartile3 | P‐value | Quartile4 | P‐value | P for trend | |
| TC (mmol/L) | <4.02 | 4.02‐<4.34 | 4.34‐<4.97 | ≥4.97 | ||||
| OR (95% CI)a | 1 | 1.72 (1.14‐2.59) | .010 | 1.97 (1.30‐2.98) | .001 | 2.13 (1.39‐3.24) | <.001 | <.001 |
| OR (95% CI)b | 1 | 1.84 (1.21‐2.80) | .004 | 2.17 (1.42‐3.32) | <.001 | 2.39 (1.55‐3.69) | <.001 | <.001 |
| HDL‐C (mmol/L) | <1.10 | 1.10‐<1.23 | 1.23‐<1.46 | ≥1.46 | ||||
| OR (95% CI)a | 1 | 1.01 (0.65‐1.57) | .955 | 0.79 (0.52‐1.21) | .282 | 0.75 (0.49‐1.14) | .174 | .304 |
| OR (95% CI)b | 1 | 1.06 (0.68‐1.64) | .805 | 0.79 (0.51‐1.21) | .269 | 0.79 (0.52‐1.21) | .791 | .293 |
| LDL‐C (mmol/L) | <2.50 | 2.50‐<2.88 | 2.88‐<3.45 | ≥3.45 | ||||
| OR (95% CI)a | 1 | 1.34 (0.88‐2.04) | .169 | 1.25 (0.82‐1.88) | .297 | 1.44 (0.95‐2.19) | .090 | .507 |
| OR (95% CI)b | 1 | 1.33 (0.87‐2.02) | .183 | 1.26 (0.83‐1.90) | .278 | 1.48 (0.97‐2.26) | .072 | .470 |
| Non‐HDL‐C (mmol/L) | <2.72 | 2.72‐<3.08 | 3.08‐<3.73 | ≥3.73 | ||||
| OR (95% CI)a | 1 | 0.95 (0.63‐1.41) | .779 | 1.71 (1.12‐2.62) | .014 | 1.91 (1.24‐2.95) | .003 | .345 |
| OR (95% CI)b | 1 | 0.93 (0.62‐1.40) | .736 | 1.73 (1.12‐2.66) | .013 | 1.99 (1.29‐3.08) | .002 | .359 |
| TC/HDL‐C | <3.11 | 3.11‐<3.50 | 3.50‐<4.22 | ≥4.22 | ||||
| OR (95% CI)a | 1 | 1.10 (0.74‐1.64) | .645 | 1.39 (0.92‐2.10) | .122 | 2.15 (1.39‐3.34) | .001 | .007 |
| OR (95% CI)b | 1 | 1.05 (0.70‐1.58) | .796 | 1.31 (0.87‐2.00) | .199 | 2.18 (1.40‐3.39) | .001 | .029 |
P‐values are obtained from a logistic regression model. Statistically significant results are in italic font.
TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC/HDL‐C, TC to HDL‐C ratio; OR, odds ratio; CI, confidence interval.
OR, adjusted for age and gender.
OR, a with additional adjustment for smoking, NIHSS, medical history, stoke etiology, and laboratory characteristics (WBC, platelet, glucose, and creatinine).
Table 3.
Adjusted odds ratios for death according to lipid levels
| Main parameters | Lipid levels | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartile1 | Quartile2 | P‐value | Quartile3 | P‐value | Quartile4 | P‐value | P for trend | |
| TC (mmol/L) | <4.02 | 4.02‐<4.34 | 4.34‐<4.97 | ≥4.97 | ||||
| OR (95% CI)a | 1 | 0.68 (0.39‐1.19) | .684 | 0.52 (0.29‐0.94) | .520 | 0.35 (0.18‐0.68) | .002 | .019 |
| OR (95% CI)b | 1 | 0.68 (0.38‐1.21) | .192 | 0.52 (0.28‐0.95) | .035 | 0.38 (0.20‐0.76) | .006 | .046 |
| HDL‐C (mmol/L) | <1.10 | 1.10‐<1.23 | 1.23‐<1.46 | ≥1.46 | ||||
| OR (95% CI)a | 1 | 1.00 (0.53‐1.90) | .993 | 0.95 (0.49‐1.81) | .869 | 1.37 (0.74‐2.54) | .312 | .638 |
| OR (95% CI)b | 1 | 1.05 (0.54‐2.02) | .895 | 0.92 (0.48‐1.78) | .806 | 1.51 (0.80‐2.87) | .207 | .670 |
| LDL‐C (mmol/L) | <2.50 | 2.50‐<2.88 | 2.88‐<3.45 | ≥3.45 | ||||
| OR (95% CI)a | 1 | 1.18 (0.67‐2.06) | .569 | 0.53 (0.27‐1.03) | .063 | 0.79 (0.43‐1.44) | .441 | .104 |
| OR (95% CI)b | 1 | 1.21 (0.69‐2.14) | .506 | 0.56 (0.28‐1.09) | .086 | 0.80 (0.44‐1.48) | .479 | .097 |
| Non‐HDL‐C (mmol/L) | <2.72 | 2.72‐<3.08 | 3.08‐<3.73 | ≥3.73 | ||||
| OR (95% CI)a | 1 | 0.62 (0.36‐1.05) | .077 | 0.32 (0.17‐0.61) | .001 | 0.28 (0.15‐0.55) | <.001 | .004 |
| OR (95% CI)b | 1 | 0.60 (0.35‐1.03) | .065 | 0.31 (0.16‐0.60) | .001 | 0.29 (0.15‐0.58) | <.001 | .008 |
| TC/HDL‐C | <3.11 | 3.11‐<3.50 | 3.50‐<4.22 | ≥4.22 | ||||
| OR (95% CI)a | 1 | 0.66 (0.38‐1.15) | .144 | 0.72 (0.41‐1.27) | .255 | 0.23 (0.11‐0.50) | <.001 | .031 |
| OR (95% CI)b | 1 | 0.61 (0.35‐1.08) | .089 | 0.66 (0.37‐1.17) | .152 | 0.23 (0.10‐0.50) | <.001 | .041 |
TC, total cholesterol; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC/HDL‐C, TC to HDL‐C ratio; OR, odds ratio; CI, confidence interval. P‐values are obtained from a logistic regression model. Statistically significant results are in italic font.
OR, adjusted for age and gender.
OR, a with additional adjustment for smoking, NIHSS, medical history, stoke etiology, and laboratory characteristics (WBC, platelet, glucose, and creatinine).
3.3. Predictive values of lipid level and TC/HDL‐C for the two endpoints
To further assess their predictive values of TC, HDL‐C, LDL‐C, non‐HDL‐C, and TC/HDL‐C in AIS patients, the receiver operating characteristic curves and the area under the curve values for TC, HDL‐C, LDL‐C, non‐HDL‐C, and TC/HDL‐C with respect to good outcomes and death are shown in Figures 1 and 2. The best predictive value for good outcomes (AUC=0.587, 95% CI=0.55‐0.63, P<.001) was observed with the TC/HDL‐C ratio, with its value of 4.09 with the largest Youden's index (sensitivity 63.9%, specificity 79.7%). For death, the similar results were also found for the TC/HDL‐C ratio, with its best value of 3.37 (AUC=0.643, 95% CI=0.59‐0.70, P<.001, sensitivity 61.3%, specificity 61.7%).
Figure 1.
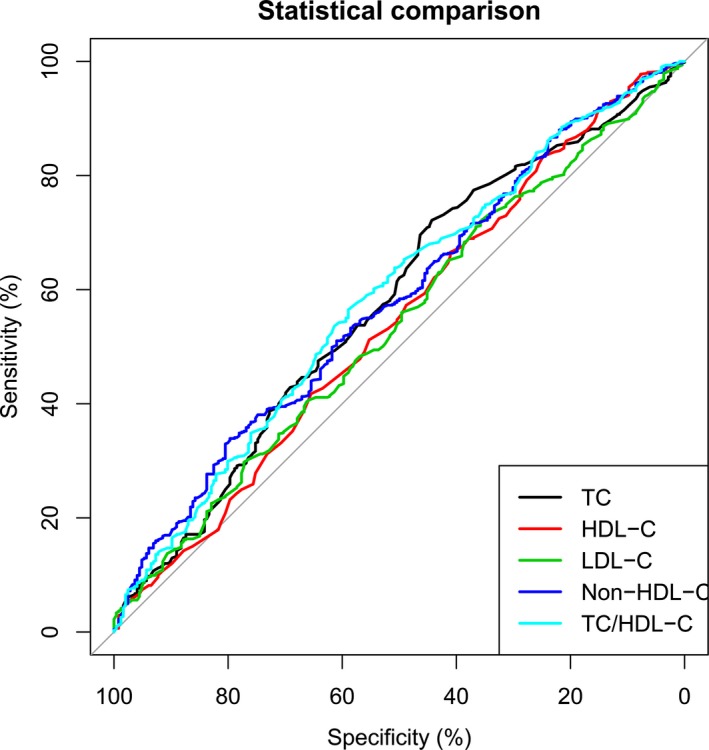
Predictive values of lipid level and total cholesterol to high‐density lipoprotein cholesterol (TC/HDL‐C) ratio for good outcomes. Their predictive values are determined by the receiver operating characteristic curves. Areas under the curves: 0.578 for TC, 0.544 for HDL‐C, 0.539 for LDL‐C, 0.581 for non‐HDL‐C, 0.587 for TC/HDL‐C
Figure 2.
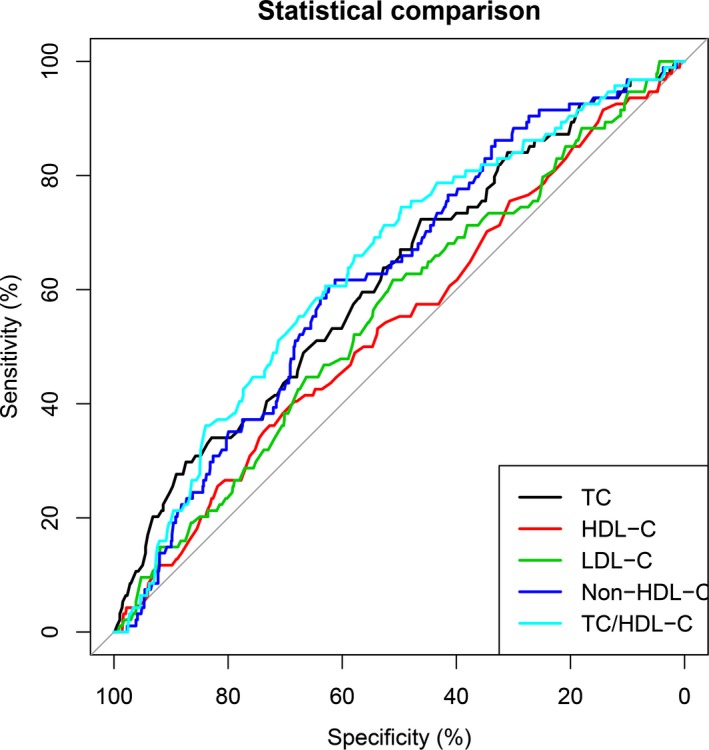
Predictive values of lipid level and total cholesterol to high‐density lipoprotein cholesterol (TC/HDL‐C) ratio for death. Their predictive values are also determined by the receiver operating characteristic curves. Areas under the curves: 0.617 for TC, 0.542 for HDL‐C, 0.558 for LDL‐C, 0.621 for non‐HDL‐C, 0.643 for TC/HDL‐C
4. Discussion
The present study has shown that 1 lower TC and TC/HDL‐C were independently associated with increased risks of poor outcomes and death, and decreased probability of good outcomes;2 the best discriminating factor for death was a TC/HDL‐C value of 3.37 or less, associated with 2.6‐fold increased risk of death in patients with AIS;3 the HDL‐C and LDL‐C was not independently associated with the two outcome measures.
The strengths of this study are that clinical information was collected in two different areas. Blood samples were drawn strictly before treatment, meaning that all data were obtained in the ideal conditions to assess the influence of lipid levels at admission on the outcome. However, blood samples were collected after treatment, sometimes several days after AIS in previous studies. Another strength is that the overall information of AIS patients enrolled in the analyses is representative of patients with ischaemic stroke according to demographic, baseline parameters, medical history, stroke etiology, and vascular risk factors.16
The TC/HDL‐C ratio is a cheap and reproducible variable that is simple to obtain from routine laboratory parameters. Recent evidence revealed that the TC/HDL‐C has important prognostic values. A recent study showed that the TC/HDL‐C ratio quantified atherogenic lipoproteins and particles superior to simply measuring lipid levels.17 However, the role of TC/HDL‐C ratio on stroke outcome remain unknown.
Our main speculation is associated with the lower TC concentration. Previous studies have demonstrated that a high TC level was a strong risk factor for coronary heart disease, but its function in stroke remained inconsistent. Although a significant association between TC level and stroke were not found in several observational reports,18, 19 another studies have indicated correlations of high serum TC concentration with an increased risk of ischaemic stroke.20, 21 In addition, there was a positive association between TC level and atherothrombotic infarction.22 Otherwise, another observational studies showed that lower concentration of serum TC was a risk factor for hemorrhagic stroke and increased the probability of death after stroke, and similar results were also observed for ischaemic stroke.23, 24 Furthermore, low serum TC levels had an increased risk of TACI and poor outcomes in patients with ischaemic stroke experiencing prestroke statin treatment in a hospital‐based study.25 In a community‐based cohort study, low TC concentration was significantly associated with increased risk of stroke and heart disease.26 In consistent with these findings, there was an inverse association between TC levels and stroke outcomes in the current study.
Another speculation is associated with the higher HDL‐C level. However, our findings supported no significant association between higher HDL‐C level and poor outcomes in AIS patients, and these results supported previous observations indicating the importance of the TC/HDL‐C ratio for stroke risk stratification.8, 27 Otherwise, several studies revealed that HDL‐C concentration was associated with 3‐month prognosis in Chinese patients with acute stroke.10 These contradictory findings may be due to methods of outcome evaluation and grouping or sample size. In the present study, predictive value of TC/HDL‐C was mainly controlled by TC compared with HDL‐C. More importantly, combined predictive values of TC and HDL‐C were significantly superior to the single predictive value of TC or HDL‐C as illustrated in Figures 1 and 2. Collectively, lower TC/HDL‐C superior to TC and HDL‐C was associated with poor prognosis after AIS.
In conclusion, these results of our study support the hypothesis that the TC/HDL‐C ratio was a significant independent predictor of poor outcomes at 3 months in patients with AIS. Lower TC/HDL‐C ratio can be risk indicator for death superior to single predictive value of TC or HDL‐C in AIS. Identification of the TC/HDL‐C ratio of this effect could facilitate us identifying new targets for neuroprotection in patients with AIS.
Competing Interests
The authors have declared no competing interests.
Author Contribution Statement
LFC and JSZ conceived and designed the project, JX, HS, and HW acquired the data, LFC and JX analyzed and interpreted the data, LFC and JSZ wrote the paper.
Acknowledgments
This study was supported by the Program for Personnel training of Jiangsu Province (no. WSW‐022).
Chen L, Xu J, Sun H, Wu H, and Zhang J. The total cholesterol to high‐density lipoprotein cholesterol as a predictor of poor outcomes in a Chinese population with acute ischaemic stroke. J Clin Lab Anal. 2017;31: e22139 10.1002/jcla.22139
References
- 1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hankey GJ. Long‐term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16(Suppl 1):14–19. [DOI] [PubMed] [Google Scholar]
- 3. Feigin VL, Lawes CM, Bennett DA, Barker‐Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population‐based studies: a systematic review. Lancet Neurol. 2009;8:355–369. [DOI] [PubMed] [Google Scholar]
- 4. Olsen TS, Christensen RH, Kammersgaard LP, Andersen KK. Higher total serum cholesterol levels are associated with less severe strokes and lower all‐cause mortality: ten‐year follow‐up of ischemic strokes in the Copenhagen Stroke Study. Stroke. 2007;38:2646–2651. [DOI] [PubMed] [Google Scholar]
- 5. Tuttolomondo A, Di Raimondo D, Di Sciacca R, et al. Effects of clinical and laboratory variables at admission and of in‐hospital treatment with cardiovascular drugs on short term prognosis of ischemic stroke. The GIFA study. Nutr Metab Cardiovasc Dis. 2013;23:642–649. [DOI] [PubMed] [Google Scholar]
- 6. Zhao W, An Z, Hong Y, et al. Low total cholesterol level is the independent predictor of poor outcomes in p™atients with acute ischemic stroke: a hospital‐based prospective study. BMC Neurol. 2016;16:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Markaki I, Nilsson U, Kostulas K, Sjostrand C. High cholesterol levels are associated with improved long‐term survival after acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23:e47–e53. [DOI] [PubMed] [Google Scholar]
- 8. Cuadrado‐Godia E, Jimenez‐Conde J, Ois A, Rodriguez‐Campello A, Garcia‐Ramallo E, Roquer J. Sex differences in the prognostic value of the lipid profile after the first ischemic stroke. J Neurol. 2009;256:989–995. [DOI] [PubMed] [Google Scholar]
- 9. Dyker AG, Weir CJ, Lees KR. Influence of cholesterol on survival after stroke: retrospective study. BMJ. 1997;314:1584–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li W, Liu M, Wu B, Liu H, Wang LC, Tan S. Serum lipid levels and 3‐month prognosis in Chinese patients with acute stroke. Adv Ther. 2008;25:329–341. [DOI] [PubMed] [Google Scholar]
- 11. Stroke–1989 . Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 12. Adams HP Jr, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: a scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1083. [DOI] [PubMed] [Google Scholar]
- 13. European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee . Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. [DOI] [PubMed] [Google Scholar]
- 14. Deng QW, Wang H, Sun CZ, et al. Triglyceride to high‐density lipoprotein cholesterol ratio predicts worse outcomes after acute ischaemic stroke. Eur J Neurol. 2016. doi: 10.1111/ene.13198. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 16. Nardi K, Engelter S, Strbian D, et al. Lipid profiles and outcome in patients treated by intravenous thrombolysis for cerebral ischemia. Neurology. 2012;79:1101–1108. [DOI] [PubMed] [Google Scholar]
- 17. Mathews SC, Mallidi J, Kulkarni K, Toth PP, Jones SR. Achieving secondary prevention low‐density lipoprotein particle concentration goals using lipoprotein cholesterol‐based data. PLoS One. 2012;7:e33692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurth T, Everett BM, Buring JE, Kase CS, Ridker PM, Gaziano JM. Lipid levels and the risk of ischemic stroke in women. Neurology. 2007;68:556–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prospective Studies C , Lewington S, Whitlock G, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta‐analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. [DOI] [PubMed] [Google Scholar]
- 20. Benfante R, Yano K, Hwang LJ, Curb JD, Kagan A, Ross W. Elevated serum cholesterol is a risk factor for both coronary heart disease and thromboembolic stroke in Hawaiian Japanese men. Implications of shared risk. Stroke. 1994;25:814–820. [DOI] [PubMed] [Google Scholar]
- 21. Lindenstrom E, Boysen G, Nyboe J. Influence of total cholesterol, high density lipoprotein cholesterol, and triglycerides on risk of cerebrovascular disease: the Copenhagen City Heart Study. BMJ. 1994;309:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–2498. [DOI] [PubMed] [Google Scholar]
- 23. Campbell GS, Bick HD, Varco RL, Paulsen EP, Lober PH, Watson CJ. Bleeding esophageal varices with polycystic liver; report of three cases. N Engl J Med. 1958;259:904–910. [DOI] [PubMed] [Google Scholar]
- 24. Suzuki K, Izumi M, Sakamoto T, Hayashi M. Blood pressure and total cholesterol level are critical risks especially for hemorrhagic stroke in Akita. Japan. Cerebrovasc Dis. 2011;31:100–106. [DOI] [PubMed] [Google Scholar]
- 25. Koton S, Molshatzki N, Bornstein NM, Tanne D. Low cholesterol, statins and outcomes in patients with first‐ever acute ischemic stroke. Cerebrovasc Dis. 2012;34:213–220. [DOI] [PubMed] [Google Scholar]
- 26. Nago N, Ishikawa S, Goto T, Kayaba K. Low cholesterol is associated with mortality from stroke, heart disease, and cancer: the Jichi Medical School Cohort Study. J Epidemiol. 2011;21:67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simundic AM, Nikolac N, Topic E, Basic‐Kes V, Demarin V. Are serum lipids measured on stroke admission prognostic? Clin Chem Lab Med. 2008;46:1163–1167. [DOI] [PubMed] [Google Scholar]


