Abstract
Background
This study aimed to investigate the miR‐126 expression in lesional skin and its correlation with clinical features in psoriasis patients and to explore the effect of upregulated miR‐126 on cells' proliferation, apoptosis, and inflammation in human keratinocytes.
Methods
A total of 102 psoriasis patients were consecutively enrolled in this study. MiR‐126 expressions in lesional skin and paired nonlesional skin were detected by quantitative polymerase chain reaction (qPCR). Human keratinocytes (HaCaT cells) were transfected with miR‐126 mimic plasmids and blank mimic plasmid. Cell Counting Kit‐8 and annexin V/propidium iodide assays were performed to assess the cells' proliferation and apoptosis, and protein levels of apoptotic markers (cleaved caspase‐3 [C‐caspase‐3] and B‐cell lymphoma‐2 [Bcl‐2]) were detected by Western blot assay. Inflammatory cytokines mRNA and protein levels were detected by qPCR and Western blot assays, respectively.
Results
MiR‐126 expression was upregulated in lesional skin tissue compared with paired nonlesional skin tissue, and its expression positively associated with Psoriasis Area and Severity Index score in psoriasis patients. MiR‐126 expression was increased in miR‐126 mimic group compared with negative control (NC) mimic group after plasmids transfection into HaCaT cells, and cells' proliferation was enhanced while cells' apoptosis rate was reduced in miR‐126 mimic group than NC mimic group. Protein expressions of C‐caspase and Bcl‐2 also indicated miR‐126 mimic decreased the cells' apoptosis. In addition, miR‐126 mimic increased TNF‐α, IFN‐γ, IL‐17A, and IL‐22 expressions while decreased IL‐10 expression.
Conclusion
In conclusion, miR‐126 correlates with elevated risk and increased disease severity in psoriasis patients, and upregulation of miR‐126 promotes cells' proliferation and inflammation while inhibits cells' apoptosis in keratinocytes.
Keywords: apoptosis, inflammation, miR‐126, proliferation, psoriasis
1. INTRODUCTION
Psoriasis, a common chronic and systemic inflammatory skin disease, affects 2%‐3% of the population worldwide.1, 2 In China, an epidemiological survey of psoriasis in 6 provinces shows that the prevalence of psoriasis is 0.47%.3 Patient with psoriasis has a higher risk of developing multiple complications, including psoriatic arthritis, cardiovascular diseases, Crohn's disease, anxiety, and depression, all of them substantially contributing to increased morbidity and mortality.4, 5 Although the application of topical therapy, physiotherapy, systemic therapy, and novel biological agents (such as tumor necrosis factor alpha [TNF‐α] inhibitor, interleukin [IL]‐12/23 inhibitor and IL‐17 inhibitor) have achieved a certain effect, the low response rate and high recurrence rate remain critical issues to clinicians.1, 6, 7, 8 Therefore, it is of great importance to investigate the underlying mechanism of psoriasis and explore novel treatment target.
MicroRNAs (miRNAs) are a class of small noncoding RNAs that play crucial roles in the regulation of biological processes in signal transduction, cells' proliferation, differentiation, and apoptosis.9, 10, 11, 12 Accumulating studies disclose that dysregulation of miRNAs including miR‐31, miR‐155, and miR‐26b is involved in the pathological processes of a variety of immune diseases including psoriasis.13, 14, 15 MiR‐126, located on human chromosome 9q34.3, has been reported to participate in the development and progression of multiple immune diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), and inflammatory bowel disease (IBD).16, 17, 18 However, to the best of our knowledge, the role of miR‐126 in the etiology of psoriasis has not been reported. Therefore, this study aimed to investigate the miR‐126 expression of lesional skin and its correlation with clinical features in psoriasis patients and to explore the effect of upregulation of miR‐126 on cells' proliferation, apoptosis, and inflammation in human keratinocytes.
2. MATERIALS AND METHODS
2.1. Participants
One hundred and two psoriasis patients were consecutively enrolled in this study between January 2016 and December 2016. The inclusion criteria consisted of: (i) Diagnosed as psoriasis. (ii) In moderate to severe disease condition which was defined as lesional body surface area (BSA; psoriasis‐affected) ≥10% and Psoriasis Area and Severity Index (PASI) score ≥8 points. (iii) Age above 18 years. Patients with: (i) solid tumor, (ii) hematological malignancy, (iii) severe infection, (iv) hepatic or renal dysfunction were excluded from our study. This study was approved by the Ethics Committee of Fuling Centre Hospital of Chongqing, and all patients signed the informed consents.
2.2. Samples and miR‐126 detection
After enrollment, 3 mm punch biopsies were taken from the lesional skin of psoriasis patients. Meanwhile, paired nonlesional skin 5 cm away from the lesion was taken as controls. Total RNA was extracted using TRIzol (Invitrogen, USA), and miR‐126 expression in tissue of lesional skin was detected by quantitative polymerase chain reaction (qPCR; Detailed methods were described in “qPCR assay” subsection).
2.3. Data collection
Patients' characteristics including age, gender, body mass index (BMI), disease duration, lesional BSA, PASI score, and treatments were documented after the enrollment.
2.4. Cells' culture
Human keratinocytes (HaCaT cells) were kindly given by Fudan University (Shanghai, China). After resuscitation, the HaCaT cells were subsequently cultured in RPMI‐1640 medium (Gibco, USA) containing 10% fetal bovine serum (FBS; Gibco) at 37°C with 5% (v/v) CO2.
2.5. miR‐126 mimic plasmids transfection and followed assays
HaCaT cells were transfected with blank mimic plasmid and miR‐126 mimic plasmid as negative control (NC) mimic group and miR‐126 mimic group, respectively. MiR‐126 expression was detected by qPCR assay at 24 hour after transfection, and cells' proliferation was determined by Cell Counting Kit (CCK)‐8 assay at 0, 24, 48, and 72 hour after transfection. And cells' apoptosis rate was measured by annexin V/propidium iodide (AV/PI) assay at 72 hour after transfection. Meanwhile, the expressions of apoptotic markers (cleaved caspase‐3 [C‐caspase‐3] and B‐cell lymphoma‐2 [Bcl‐2]) were detected by Western blot assay at 72 hour after transfection; In addition, tumor necrosis factor‐α (TNF‐α), interferon‐γ (IFN‐γ), interleukin (IL)‐10, IL‐17A, and IL‐22 mRNA expressions were detected by qPCR assay and their protein levels were detected by Western blot assay at 24 hour after transfection.
2.6. qPCR assay
Total RNA in lesional skin tissue, nonlesional skin tissue or HaCaT cell line was extracted using TRIzol (Invitrogen) according to manufacturer's instructions. And 1 ug total RNA was reversely transcribed into cDNA using ReverTra Ace® qPCR RT Kit (Toyobo, Japan), then the cDNA was used for qPCR amplification by SYBR® Green Quantitative RT‐qPCR Kit (KAPA, Japan). U6 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) were used as internal reference for miR‐126 and mRNA, respectively. qPCR was performed as follows: heat activated at 95°C for 5 minutes followed by 40 cycles of denaturation at 95°C for 5 seconds, annealing, and extension at 61°C for 30 seconds. And the PCR amplification was performed in triplicate. Subsequently, the qPCR results were calculated using 2−ΔΔCt method. The primer sequences were exhibited in Table 1.
Table 1.
Primers used in this study
| Genes | Sequence (5′→3′) |
|---|---|
| miR‐126 | F: GCTGTCAGTTTGTCAAATA |
| R: GTGCAGGGTCCGAGGT | |
| TNF‐α | F: TTCCTCAGCCTCTTCTCCTTCCT |
| R: ATCTCTCAGCTCCACGCCATTG | |
| IFN‐γ | F: TCGGTAACTGACTTGAATGTCCA |
| R: TCGCTTCCCTGTTTTAGCTGC | |
| IL‐10 | F: TGTTGCCTGGTCCTCCTGACT |
| R: GCCTTGATGTCTGGGTCTTGGTT | |
| GAPDH | F: TGACCACAGTCCATGCCATCAC |
| R: GCCTGCTTCACCACCTTCTTGA | |
| U6 | F: CTCGCTTCGGCAGCACATATACTA |
| R: ACGAATTTGCGTGTCATCCTTGC |
GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; IFN‐γ, interferon‐γ; IL‐10, interleukin 10; TNF‐α, tumor necrosis factor α.
2.7. Western blot assay
RIPA (Thermo fisher, MA, USA) was used to extract total protein from tissue or cells. The extracted total protein was then quantified using Pierce™ BCA Protein Assay Kit (Thermo fisher). 20 μg of total protein after denaturation at 95°C for 5 minutes was used for sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE), and the isolated protein was transferred into nitrocellulose membranes (Millipore, USA). After that, the nitrocellulose membranes were blocked in 5% nonfat powdered milk at room temperature for 2 hour and were incubated with primary antibodies overnight at 4°C overnight, followed by incubation with appropriate horseradish peroxidase (HRP)‐conjugated secondary antibodies at room temperature for 1 hour. Afterward, the bands were visualized using an enhanced chemiluminescence (ECL) kit (Millipore, USA) under X‐ray. Additionally, the antibodies information was shown in Table 2.
Table 2.
Antibody information
| Antibody name | Company | Country | Dilution ratio |
|---|---|---|---|
| Primary antibody | |||
| Rabbit monoclonal anticleaved caspase 3 antibody | Abcam | USA | 1:2000 |
| Rabbit polyclonal anticaspase 3 antibody | Abcam | USA | 1:2000 |
| Rabbit monoclonal anti‐BCL‐2 antibody | Abcam | USA | 1:1000 |
| Rabbit polyclonal anti‐TNF‐α antibody | Abcam | USA | 1:2000 |
| Rabbit polyclonal anti‐IFN‐γ antibody | Abcam | USA | 1:1000 |
| Rabbit polyclonal anti‐IL‐10 antibody | Abcam | USA | 1:2000 |
| Rabbit monoclonal anti‐GAPDH antibody | Abcam | USA | 1:10 000 |
| Second antibody | |||
| Goat anti‐rabbit IgG H&L (HRP) antibody | Abcam | USA | 1:2000 |
Bcl‐2, B‐cell lymphoma‐2; GAPDH, glyceraldehyde‐3‐phosphate dehydrogenase; HRP, horse radish peroxidase; IFN‐γ, interferon‐γ; IgG, immunoglobulin G, IL‐10, interleukin 10; TNF‐α, tumor necrosis factor α.
2.8. CCK‐8 assay
Cells' proliferation was assessed using the CCK‐8 kit (Dojindo, Japan). After transfection, the cells were cultured with 100 μL RPMI‐1640 medium (Gibco) in 96‐well plates, and 10 μL CCK‐8 dye solution (Dojindo, Japan) was added into each well at 0, 24, 48, and 72 hour after transfection. The cells were incubated for 2 hour in the dark, then using a Microplate reader (BioTek, USA) to read the fluorescence value of the plates.
2.9. AV/PI assay
Cells' apoptosis rate of HaCaT cells was quantified by flow cytometry (FCM; BD, USA) using the FITC AV apoptosis detection kit with PI (BD, USA) according to the manufacturer's protocol. Briefly, HaCaT cells were collected at 72 hour after transfection, and then were washed by PBS and resuspended in binding buffer. Afterward, AV solution was added and stand in the dark at room temperature for 15 minutes, and the PI solution was added before setting in the FCM. Then the results green (AV) and red (PI) fluorescence of each sample were quantitatively analyzed by FCM.
2.10. Statistics
Statistical analysis was performed by SPSS software 22.0 (IBM, USA) and GraphPad Prism software 5.01 (GraphPad, USA). Data were mainly presented as mean ± standard deviation, median (1/4‐3/4 quantiles) or count (%). Comparison between 2 groups was determined by t test, Wilcoxon signed rank sum test or Wilcoxon rank sum test. The association of miR‐126 expression with continuous clinicopathological characteristics was evaluated by Spearman test. P < .05 was considered as significant.
3. RESULTS
3.1. Characteristics of psoriasis patients
As presented in Table 3, 102 psoriasis patients with mean of age 44.11 ± 10.92 years were enrolled in this study, among whom 61 patients were males and 41 patients were females. The mean BMI was 24.68 ± 2.63 kg/m2, and the mean disease duration was 11.32 ± 6.83 years. The mean lesional BSA and PASI Score were 20.34% ± 8.38% and 12.52 ± 4.73 points, respectively. In addition, the numbers of cases treated by topical therapy, phototherapy, systemic nonbiologic treatment, and systemic biologic treatment were 93 (91.2%) cases, 86 (84.3%) cases, 70 (68.6%) cases, and 11 (10.8%) cases, respectively.
Table 3.
Characteristics of psoriasis patients
| Parameters | Psoriasis patients (N = 102) |
|---|---|
| Age (y) | 44.11 ± 10.92 |
| Gender (male/female) | 61/41 |
| BMI (kg/m2) | 24.68 ± 2.63 |
| Disease duration (y) | 11.32 ± 6.83 |
| Lesional BSA (%) | 20.34 ± 8.38 |
| PASI Score | 12.52 ± 4.73 |
| Treatment | |
| Topical therapy (n/%) | 93 (91.2) |
| Phototherapy (n/%) | 86 (84.3) |
| Systemic nonbiologic treatment (n/%) | 70 (68.6) |
| Systemic biologic treatment (n/%) | 11 (10.8) |
BMI, body mass index; BSA, body surface area (affected by psoriasis); PASI, Psoriasis Area and Severity Index.
Data were presented as mean ± standard deviation or count (%).
3.2. MiR‐126 expression in lesional skin tissue and paired nonlesional skin tissue
Wilcoxon signed rank sum test was used to determine the difference in miR‐126 expression between lesional skin tissue and paired nonlesional skin tissue, and the result showed that miR‐126 expression in lesional skin tissue was higher compared with paired nonlesional skin tissue (P < .001, Figure 1).
Figure 1.
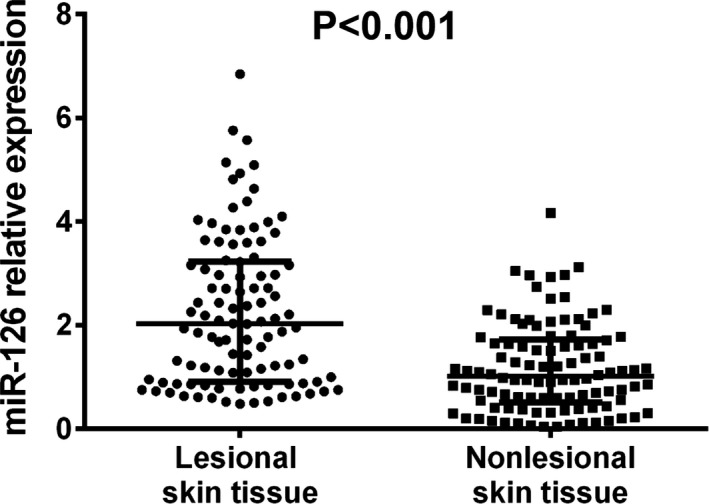
The miR‐126 expression in lesional skin tissue and nonlesional skin tissue. The expression of miR‐126 was increased in lesional skin tissue compared with nonlesional skin tissue. Comparison between 2 groups was determined by Wilcoxon signed rank sum test. P < .05 was considered significant
3.3. The association of miR‐126 expression with clinical features
MiR‐126 expression was positively associated with PASI score (P = .003, Figure 2F), however, no correlation of miR‐126 expression with age (P = .212, Figure 2A), gender (P = .195, Figure 2B), BMI (P = .629, Figure 2C), disease duration (P = .090, Figure 2D) or lesional BSA (P = .086, Figure 2E) was found.
Figure 2.
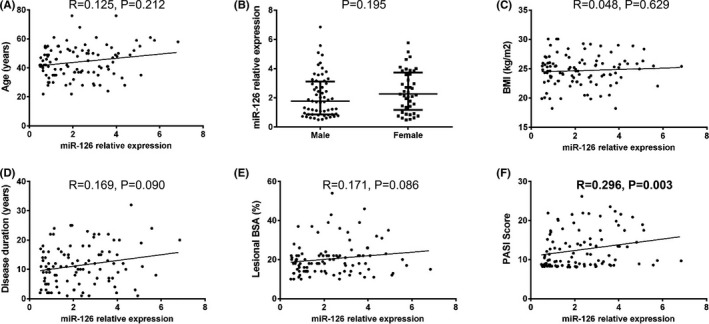
The association of miR‐126 expression with pathological features in psoriasis patients. MiR‐126 level in lesional skin tissue positively correlated with PASI Score (F) but did not associate with age (A), gender (B), BMI (C), disease duration (D), or lesional BSA (E). The correlation of miR‐126 expression in lesional skin tissue with age, BMI, disease duration, and lesional BSA was determined by Spearman test. Comparison between 2 groups was determined by Wilcoxon signed rank sum test. P < .05 was considered significant. BMI, body mass index; BSA, body surface area; PASI Score, Psoriasis Area and Severity Index Score
3.4. The effect of upregulated miR‐126 on HaCaT cells' proliferation
At 24 hour after transfection, miR‐126 expression in miR‐126 mimic group was increased compared with NC mimic group (P < .001, Figure 3A), which indicated that miR‐126 mimic plasmid successfully increased the miR‐126 expression. And at 72 hour after transfection, cells' proliferation by CCK8 assay was elevated in miR‐126 mimic group compared to NC mimic group (P < .05, Figure 3B), which implied that miR‐126 enhanced HaCaT cells' proliferation.
Figure 3.
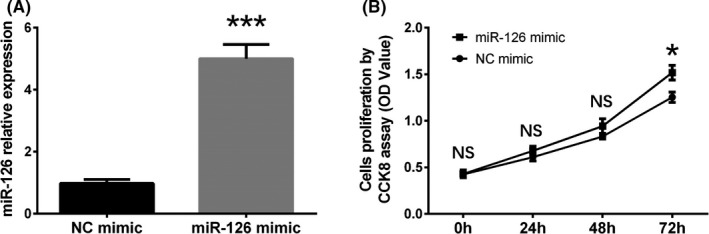
The effect of miR‐126 upregulation on proliferation in HaCaT cells. MiR‐126 expression in HaCaT cells was increased in miR‐126 mimic group compared with NC mimic group at 24 h after transfection (A). And the cells' proliferation was increased in miR‐126 mimic group compared with NC mimic group at 72 h after transfection (B). Comparison between 2 groups was determined by t test. P < .05 was considered significant. *P < .01, ***P < .001. HaCaT, human keratinocytes; NC, negative control
3.5. The effect of upregulated miR‐126 on HaCaT cells' apoptosis
At 72 hour after transfection, cells' apoptosis rate was detected by AV/PI assay (Figure 4A), and the results showed that cells' apoptosis rate was reduced in miR‐126 mimic group compared with NC mimic group (P < .01, Figure 4B). In addition, apoptotic markers (C‐caspase 3 and Bcl‐2) were also detected by western blot assay, which displayed that C‐caspase 3 expression was decreased while Bcl‐2 expression was increased in miR‐126 mimic group compared to NC mimic group (Figure 4C). These results suggested that miR‐126 inhibited HaCaT cells' apoptosis.
Figure 4.
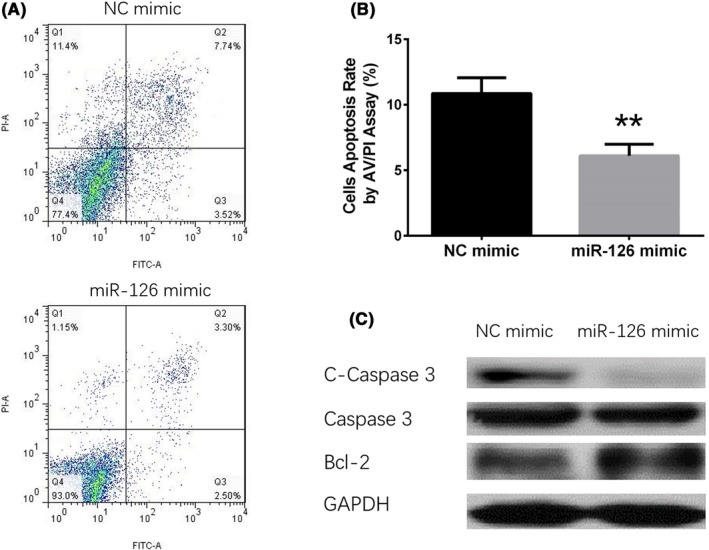
The effect of miR‐126 upregulation on apoptosis in HaCaT cells. At 72 h after transfection, the cells' apoptosis was detected by AV/PI (A), and compared with NC mimic group, the cells' apoptosis rate in miR‐126 mimic group was decreased (B). The levels of C‐caspase 3 and caspase 3 in miR‐126 mimic group were higher than that in NC mimic group, while Bcl‐2 expression was decreased in miR‐126 mimic group compared with NC mimic group (C). Comparison between 2 groups was determined by t test. P < .05 was considered significant. **P < .01. AV/PI, annexin V/propidium iodide; Bcl‐2, B‐cell lymphoma‐2; C‐caspase 3, cleaved caspase 3; HaCaT, human keratinocytes; NC, negative control
3.6. The effect of upregulated miR‐126 on inflammatory cytokines expressions in HaCaT cells
To explore the influence of miR‐126 on regulating inflammation in HaCaT cells, mRNA and protein expressions of TNF‐α, IFN‐γ, IL‐10, IL‐17A, and IL‐22 were determined after transfection by qPCR assay and Western blot assay (Figure 5F) respectively, which disclosed that pro‐inflammatory cytokines (TNF‐α, IFN‐γ, IL‐17A, and IL‐22) were increased (Figure 5A‐B,D‐E) while anti‐inflammatory cytokine (IL‐10) was decreased (Figure 5C) in miR‐126 mimic group compared with NC mimic group. These results indicated that miR‐126 promoted inflammation in HaCaT cells.
Figure 5.
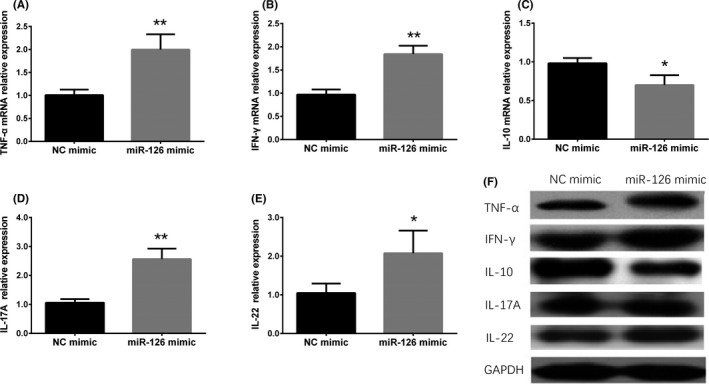
Correlation of miR‐126 expression with the mRNA and protein levels of inflammatory cytokines. Compared with NC mimic group, TNF‐α (A), IFN‐γ (B), IL‐17A (D), and IL‐22 (E) mRNA levels were increased while IL‐10 (C) level was reduced in miR‐126 mimic group. Besides, compared with NC mimic group, TNF‐α, IFN‐γ, IL‐17A, and IL‐22 proteins were increased but IL‐10 protein was decreased in miR‐126 mimic group (F). Comparison between 2 groups was determined by t test. P < .05 was considered significant. *P < .01, **P < .01. IFN‐γ, interferon‐γ; IL‐10, interleukin 10; IL‐17A, interleukin 17a; IL‐22, interleukin 22; NC, negative control; TNF‐α, tumor necrosis factor α
3.7. The association of miR‐126 expression with therapeutic methods
As listed in Table S1, the miR‐126 expression was not associated with topical therapy (P = .892), phototherapy (P = .451), systemic nonbiological treatment (P = .530) or systemic biologic treatment (P = .859) in psoriasis patients.
3.8. The effect of downregulation of miR‐126 on HaCaT cells' proliferation and apoptosis
MiR‐126 expression in miR‐126 inhibitor group was lower than that in NC inhibitor group (P < .001) (Figure S1A). And there was no difference in cells' proliferation (P < .05) (Figure S1B) or cells' apoptosis (P < .05) (Figure S1C,D) between miR‐126 inhibitor group and NC inhibitor group.
4. DISCUSSION
The results of our study presented that: (i) MiR‐126 expression was elevated in lesional skin tissue compared with paired nonlesional skin tissue in psoriasis patients, and it was positively associated with PASI score. (ii) Upregulated miR‐126 promoted cells' proliferation and inflammation while reduced cells' apoptosis in HaCaT cells.
Although the etiology of psoriasis is still obscure, it is generally accepted that psoriasis is a T cell‐mediated inflammatory dermatosis, and hyperproliferation of keratinocytes and sustained inflammation in skin tissue are the major pathological processes of psoriasis.19, 20 Inflammatory myeloid dendritic cells release IL‐12 and IL‐23 to activate IL‐17‐producing T cells, Th1 cells, and Th22 cells to secrete multiple inflammatory cytokines related to psoriasis consisting of IL‐17, IFN‐γ, TNF, and IL‐22, which mediate keratinocytes to amplify psoriatic inflammation.19 However, the molecular mechanisms behind these processes are still not fully understood. As regulatory elements, miRNAs play critical roles in immune diseases including psoriasis via participating in the development of immune cells, maintaining immune homeostasis, regulating innate and adaptive immunity, as well as regulating inflammatory factors.21, 22, 23, 24
MiR‐126 is a member of miRNAs that are reported to be involved in the development and progression of several autoimmune diseases such as RA and SLE.16, 25 A study elucidates that miR‐126 expression is increased in the CD4+ T cells of SLE patients and promotes the progression of SLE by enhancing T‐cell autoreactivity through directly targeting DNA methyltransferase 1 (Dnmt1).25 And another study illustrates that upregulation of miR‐126 enhances cells proliferation and diminishes apoptosis of RA synovial fibroblasts by targeting phosphoinositide‐3‐kinase regulatory subunit 2 (PIK3R2) and regulating phosphoinositide‐3‐kinase regulatory subunit 1 (PI3K)—serine/threonine kinase 1 (AKT) signal pathway.16 Feng et al exhibit that miR‐126 level in colonic tissue is increased in active ulcerative colitis (UC) patients compared to inactive UC patients, irritable bowel syndrome patients and healthy controls, and their in vitro experiments reveal that elevated miR‐126 expression decreases the level of NF‐κB inhibitor alpha (IκBα), which is an inhibitor of the pro‐inflammatory factor nuclear transcription factor kappa B (NF‐κB), in colonic cancer cells, thus promoting the inflammatory responses in UC patients.26 These previous studies indicate that miR‐126 plays an important role in the development of autoimmune diseases. However, to the best of our knowledge, no study has disclosed the function of miR‐126 in modulating disease risk and severity of psoriasis. In this study, we found that the expression of miR‐126 in the lesional skin tissue was upregulated than that in paired nonlesional skin tissue, and its expression positively correlated with the PASI score in psoriasis. The possible reason might be that upregulation of miR‐126 enhanced the inflammation and promoted proliferation of keratinocytes in psoriasis, which was validated in our subsequent experiments.
Abnormal cells' proliferation of human keratinocytes is a predominant feature of psoriasis, and exaggerated keratinocytes proliferation is closely related to the occurrence of this disease. Keratinocyte act as a crucial layer in the pathology of psoriasis by initiating, sustaining, and amplifying the inflammatory responses via expressing molecules involved in T‐cell recruitment, retention, and activation.27, 28 To further illustrate the effect of miR‐126 on keratinocytes proliferation, apoptosis, and inflammatory cytokines expressions in psoriasis, we upregulated the expression of miR‐126 by transfecting miR‐126 mimic in HaCaT cells, and the results disclosed that the cells' proliferation was increased while cells' apoptosis was decreased in HaCaT cells' in miR‐126 mimic group compared with NC mimic group. In addition, TNF‐α, IFN‐γ, IL‐17A and IL‐22 levels were elevated while IL‐10 level was lowered in miR‐126 mimic group compared with NC mimic group. Here are several possible explanations for our results: (i) according to previous studies, the overexpression of miR‐126 could promote cells' proliferation and inhibits cells' apoptosis by regulating multiple pathways16; (ii) and miR‐126 also upregulates the expression of pro‐inflammatory cytokines by modulating immune‐related factors such as Dnmt1 and NF‐κB.25, 26 These results in our study indicated that miR‐126 could be served as a potential treatment target in psoriasis patients.
In conclusion, miR‐126 correlates with elevated risk and increased disease severity in psoriasis patients, and upregulated miR‐126 promotes cells' proliferation and inflammation while inhibits cells' apoptosis in keratinocytes.
Supporting information
Feng S, Wang L, Liu W, Zhong Y, Xu S. MiR‐126 correlates with increased disease severity and promotes keratinocytes proliferation and inflammation while suppresses cells' apoptosis in psoriasis. J Clin Lab Anal. 2018;32:e22588 10.1002/jcla.22588
Feng and Wang equally contributed as first author.
REFERENCES
- 1. Boehncke WH, Schon MP. Psoriasis. Lancet. 2015;386:983‐994. [DOI] [PubMed] [Google Scholar]
- 2. Parisi R, Symmons DP, Griffiths CE, Ashcroft DM, Identification and Management of Psoriasis Associated ComorbidiTy (IMPACT) project team . Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377‐385. [DOI] [PubMed] [Google Scholar]
- 3. Ding X, Wang T, Shen Y, et al. Prevalence of psoriasis in China: a population‐based study in six cities. Eur J Dermatol. 2012;22:663‐667. [DOI] [PubMed] [Google Scholar]
- 4. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957‐970. [DOI] [PubMed] [Google Scholar]
- 5. Liakou AI, Zouboulis CC. Links and risks associated with psoriasis and metabolic syndrome. Psoriasis (Auckl). 2015;5:125‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs. An Bras Dermatol. 2017;92:668‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non‐steroidal anti‐inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta‐analysis. Ann Rheum Dis. 2015;74:480‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Geller S, Xu H, Lebwohl M, Nardone B, Lacouture ME, Kheterpal M. Malignancy risk and recurrence with psoriasis and its treatments: a concise update. Am J Clin Dermatol. 2018;19:363‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bracken CP, Scott HS, Goodall GJ. A network‐biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet. 2016;17:719‐732. [DOI] [PubMed] [Google Scholar]
- 10. Zhao L, Lu X, Cao Y. MicroRNA and signal transduction pathways in tumor radiation response. Cell Signal. 2013;25:1625‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shenoy A, Blelloch RH. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat Rev Mol Cell Biol. 2014;15:565‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Otsuka K, Ochiya T. Genetic networks lead and follow tumor development: microRNA regulation of cell cycle and apoptosis in the p53 pathways. Biomed Res Int. 2014;2014:749724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan S, Xu Z, Lou F, et al. NF‐kappaB‐induced microRNA‐31 promotes epidermal hyperplasia by repressing protein phosphatase 6 in psoriasis. Nat Commun. 2015;6:7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu L, Leng H, Shi X, Ji J, Fu J, Leng H. MiR‐155 promotes cell proliferation and inhibits apoptosis by PTEN signaling pathway in the psoriasis. Biomed Pharmacother. 2017;90:524‐530. [DOI] [PubMed] [Google Scholar]
- 15. Cheung L, Fisher RM, Kuzmina N, et al. Psoriasis skin inflammation‐induced microRNA‐26b targets NCEH1 in underlying subcutaneous adipose tissue. J Invest Dermatol. 2016;136:640‐648. [DOI] [PubMed] [Google Scholar]
- 16. Qu Y, Wu J, Deng JX, et al. MicroRNA‐126 affects rheumatoid arthritis synovial fibroblast proliferation and apoptosis by targeting PIK3R2 and regulating PI3K‐AKT signal pathway. Oncotarget. 2016;7:74217‐74226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liang Y, Zhao S, Liang G, Zhao M, Lu Q. [DNA methylation status of miR‐126 and its host gene EGFL7 in CD4+ T cells from patients with systemic lupus erythematosus]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2013;38:793‐797. [DOI] [PubMed] [Google Scholar]
- 18. Thorlacius‐Ussing G, Schnack Nielsen B, Andersen V, Holmstrom K, Pedersen AE. Expression and localization of miR‐21 and miR‐126 in mucosal tissue from patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23:739‐752. [DOI] [PubMed] [Google Scholar]
- 19. Lowes MA, Suarez‐Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol. 2014;32:227‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jadali Z, Eslami MB. T cell immune responses in psoriasis. Iran J Allergy Asthma Immunol. 2014;13:220‐230. [PubMed] [Google Scholar]
- 21. Cheng NL, Chen X, Kim J, et al. MicroRNA‐125b modulates inflammatory chemokine CCL4 expression in immune cells and its reduction causes CCL4 increase with age. Aging Cell. 2015;14:200‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee GJ, Hyun S. Multiple targets of the microRNA miR‐8 contribute to immune homeostasis in Drosophila. Dev Comp Immunol. 2014;45:245‐251. [DOI] [PubMed] [Google Scholar]
- 23. Zhu S, Pan W, Qian Y. MicroRNA in immunity and autoimmunity. J Mol Med (Berl). 2013;91:1039‐1050. [DOI] [PubMed] [Google Scholar]
- 24. Alsaleh G, Francois A, Philippe L, et al. MiR‐30a‐3p negatively regulates BAFF synthesis in systemic sclerosis and rheumatoid arthritis fibroblasts. PLoS One. 2014;9:e111266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao S, Wang Y, Liang Y, et al. MicroRNA‐126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum. 2011;63:1376‐1386. [DOI] [PubMed] [Google Scholar]
- 26. Feng X, Wang H, Ye S, et al. Up‐regulation of microRNA‐126 may contribute to pathogenesis of ulcerative colitis via regulating NF‐kappaB inhibitor IkappaBalpha. PLoS One. 2012;7:e52782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Volpe E, Pattarini L, Martinez‐Cingolani C, et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell‐derived IL‐23 in patients with psoriasis. J Allergy Clin Immunol. 2014;134:373‐381. [DOI] [PubMed] [Google Scholar]
- 28. Lowes MA, Russell CB, Martin DA, Towne JE, Krueger JG. The IL‐23/T17 pathogenic axis in psoriasis is amplified by keratinocyte responses. Trends Immunol. 2013;34:174‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


