Abstract
Background
Inflammation plays an important role in the initiation and progression of acute aortic dissection (AAD). New inflammatory indices derived from full cell blood count and its differential may be associated with increased risk. We evaluated platelet‐lymphocyte (PLR), red cell distribution width (RDW) and RDW/PLT's (platelets) (RPR) in AAD.
Methods
We studied 120 consecutive patients with AAD type I admitted for emergency surgery (group I), 121 consecutive patients with aortic aneurysms of the ascending aorta prior to elective repair (group II) and 121 controls (group III), age and sex matched.
Results
PLR was significantly higher in group I vs both groups II and III (P < .001). There was an excellent correlation of PLR with neutrophil/lymphocyte ratio (NLR) in all three groups (P < .001 for all). After adjustment for hemoglobin, RDW did not differ but RPR remained significantly higher in group I compared to groups II and III (P < .001). The best cutoff value of PLR to predict dissection was 159 with 53% sensitivity and 86% specificity. No association between PLR, RDW, and RPR and mortality in group I was found.
Conclusions
Indices derived from full cell blood count may provide diagnostic information in patients with AAD; whether these indices may contribute to prognosis assessment should be further investigated.
Keywords: acute aortic dissection, aortic aneurysms, biomarkers, full blood count
1. INTRODUCTION
Inflammation plays an important role in the initiation and progression of cardiovascular diseases. Inflammation may also contribute to the pathogenesis of aortic aneurysm formation and rupture. C‐reactive protein (CRP) is increased in acute aortic dissection (AAD) and correlates with markers of ischemia.1, 2 Likewise, white cell blood count (WBC) is higher in AAD compared to aortic aneurysms and normal subjects and is associated with higher mortality.3, 4 WBC subtypes and especially neutrophil to lymphocyte ratio (NLR) have been associated with adverse clinical outcomes in various clinical settings. We have previously reported that NLR is also significantly higher in AAD.4 In addition, we have found significant thrombocytopenia and alterations in platelet parameters—platelet size distribution width and mean platelet volume to platelets (PLTs) ratio—in patients with AAD.5
Platelet to lymphocyte ratio (PLR) is an emerging inflammatory index, which is associated with adverse outcomes.6, 7, 8, 9, 10, 11, 12 It integrates the risk prediction of PLTs and lymphocytes, reflecting the activation of both hemostatic and inflammatory pathways.13 Similarly, red cell distribution width (RDW), a measure of variability in size of circulating erythrocytes, is a novel risk factor for total and cardiovascular mortality.14, 15 RDW indicates the underlying inflammatory state which leads to impaired erythrocyte maturation. The aim of this study was to evaluate alterations in PLR, RDW, and RDW/PLT's ratio (RPR) and any possible association with mortality in AAD.
2. METHODS
We studied 120 consecutive patients with AAD type A admitted for emergency surgery (group I) between January 2005 and November 2013, 121 consecutive patients with aortic aneurysms of the ascending aorta prior to elective repair (group II), performed between March 2005 and November 2013 and compared them with 121 controls (group III), age and sex matched. Our hospital is regional referral center for AAD. The study protocol was approved by the Ethics Committee of our institution (Ref: 472/13.01.12), and all patients gave informed consent. The diagnosis of acute dissection was confirmed in all patients of group I with computed tomography (CT); likewise, all patients in group II underwent CT (Somatom Definition CT, Siemens, Erlangen, Germany). Dissection was classified according to the Stanford criteria.
We measured WBC, hemoglobin (Hb), PLTs, D‐dimer, creatinine, CRP, and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) at baseline. D‐dimer was measured with Elisa (Vidas D‐dimer, Biomerieux, France), CRP with an immunoturbidimetric method (Cobas Integra, Roche, Mannheim, Germany) and NT‐proBNP with immunoassay (Elecsys proBNP, Roche, Mannheim, Germany). Creatine kinase (CPK) was evaluated on a Cobas Integra 800 analyzer (Roche, Bazel, Switzerland), and creatine kinase‐MB (CPK‐MB) and troponin I were evaluated by immunoassay on the Dimension system (Dade Behring, Deerfield, IL, USA).
For patients in group I, blood samples were obtained as soon as the patient came to the intensive care unit and before surgery and for patients in group II, the morning of the admission and prior to operation. The measurement of the diameter of the ascending aorta in patients of groups I and II was based on CT angiograms. Left ventricular ejection fraction was evaluated with transthoracic echocardiogram (Vivid E9 ultrasound system, GE Healthcare, Wauwatosa, USA). In‐hospital mortality in groups I and II was retrieved from hospital records.
Kruskal‐Wallis was applied to compare all continuous variables between groups; when P was <.05, post hoc analysis was performed with Bonferroni correction. Normality assumption was assessed using histograms and the Kolmogorov‐Smirnov test. We used the Spearman correlation coefficient to investigate associations between continuous variables. A receiver operating characteristic curve (ROC) analysis was performed to investigate the diagnostic performance of PLR.
3. RESULTS
The demographic and clinical characteristics of study population are reported in Table 1. There was no difference in age and gender among the three groups. As expected, patients in groups I and II were more often hypertensive and smokers compared to controls. Left ventricular ejection fraction was preserved in all three groups, but was lower in group I (P = .002). Of note, aortic diameter was smaller in group I than in group II (P = .009).
Table 1.
Demographic and clinical characteristics of patients with type A acute aortic dissection (group I), aortic aneurysms (group II), and controls (group III)
| Group I (n = 120) | Group II (n = 121) | Group III (n = 121) | P value | |
|---|---|---|---|---|
| Age (y) | 63 ± 14 | 63 ± 11 | 63 ± 14 | .962 |
| Male/female | 89/31 | 90/31 | 90/31 | .993 |
| Hypertension | 91 (81) | 102 (86) | 55 (47) | <.001 |
| Diabetes | 6 (5) | 10 (8) | 19 (17) | .012 |
| Dyslipidemia | 36 (32) | 64 (54) | 45 (39) | .003 |
| Smoking | 52 (46) | 50 (42) | 29 (29) | .024 |
| Ejection Fraction (%) | 54 ± 7 | 58 ± 7 | 57 ± 7 | .002 |
| Aortic diameter (cm) | 5.2 ± 1.1 | 5.5 ± 0.7 | .009 | |
| Aortic regurgitation | 53 (62) | 70 (61) | .892 | |
| Extent of dissection or dilatation | ||||
| Thoracic aorta | 57 (47) | 117 (97) | <.001 | |
| Thoracic and abdominal aorta | 26 (22) | 2 (3) | ||
| Thoracic and abdominal aorta and iliac arteries | 29 (24) | 0 (0) | ||
| Time from onset of symptoms (h) | 16 ± 15 | |||
Data are expressed as mean ± SD or n (%).
P value refers to Kruskal‐Wallis test between all 3 groups.
Hospital stay was significantly longer in group I compared to group II {median [25%‐75% interquartile range], 9 (6, 13) vs 8 (7, 9) days, P = .042}. Aortic cross‐clamping time and cardiopulmonary bypass time were significantly longer in group I compared to group II (131 ± 90 vs 99 ± 39 minutes, P = .001 and 250 ± 192 vs 137 ± 53 minutes, P < .001, respectively). Within the group I, five patients were managed conservatively and two of them died. Ten patients died before surgery, 5 died during surgery, and 10 shortly after, in the intensive care unit. As expected, in‐hospital mortality was higher in group I compared to group II (22.5 vs 1.7%, P < .001).
We found that PLR is significantly higher in AAD compared to both aortic aneurysms and controls (P < .001), with no difference between the last two (Table 2). The best cutoff value of PLR to predict dissection was 159 with 53% sensitivity and 86% specificity (Figure 1). In dissection, WBC was higher as were the neutrophils and the NLR (P < .001 for both); inversely, lymphocytes were lower (P < .001). Likewise, marked thrombocytopenia was observed (P < .001). As expected, D‐dimers, NT‐proBNP, CRP, cardiac enzymes, and creatinine were increased and Hb decreased (Table 2).
Table 2.
Laboratory results in acute aortic dissection (group I), aortic aneurysms (group II), and controls (group III)
| Group I (n = 120) | Group II (n = 121) | Group III (n = 121) | P value | |
|---|---|---|---|---|
| Hb (g/dL) | 12.6 (11.1, 13.7) | 14 (13.1, 15.2) | 13.9 (12.8, 15) | <.001 |
| WBC (K/μL) | 12200 (10000, 16000) | 7400 (6400, 8600) | 7300 (6100, 8700) | <.001 |
| Neutrophils (%) | 85 (79, 89) | 61 (55, 67) | 59 (53, 67) | <.001 |
| Lymphocytes (%) | 8.5 (6, 14) | 28 (23, 33) | 29 (22, 36) | <.001 |
| NLR | 10.1(5.9, 14.3) | 2.2 (1.7, 2.9) | 2.0 (1.4, 3.1) | <.001 |
| PLT's (K/μL) | 187500 (148500, 219000) | 222000 (187000, 251000) | 222000 (190000, 259000) | <.001 |
| PLR | 165.2(113.1, 240.5) | 107.5 (83.1,139.6) | 107.9(83.1, 138.7) | <.001 |
| RDW (fl) | 14 (13.5,15.1) | 13.8 (13.3, 14.8) | 13.7 (13.1, 14.3) | .010 |
| RDW/PLT's | 7.9 (6.3, 9.6) | 6.3(5.6, 7.8) | 6.1 (5.2, 7.7) | <.001 |
| D‐dimers (ng/mL) | 5434 (2586, 8709) | 426 (251, 972) | 234 (173, 563) | <.001 |
| NT‐proBNP (pg/mL) | 522 (201, 1030) | 160.5 (67, 690) | 23 (20,108) | <.001 |
| CRP (mIU/mL) | 8.6 (3.0, 19.8) | 2.3 (1.2, 5.8) | 2.1 (1.1, 5.6) | <.001 |
| CPK (ng/mL) | 114 (65, 264) | 161 (44, 232) | 73 (53, 107) | .001 |
| CPK‐MB (ng/mL) | 1.3 (0.8, 3.3) | 0.7 (0.3, 4.6) | 0.7 (0.3, 1.1) | <.001 |
| Tn‐I (ng/mL) | 0.1 (0.0, 0.2) | 0 (0.0, 0.1) | 0.0 (0.0, 0.0) | <.001 |
| Creatinine (mg/dL) | 1.1 (0.8, 1.4) | 1.0 (0.8, 1.1) | 0.9 (0.8, 1.1) | <.001 |
CPK, creatine kinase; CPK‐MB, the MB isoenzyme of creatine kinase; CRP, C‐reactive protein; Hb, hemoglobin; NLR, neutrophil/lymphocyte ratio; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; PLR, platelet/lymphocyte ratio; PLT's, platelets count; RDW, red cell distribution width; Tn‐I, troponin I; WBC, white cell blood count.
Data are expressed as median (25%‐75% interquartile range).
P value refers to Kruskal‐Wallis test between all 3 groups.
Figure 1.
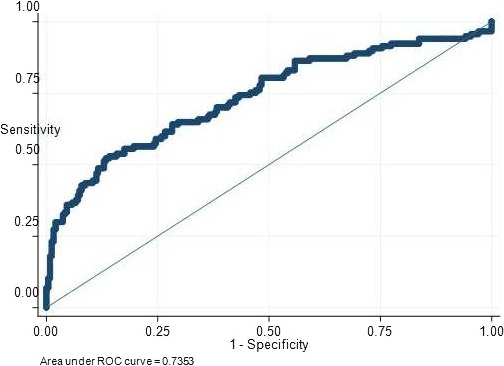
ROC curve of PLR for diagnosis of type A acute aortic dissection
There was an excellent correlation of PLR with NLR in all three groups (P < .001 for all). PLR correlated significantly with the number of PLTs in all three groups (P < .001 for I and II, P = .003 for III) and inversely with the number of lymphocytes in all three groups (P < .001). RDW was higher in group I compared to group III (P = .007) and similar between I vs II and II vs III; after adjustment, however, for Hb values there was no longer statistical difference between our groups (P = .131). The ratio RPR was significantly increased in group I vs both II and III (P < .001), without difference between II and III (P = .64), and this difference remained significant when taking into account the Hb levels (P < .001 and P = .001, respectively). PLR, RDW, and RPR were not associated with mortality in group I.
4. DISCUSSION
We have shown, for the first time that PLR and RPR were significantly higher in AAD compared to uncomplicated aortic aneurysms and controls. PLR demonstrated a high degree of specificity but fairly low sensitivity in predicting AAD. For patients with symptoms suggestive of acute aortic syndrome, with or without a history of preexisting aneurysm, mainstream biomarkers exhibiting high specificity may be helpful to the clinician to rule out AAD.
PLR is readily available, widely applied, inexpensive, and reproducible. Thrombocytopenia is presumably due to platelet consumption associated with enhanced fibrinolysis within the false lumen of the dissecting aneurysm. Hypothalamic‐pituitary‐adrenal axis activation during stress induces cortisol secretion and subsequent relative lymphopenia. Leucocytosis occurs as part of the acute inflammatory process in the vessel wall and carries prognostic significance.
Increased NLR implies higher inflammatory burden, it is due to higher neutrophils as well as lower lymphocytes and may be associated with higher risk. Higher PLR in AAD is not associated with thrombocytosis, which could occur because of inflammation and bleeding; in AAD, true thrombocytopenia is noted which, however, in conjunction with severe lymphopenia results in increased PLR. Lymphopenia is a predictor of adverse outcomes in chronic ischemic disease, heart failure, and acute coronary syndromes.16, 17 Our best cutoff value of PLR with a high degree of specificity but fairly low sensitivity was 159; of note, PLR > 150 was associated with an odds ratio of 1.9 for critical limb ischemia in patients with peripheral arterial disease.10 In addition, preoperative PLR > 142 was observed in coronary patients with post‐coronary bypass grafting complications, PLR > 128 was an independent predictor of future events in patients with NSTEMI, and PLR > 171 in patients with stable CAD was associated with more severe coronary disease and worse prognosis.12, 18, 19, 20, 21 PLR is, therefore, a novel parameter which indicates inflammation and carries prognostic significance in a variety of clinical conditions.
Recently, Morello et al showed that WBC and PLT count were helpful in risk assessment of patients at low pretest probability for AAD by clinical evaluation.22 We also found high WBC and low PLTs; likewise, CRP and D‐dimers were also high as previously described.
RDW may be a proinflammatory biomarker; high RDW decreases red cell deformability and possibly compromises blood flow and oxygen supply in the microcirculation, although this has been reported for values >14%.23 Although the role of anisocytosis in the pathogenesis of cardiovascular diseases is not clear, emerging evidence suggests that it may be important in diagnosis and prognosis of various disorders, independent of Hb values.24 We found no significant difference in RDW when corrected for Hb values, but the ratio RPR was significantly higher in AAD even after adjustment for Hb.
We compared AAD with aortic aneurysms, although hemostatic and inflammatory indices obviously differ significantly between the two, because these patients share pathology in relation to aortic wall changes and, in addition, this could be of help in differentiating the cause of chest pain in patients with a known aortic aneurysm. Our control group does not include normal volunteers but age and sex‐matched patients with established risk factors for vascular disease; this may, however, be more relevant in every day clinical practice.
5. CONCLUSION
PLR is significantly higher in AAD compared to aortic aneurysms and controls and carries a high degree of specificity but fairly low sensitivity in predicting AAD; PLR is an inflammatory biomarker which might be useful in the diagnosis of AAD. In addition, RDW/PLT's is also higher in AAD. No association between PLR, RDW, and RPR and mortality in AAD was found in this sample size.
Sbarouni E, Georgiadou P, Kosmas E, Analitis A, Voudris V. Platelet to lymphocyte ratio in acute aortic dissection. J Clin Lab Anal. 2018;32:e22447 10.1002/jcla.22447
REFERENCES
- 1. Sbarouni E, Georgiadou P, Marathias A, Geroulanos S, Kremastinos DT. D‐dimer and BNP in acute aortic dissection. Int J Cardiol. 2007;122:170‐172. [DOI] [PubMed] [Google Scholar]
- 2. Sbarouni E, Georgiadou P, Marathias A, Panagiotakos D, Geroulanos S, Voudris V. Ischemia‐modified albumin in acute aortic dissection. J Clin Lab Anal. 2010;24:399‐402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sbarouni E, Georgiadou P, Analitis A, et al. High homocysteine and low folate concentrations in acute aortic dissection. Int J Cardiol. 2013;168:463‐466. [DOI] [PubMed] [Google Scholar]
- 4. Sbarouni E, Georgiadou P, Analitis A, Voudris V. High neutrophil to lymphocyte ratio in type A acute aortic dissection facilitates diagnosis and predicts worse outcome. Expert Rev Mol Diagn. 2015;15:965‐970. [DOI] [PubMed] [Google Scholar]
- 5. Sbarouni E, Georgiadou P, Analitis A, Voudris V. Significant changes in platelet count, volume and size in acute aortic dissection. Int J Cardiol. 2013;168:4349‐4350. [DOI] [PubMed] [Google Scholar]
- 6. Kurtul A, Yarlioglues M, Murat SN, et al. Usefulness of the platelet‐lymphocyte ratio in predicting angiographic reflow after PPCI in patients with acute STEMI. Am J Cardiol. 2014;114:342‐347. [DOI] [PubMed] [Google Scholar]
- 7. Azab B, Shah N, Akerman M, McGinn JT Jr. Value of platelet/lymphocyte ratio as a predictor of all‐cause mortality after non‐ST‐elevation myocardial infarction. J Thromb Thrombolysis. 2012;34:326‐334. [DOI] [PubMed] [Google Scholar]
- 8. Sunbul M, Gerin F, Durmus E, et al. Neutrophil to lymphocyte and platelet to lymphocyte ratio in patients with dipper versus non‐dipper hypertension. Clin Exp Hypertens. 2014;36:217‐221. [DOI] [PubMed] [Google Scholar]
- 9. Açar G, Kalkan ME, Avci A, et al. The relation of platelet‐lymphocyte ratio and coronary collateral circulation in patients with stable angina pectoris and chronic total occlusion. Clin Appl Thromb Hemost. 2015;21:462‐468. [DOI] [PubMed] [Google Scholar]
- 10. Gary T, Pichler M, Belaj K, et al. Platelet‐to‐lymphocyte ratio: a novel marker for critical limb ischemia in peripheral arterial occlusive disease patients. PLoS ONE. 2013;8:e67688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ciçek G, Açıkgoz SK, Bozbay M, et al. Neutrophil‐Lymphocyte Ratio and Platelet‐Lymphocyte Ratio Combination Can Predict Prognosis in Patients With ST‐Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Angiology. 2015;66:441‐447. [DOI] [PubMed] [Google Scholar]
- 12. Cho KI, Ann SH, Singh GB, Her AY, Shin ES. Combined usefulness of the platelet‐to‐lymphocyte ratio and the neutrophil‐to‐lymphocyte ratio in predicting the long term adverse events in patients who have undergone percutaneous coronary intervention with a DES. PLoS ONE. 2015;10:e0133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yildiz A, Yuksel M, Oylumlu M, et al. The utility of the platelet‐lymphocyte ratio for predicting no reflow in patients with ST‐segment elevation myocardial infarction. Clin Appl Thromb Hemost. 2015;21:223‐228. [DOI] [PubMed] [Google Scholar]
- 14. Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle‐aged and older adults. Arch Intern Med. 2009;169:515‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perlstein TS, Weuve J, Pfeffer MA, Beckman JA. Red blood cell distribution width and mortality risk in a community‐based prospective cohort. Arch Intern Med. 2009;169:588‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997;79:812‐814. [DOI] [PubMed] [Google Scholar]
- 17. Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19‐22. [DOI] [PubMed] [Google Scholar]
- 18. Şaşkın H, Düzyol Ç, Özcan KS, Aksoy R, Idiz M. Preoperative platelet to lymphocyte ratio is associated with early morbidity and mortality after coronary artery bypass surgery. Heart Surg Forum. 2016;18:E255‐E262. [DOI] [PubMed] [Google Scholar]
- 19. Zhou D, Wang G, Fan Y, Wan Z, Liu X. Platelet to lymphocyte ratio is associated with the severity of coronary artery disease and clinical outcomes of percutaneous coronary intervention in the Chinese Han population. Exp Ther Med. 2017;13:731‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Acet H, Ertaş F, Akıl MA, et al. Relationship Between Hematologic Indices and Global Registry of Acute Coronary Events Risk Score in Patients With ST‐Segment Elevation Myocardial Infarction. Clin Appl Thromb Hemost. 2016;22:60‐68. [DOI] [PubMed] [Google Scholar]
- 21. Acet H, Ertaş F, Akıl MA, et al. Novel predictors of infarct‐related artery patency for ST‐segment elevation myocardial infarction: platelet‐to‐lymphocyte ratio, uric acid, and neutrophil‐to‐lymphocyte ratio. Anatol J Cardiol. 2015;15:648‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Morello F, Cavalot G, Giachino F, et al. White blood cell and platelet count as adjuncts to standard clinical evaluation for risk assessment in patients at low probability of acute aortic syndrome. Eur Heart J Cardiovasc Care. 2017;6:389‐395. [DOI] [PubMed] [Google Scholar]
- 23. Patel KV, Mohanty JG, Kanapuru B, Hesdorffer C, Ershler WB, Rifkind JM. Association of the red cell distribution width with red blood cell deformability. Adv Exp Med Biol. 2013;765:211‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Cardiovasc Dis. 2015;7:402‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]


