Abstract
Background
To evaluate the application of interferon gamma release assay (IGRA), rifampicin resistant real‐time fluorescence quantitative PCR technique Xpert Mycobacterium tuberculosis/rifampicin (Xpert MTB/RIF), and the levels of TNF‐α and TGF‐β in the diagnosis of bone and joint tuberculosis.
Methods
Eighty‐six patients with bone and joint tuberculosis, diagnosed by pathology or microbiology, were examined by Xpert MTB/RIF and IGRA (T‐SPOT. TB) for Mycobacterium tuberculosis infection, and the TNF‐α and TGF‐β levels of the patients were measured.
Results
The sensitivity of IGRA in diagnosing bone and joint tuberculosis was 81.4%; Xpert MTB/RIF's sensitivity was 70.9%. The combined sensitivity of the two methods was 91.9%. The combined detection sensitivity of the two methods was higher than individual IGRA or Xpert MTB/RIF detection sensitivity. The TNF‐α and TGF‐β levels in bone and joint tuberculosis patients were higher than those in the control group.
Conclusion
Xpert MTB/RIF, IGRA, TNF‐α, and TGF‐βs expression have value in the rapid diagnosis of bone and joint tuberculosis, and the sensitivity and accuracy of bone and joint tuberculosis diagnosis by combining them can improve it.
Keywords: bone and joint tuberculosis, TGF‐β, TNF‐α, Xpert, γ‐interferon
1. INTRODUCTION
On a global scale, tuberculosis remains a major infectious disease, presenting approximately nine million new cases and two to three million deaths annually. About one‐eighth of these are extrapulmonary tuberculosis,1 which includes an estimated 11.3%‐34.5% of bone and joint tuberculosis patients.2 Because bone and joint tuberculosis onset is often occult and progression is slow, it is difficult to diagnose. In addition, bacteria of articular effusion in lesion sites are less frequent, and specimens are not easy to obtain, which further reduces the positive rate of puncture fluid or joint surgical specimens.3 These factors make the early diagnosis of bone and joint tuberculosis extremely challenging. However, the early diagnosis of bone and joint tuberculosis is essential as Mycobacterium tuberculosis can cause inflammation after the invasion of the joint and mass proliferation in the cells, leading to limited mobility and destruction of immune system functions.4 However, early treatment can reduce the incidence of physical disability and injury. Early symptoms of bone and joint tuberculosis are not obvious, and signs and imaging features are atypical, making diagnosis difficult, so that diagnosis of the disease is often overlooked.5, 6 The gold standard for the diagnosis of human tuberculosis is the detection of Mycobacterium tuberculosis using microbiological methods. However, traditional solid and liquid culture methods may take several weeks to produce a positive result, and the positive rate of joint tuberculosis detection is very low,7 which often delays the treatment period or even leads to a misdiagnosis. In this case, rapid and sensitive indicators are needed to improve the level of diagnosis, especially in the difficult diagnoses, misdiagnoses, and completely missed diagnoses. It is imperative to establish sensitive and specific laboratory testing technologies to assist in clinical diagnosis.
In December 2010, the World Health Organization (WHO) issued its official Xpert Mycobacterium tuberculosis/rifampicin (Xpert MTB/RIF) test for tuberculosis diagnosis.8 This detection technique is a rapid diagnostic method for tuberculosis and drug‐resistant tuberculosis that integrates specimen processing, DNA extraction, nucleic acid amplification, Mycobacterium tuberculosis‐specific nucleic acid detection, and rifampicin resistance gene rpoB mutation detection. The entire process takes only 90 minutes, and it can achieve simultaneous detection of Mycobacterium tuberculosis and rifampicin resistance,9 This has been hailed as a breakthrough in the diagnosis of tuberculosis by the WHO.
Interferon gamma release assay (IGRA) guidelines were updated in 2010.10 The IGRA is based on enzyme‐linked immunospot (ELISPOT) assay formats. ELISPOT technology is an innovative immune enzyme technology which detects antibody secretion cells or cytokine cells at the single‐cell level. T‐SPOT. TB, developed by ELISPOT, stimulates peripheral mononuclear blood cells using Mycobacterium tuberculosis‐specific antigens, detects antigen‐specific T‐lymphocyte response by secretion of cytokine interferon‐γ with a high sensitivity and specificity,11, 12 and, thus, determines the state of tuberculosis infection. It is the most successful application of Mycobacterium tuberculosis genomics and its cellular immune research in the field of tuberculosis in the last 10 years.13
Tumor necrosis factor alpha (TNF‐α) has immunomodulatory effects on leukocytes, vascular endothelial cells, and the cytokines of connective tissue. It can stimulate cells to produce prostaglandins and proteases, and it can also enhance vascular permeability with a pro‐inflammatory effect.14 Transforming growth factor beta(TGF‐β) is primarily involved in inflammatory reaction and damage repair affecting cell proliferation and differentiation. It plays an immunomodulatory role in tuberculosis tissue and simultaneously inhibits T‐cell response by the inactivation of macrophages.15 The expression of TNF‐α and TGF‐β plays an important role in the process of protective immune response and allergic reaction, which is closely related to the injury and repair of bone and joint tissue.16
The purpose of this study is to evaluate the application of Xpert MTB/RIF and IGRA tests in the diagnosis of bone and joint tuberculosis, and to explore the diagnostic value of TNF‐α and TGF‐β levels.
2. METHODS AND MATERIALS
2.1. Subjects
Eighty‐six patients who were diagnosed with bone and joint tuberculosis at the Third Affiliated Hospital of Guangxi University of traditional Chinese Medicine, China from September 2014 to August 2016 were divided into a hyperplastic lesion group (38 cases) and a caseous necrosis group (48 cases). The study was carried out in accordance with the Declaration of Helsinki. Diagnosis was based on (i) a positive acid‐fast bacilli (AFB) smear or culture, (ii) tuberculous granuloma indicated by histopathology (the presence of hyperplastic or caseous necrosis), and (iii) radiological features compatible with ultrasound or CT scan of the osteoarticular. The 86 patients included 38 cases of knee tuberculosis, 26 cases of hip tuberculosis, 11 cases of elbow tuberculosis, four cases of sternoclavicular joint tuberculosis, and seven cases of shoulder tuberculosis. Patients were aged 20‐57 years with a mean age of 36±14 years; 49 patients were male, and 37 were females. There were 30 healthy people in the control group, 13 males and 17 females aged 29‐59 years with a mean age of 37±9 years. Physical indicators were normal, excluding AIDS, hypertension, cardiac dysfunction, and other diseases, and no patient had a history of living with a family member who was tuberculosis patient. There were no statistically significant differences in age and gender (P>.05). The study protocol was approved by the Institute and National Scientific and Ethical Committees, and consent to participate in the study was obtained from each individual.
2.2. Samples and reagents
A 2 mL sample of lesions joint pus was taken from each patient with bone and joint tuberculosis, and a 2 mL sample of randomized joint fluid was taken from each of the healthy people. These samples were centrifuged at 1509 g for 15 minutes and then reserved for Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA) assays at a later time. Treatment of joint fluid samples was performed with reference to the sputum method.9 A fasting peripheral venous blood sample of 2 mL was collected from all patients, and these were then stored at −20°C for reserving, after separating plasma with centrifuging for 5 minutes at 1509 g, in order to perform IGRA (Oxford Immunotech, Abingdon, UK) and to measure TNF‐α and TGF‐β levels. The specific operational processes of IGRA test can be queried.17 TNF‐α and TGF‐β levels were detected by enzyme‐linked immunosorbent assay (ELISA), which was done according to the manufacturer's (R&D Systems, Minneapolis, MN, USA) instructions. The standard curve was plotted against the standard value of A, and then plasma TNF‐α and TGF‐β levels were calculated by the ELISA method, using matched antibody pairs and their corresponding standards.
2.3. Statistical analysis
Statistical analysis was calculated using IBM SPSS ver.19.0 (IBM, Armonk, NY, USA) software. Continuous variables were compared using the Mann‐Whitney test. Categorical variables were compared using Pearson chi‐square. Spearman's correlation coefficient was used to assess the association between the levels of TNF‐α and TGF‐β in the bone and joint tuberculosis patients. Receiver operating characteristic (ROC) curves were constructed to assess the sensitivity and specificity of the obtained TNF‐α and TGF‐β measurements to compare their ability to diagnose bone and joint tuberculosis. All reported P values in the study were two‐tailed, and there was a statistically significant difference when P<.05.
3. RESULTS
The Xpert MTB/RIF and IGRA test results are shown in Table 1. The sensitivity and specificity of the two test methods are given in Table 2. Eighty‐six bone and joint tuberculosis patients were examined by the pus Xpert MTB/RIF and plasma IGRA detection methods, respectively. The sensitivity of the IGRA in the diagnosis of bone and joint tuberculosis was 81.4%, and the sensitivity of the Xpert MTB/RIF was 70.9%. Results were considered positive when two methods were positive or one of them was positive (79 cases); results were negative when the two detection methods were negative (7 cases). The sensitivity of the combined detection of the two methods was 91.9%, and the combined specificity was 96.7%. With 100% and 96.7% specificity, the positive likelihood ratio (PLR) and positive predictive value (PPV) for the two methods were excellent (Table 2), and the Youden Index (YI) of the Xpert MTB/RIF and the IGRA were 0.709 and 0.781, respectively. The Xpert MTB/RIF and IGRA tests offer excellent value in the diagnosis of bone and joint tuberculosis. The sensitivity of the IGRA was higher than that of the Xpert MTB/RIF, but the difference was not statistically significant (χ2=2.594, P=.107). The sensitivity of the combined detection of the two methods was higher than either individually, and the difference was significant (χ2=12.439, P=.000 and χ2=4.065, P=.044, respectively). Both the Xpert MTB/RIF and IGRA tests were positive in 52 cases, and the results of the two methods were inconsistent in a total of 27 cases. The consistency of the two methods was 68.6% (59/86).
Table 1.
Test results of Xpert MTB/RIF and IGRA
| Test method | All tuberculosis patients | Hyperplastic lesion | Caseous necrosis | Control | ||||
|---|---|---|---|---|---|---|---|---|
| Number=86 | Number= 38 | Number= 48 | Number= 30 | |||||
| positive | negative | positive | negative | positive | negative | positive | negative | |
| Xpert MTB/RIF | 61 | 25 | 26 | 12 | 35 | 13 | 0 | 30 |
| IGRA | 70 | 16 | 30 | 8 | 40 | 8 | 1 | 29 |
| Xpert MTB combined with IGRA (if Xpert MTB/RIF or IGRA positive) | 79 | 7 | 35 | 3 | 44 | 4 | 1 | 29 |
| Xpert MTB combined with IGRA (both Xpert MTB/RIF and IGRA were positive) | 52 | 34 | 21 | 17 | 31 | 17 | 0 | 30 |
IGRA, Interferon‐gamma release assays; Xpert MTB/RIF, Xpert Mycobacterium tuberculosis/rifampicin.
Table 2.
The sensitivity and specificity of the two test methods
| Test method | Sensitivitys | Specificity | Positive predictive value, PPV | Negative predictive value, NPV | Positive likelihood ratio, PLR | Negative likelihood ratio, NLR | Youden Index, YI |
|---|---|---|---|---|---|---|---|
| Xpert MTB/RIF | 70.9%* | 100% | 100% | 54.50% | max | 0.291 | 0.709 |
| IGRA | 81.4%* | 96.70% | 98.60% | 64.40% | 24.67 | 0.192 | 0.781 |
| Xpert MTB combined with IGRA (if Xpert MTB/RIF or IGRA positive) | 91.9%* | 96.70% | 98.80% | 80.60% | 27.85 | 0.084 | 0.886 |
IGRA/Xpert MTB/RIF compared with Xpert MTB combined with IGRA, * P<.05; IGRA, Interferon‐gamma release assays; Xpert MTB/RIF, Xpert Mycobacterium tuberculosis/rifampicin; max, not calculable as denominator equals zero.
There was a positive correlation between the expressions of TNF‐α and TGF‐β in the hyperplastic lesion group (Figure 1), and the expression of TNF‐α was significantly higher in the hyperplastic lesion group than in the caseous necrosis lesion group (P<.001). There was a negative correlation between the expression of TNF‐α and TGF‐β in the caseous necrosis lesion group (Figure 2), and the expression of TGF‐β was significantly higher in the caseous necrosis lesion group than in the hyperplastic lesion group (P<.001). Compared with the control group, the levels of TNF‐α and TGF‐β were significantly higher in the cases of hyperplastic lesion and caseous necrosis lesions (Table 3), and the differences were statistically significant (P<.001). To further evaluate the predictive ability of TNF‐α and TGF‐β for bone and joint tuberculosis, ROC curve and area under ROC curve (AUC) analyses were performed. The AUC analyses of TNF‐α and TGF‐β in bone and joint tuberculosis patients were 0.883 and 0.897, respectively (Figure 3).
Figure 1.
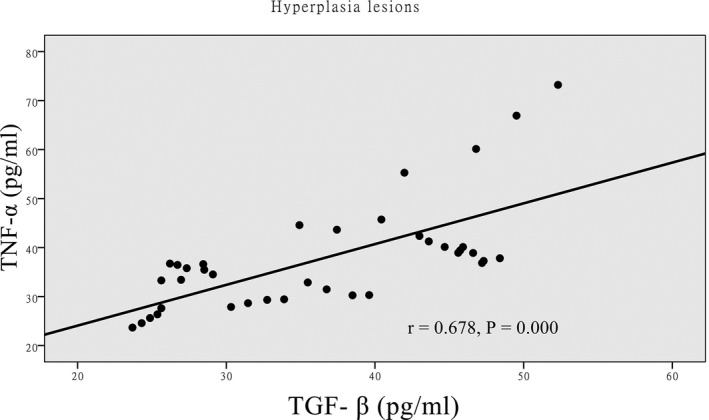
Correlation between TGF‐β and TNF‐α in hyperplasia lesions group analysis
Figure 2.
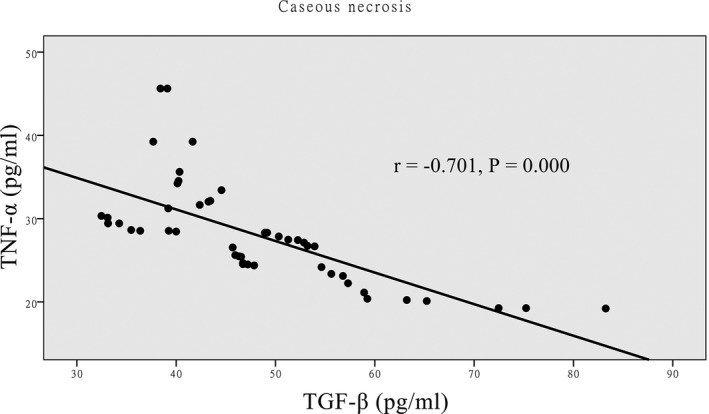
Correlation between TGF‐β and TNF‐α in caseous necrosis group analysis
Table 3.
Baseline characteristics of the patients
| Characteristic | All tuberculosis patients | Hyperplastic lesion | Caseous necrosis | Control |
|---|---|---|---|---|
| Number=86 | Number=38 | Number=48 | Number=30 | |
| Age (years) | 36±14 | 38±12 | 35±13 | 37±9 |
| Male (number, %) | 49 (57) | 21 (55) | 28 (58) | 17 (57) |
| Female (number, %) | 37 (43) | 17 (45) | 20 (42) | 13 (43) |
| TGF‐β (pg/mL) | 42.76±11.70* | 36.39±8.9* , ** | 47.8±11.25* , ** | 28.1±5.29* |
| TNF‐α (pg/mL) | 32.37±9.76* | 37.71±10.93* , ** | 28.14±6.08* , ** | 15.26±5.25* |
Figure 3.

Evaluation of serum TGf‐β and TNF‐α for the diagnosis of bone and joint tuberculosis by roc curve analysis
4. DISCUSSION
Over time, more and more studies have been done on the mechanisms of pulmonary tuberculosis damage to the body, but less research exists on bone and joint tuberculosis. As a common extrapulmonary tuberculosis, bone and joint tuberculosis can cause limb deformity, limited mobility, and even paraplegia if improperly treated,18 and it is divided into hyperplastic and caseous necrotic tuberculosis, according to the main pathological changes of tuberculosis.19 At present, the early diagnosis and differential diagnosis of atypical cases are also more challenging as no single test can diagnose all bone and joint tuberculosis patients. The current study shows that the Xpert MTB/RIF has demonstrated superior performance in sputum samples of tuberculosis.9, 20 The IGRA also showed a surprising performance in tuberculosis patients.21 In this study, 86 patients diagnosed with bone and joint tuberculosis by histopathology and Mycobacterium tuberculosis by microbiological methods, were examined by the pus Xpert MTB/RIF and the plasma IGRA tests, respectively. The results showed that the sensitivity of the Xpert MTB/RIF test in the diagnosis of bone and joint tuberculosis was 70.9%, and specificity was 100%, which is consistent with previous reports9, 20 (the sensitivity was 67%‐98%, the specificity was 98%). Moure et al. 22 proposed that the sensitivity of the Xpert MTB/RIF test to sterile body fluid specimens was lower than in sputum samples, and they showed that it was 76.5% in abscess inhalers. The positive detection rate of the IGRA in bone and joint tuberculosis patients was higher than with the Xpert MTB/RIF, sensitivity was 81.4%, and specificity was 96.7%. This result is consistent with previous reports in the literature,23, 24 having the same sensitivity as the gold standard pathology (72%~97%)25 and indicating that the Xpert MTB/RIF and IGRA detection technologies play an important supporting role in diagnosing bone and joint tuberculosis. The IGRA offers superior accuracy for the diagnosis of microbiologically confirmed bone and joint tuberculosis, compared to the Xpert MTB/RIF test. However, the IGRA test cannot distinguish latent TB infections or active tuberculosis. In addition, the agreement between the Xpert MTB/RIF and IGRA test results was 68.6%, with 31.4% inconsistent results. Therefore, the IGRA should be measured in conjunction with the Xpert MTB/RIF test in the diagnosis of bone and joint tuberculosis. The sensitivity of the combined detection of the two methods was 91.9%, and the specificity was 96.7%, which was higher than all others (P=.000), providing an excellent diagnostic value in bone and joint tuberculosis diagnosis.
As a multifunctional cytokine, TNF‐α can increase the phagocytosis of macrophages, chemotaxis, and adhesion of many immune cells; thus, when it is located in the site of infection of tuberculosis, it plays a very important role in preventing the spread of infection.26 Recent studies have shown that TNF‐α participates in osteoclast differentiation and biological activity regulation in a variety of ways.27 Additionally, TNF‐α can promote the macrophage apoptosis of Mycobacterium tuberculosis while inhibiting the replication of Mycobacterium tuberculosis. It can be said TNF‐α has an important role in stability in the maintenance of tuberculosis in the human body.28 The results showed that the levels of TNF‐α in hyperplasia and caseous necrosis lesions were significantly higher than those in normal controls (P<.001), suggesting that TNF‐α is involved in anti‐infective immunoregulation in bone and joint tuberculosis patients. The expression of TNF‐α in the hyperplastic lesion group was significantly higher than that in the caseous necrosis group (P<.001). It is suggested that TNF‐α overexpression is a factor in maintaining the integrity of the protective cell immune system and that this may have an anti‐cheese necrosis effect in the persistent infection of bone and joint tuberculosis. Zande et al.29 used immunohistochemistry to study the expression of cytokines in lymphogranulomatous granulomas, and they found that TNF‐α expression was low in necrotizing granulomas, which is consistent with the results of this study. Low expression of TNF‐α inhibits macrophages apoptosis, an important way to kill cells of Mycobacterium tuberculosis. Overgrowth of Mycobacterium tuberculosis in macrophages inhibited prolonged cell necrosis is a factor in cheese necrosis.
TGF‐β, a multifunctional cytokine, can inhibit the cellular immune response and cause macrophage failure by down‐regulating the production of TNF‐α, and, thus, promote the replication of Mycobacterium tuberculosis in monocytes.30 Studies have reported31 that the expression of TGF‐β in lymph node tuberculous granuloma is positively correlated with the presence of Mycobacterium tuberculosis in granulomatous multinucleated giant cells by using immunohistochemical methods. TGF‐β also inhibits the apoptosis of multinucleated giant cells, which is a negative factor in controlling the tuberculosis infection. This study shows that the levels of TGF‐β in the bone and joint tuberculosis hyperplasia lesion and caseous necrosis groups were higher than in the normal control group (P<.001). The expression of TGF‐β is related to the type of granuloma and the pathological manifestation in the individual. Low expression of TGF‐β was observed in hyperplasia lesions, and high expression was observed in caseous necrosis lesions. Excessive TGF‐β will further chemotaxis monocytes and neutrophils gathering in the site of the tuberculosis infection, increasing collagenase and elastase production, and leading to other complications such as extensive tuberculosis tissue damage, vacuolation, and fibrosis.32 Some studies suggest that TGF‐β and TNF‐α have a synergistic effect by formatting tissue damages with a characteristic of tuberculosis.33 This study showed that the high expressions of TGF‐β and TNF‐α were associated with each other in the hyperplastic lesion group, and the correlation analysis showed that the expression levels of TGF‐β and TNF‐α were positively correlated. However, the high expressions of TGF‐β and TNF‐α were not associated with each other in the caseous necrosis lesion group; the correlation analysis showed that the expression levels of TGF‐β and TNF‐α were negatively correlated. The reason may be that, in monocytes and macrophages, high levels of TGF‐β not only down‐regulate the production and activity of TNF‐α, but also reduce the generation of reactive oxygen intermediates, leading to inactivation of monocytes macrophages34 and to further deterioration of the patient's condition. Therefore, high expression of TGF‐β and low expression of TNF‐α may be factors leading to necrosis and ulceration of granuloma, indicating that TGF‐β is involved in tissue damage that leads to the disease's progression. The results of ROC analyses indicated that the levels of TGF‐β and TNF‐α are specific and sensitive for the diagnosis of bone and joint tuberculosis in patients. They might be suitable diagnostic markers of bone and joint tuberculosis with a distinct difference in levels between the hyperplasia lesion and caseous necrosis groups and the controls.
Thus, it can be seen that the combination of Xpert and IGRA tests can better improve the detection rate of bone and joint tuberculosis. Our findings also show that TGF‐β and TNF‐α may be involved in the pathogenesis of bone and joint tuberculosis. Recent studies have shown that TNF‐α was associated with a predisposition to bone and joint tuberculosis, which could aid clinical detection, prevention, and prognosis of bone and joint tuberculosis.35 This is consistent with our research. However, it must be noted that TGF‐β and TNF‐α levels will require further research as to their potential to be of value in the diagnosis of bone and joint tuberculosis. Studies have shown that TNF‐α is also increased in ankylosing spondylitis, rheumatoid arthritis, and other diseases.36, 37, 38 Therefore, it has been proven that TNF‐α participates in the pathogenesis of various diseases as a multifunctional cytokine. However, although it does not belong to the specific diagnostic indicator of bone and joint tuberculosis at this time, it may nevertheless be helpful in the diagnosis of bone and joint tuberculosis in the future. In particular, expression of TGF‐β and TNF‐α levels were negatively correlated in caseous necrotic lesions in our study, which may be differentiated from other diseases. However, these findings are still subject to further experimental observations. Despite these factors, in the case of a difficult diagnosis of bone and joint tuberculosis, the clinician who is trying to achieve the highest possible probability of the correct diagnosis must always attempt to employ every possible diagnostic tool that is available. Therefore, detection that combines the Xpert and IGRA tests with analysis of TNF‐α and TGF‐β levels offers a distinct possibility to improve the accuracy of the diagnosis.
Our study has some limitations. First, the study was conducted in a high burden setting of tuberculosis, which may limit the universality of the findings, so the data may not be fully representative of the situation in other countries. Second, the sample number of this study is limited, and the current literature on bone and joint tuberculosis reports is scant. Also, the serum indicators used in this study for diagnosis of bone and joint tuberculosis are still insufficient, several diagnostic strategies and tools need to be examined and confirmed in future studies.
5. CONCLUSION
In summary, no single test exists that is sufficient to diagnose all bone and joint tuberculosis patients. However, our results show that the Xpert and IGRA tests, along with an analysis of TNF‐α and TGF‐β levels, can be regarded as rapid indicators of bone and joint tuberculosis which have the potential to greatly improve diagnosis. When early diagnosis is difficult, the combination of the above‐referenced tests and levels can provide clinicians with information more efficient in the diagnosis of bone and joint tuberculosis, which would enable patients to receive the most effective treatment in the shortest amount of time.
ACKNOWLEDGMENTS
We thank all volunteers for their participation in the study, and appreciate the help on the reagent provided by the Kingmed Center for Clinical. Conceived and designed the experiments: LL Yin. Performed the experiments and drafting the article: YH Tang. Analyzed the data: HY Zhang. Revising it critically for important intellectual content: SF Tang. Without their support the project would not have been conceivable. Final approval of the version to be submitted: LL Yin.
Tang Y, Yin L, Tang S, Zhang H, Lan J. Application of molecular, microbiological, and immunological tests for the diagnosis of bone and joint tuberculosis. J Clin Lab Anal. 2018;32:e22260 10.1002/jcla.22260
REFERENCES
- 1. Misra SP, Misra V, Dwivedi M, Gupta SC. Colonic tuberculosis: clinical features, endoscopic appearance and management. J Gastroenterol Hepatol. 1999;14:723‐729. [DOI] [PubMed] [Google Scholar]
- 2. Kulchavenya E. Extrapulmonary tuberculosis: are statistical reports accurate? Ther Adv Infect Dis. 2014;2:61‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Theron G, Peter J, Calligaro G, et al. Determinants of PCR performance (Xpert MTB/RIF), including bacterial load and inhibition, for TB diagnosis using specimens from different body compartments. Sci Rep. 2014;4:5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greco E, Quintiliani G, Santucci MB, et al. Janus‐faced liposomes enhance antimicrobial innate immune response in Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 2012;109:E1360‐E1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lesic A, Bumbasirevic M, Savic B, Cobeljic G, Pesut D. Current diagnosis and treatment of the osteoarticular tuberculosis. Srp Arh Celok Lek 2004;132:345‐351. [DOI] [PubMed] [Google Scholar]
- 6. Sequeira W, Co H, Block JA. Osteoarticular tuberculosis: current diagnosis and treatment. Am J Ther. 2000;7:393‐398. [PubMed] [Google Scholar]
- 7. Boehme CC, Nicol MP, Nabeta P, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495‐1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . (2010) News release: WHO endorses new rapid tuberculosis test. Available:http://wwwwhoint/mediacentre/news/releases/2010/tb_test_20101208/en/. Accessed August 03, 2016.
- 9. Boehme CC, Nabeta P, Hillemann D, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. U.S. Food and Drug Administration . (2001) Device approvals and clearances:Quanti FERON ‐TB ‐ P010033. Available:http://wwwfdagov/downloads/advisorycommittees/committeesmeetingmaterials/medicaldevices/medicaldevicesadvisorycommittee/microbiologydevicespanel/ucm260832pdf. Accessed June 23, 2014.
- 11. Al‐Zamel FA. Detection and diagnosis of Mycobacterium tuberculosis. Expert Rev Anti Infect Ther. 2009;7:1099‐1108. [DOI] [PubMed] [Google Scholar]
- 12. Lalvani A, Pareek M. Interferon gamma release assays: principles and practice. Enferm Infecc Microbiol Clin. 2010;28:245‐252. [DOI] [PubMed] [Google Scholar]
- 13. Dillon DC, Alderson MR, Day CH, et al. Molecular and immunological characterization of Mycobacterium tuberculosis CFP‐10, an immunodiagnostic antigen missing in Mycobacterium bovis BCG. J Clin Microbiol. 2000;38:3285‐3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nemeth J, Winkler HM, Boeck L, et al. Specific cytokine patterns of pulmonary tuberculosis in Central Africa. Clin Immunol. 2011;138:50‐59. [DOI] [PubMed] [Google Scholar]
- 15. Chowdhury IH, Ahmed AM, Choudhuri S, et al. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti‐tuberculosis drug therapy. Mol Immunol. 2014;62:159‐168. [DOI] [PubMed] [Google Scholar]
- 16. Vidyarani M, Selvaraj P, Prabhu Anand S, Jawahar MS, Adhilakshmi AR, Narayanan PR. Interferon gamma (IFNgamma) & interleukin‐4 (IL‐4) gene variants & cytokine levels in pulmonary tuberculosis. Indian J Med Res 2006;124:403‐410. [PubMed] [Google Scholar]
- 17. T‐SPOT .TB 96 Package Insert for In Vitro Diagnostic Use [EB/OL]. http://wwwoxfordimmunoteccom/international/products-services/t-spot-tb-test/how-it-works/. Accessed January 15, 2012.
- 18. de Oliveira LR, Peresi E, Golim Mde A, et al. Analysis of Toll‐like receptors, iNOS and cytokine profiles in patients with pulmonary tuberculosis during anti‐tuberculosis treatment. PLoS ONE. 2014;9:e88572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schierloh P, Landoni V, Balboa L, et al. Human pleural B‐cells regulate IFN‐gamma production by local T‐cells and NK cells in a Mycobacterium tuberculosis‐induced delayed hypersensitivity reaction. Clin Sci. 2014;127:391‐403. [DOI] [PubMed] [Google Scholar]
- 20. Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev. 2014;1:CD009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. King TC, Upfal M, Gottlieb A, et al. T‐SPOT.TB Interferon‐gamma Release Assay Performance in Healthcare Worker Screening at Nineteen U.S. Hospitals. Am J Respir Crit Care Med. 2015;192:367‐373. [DOI] [PubMed] [Google Scholar]
- 22. Moure R, Martin R, Alcaide F. Effectiveness of an integrated real‐time PCR method for detection of the Mycobacterium tuberculosis complex in smear‐negative extrapulmonary samples in an area of low tuberculosis prevalence. J Clin Microbiol. 2012;50:513‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapman HJ, Lauzardo M. Advances in diagnosis and treatment of latent tuberculosis infection. J Am Board Fam Med. 2014;27:704‐712. [DOI] [PubMed] [Google Scholar]
- 24. Trajman A, Steffen RE, Menzies D. Interferon‐Gamma Release Assays versus Tuberculin Skin Testing for the Diagnosis of Latent Tuberculosis Infection: An Overview of the Evidence. Pulm Med. 2013;2013:601737.23476763 [Google Scholar]
- 25. Saxena PS, Sharma RK. Value of histopathology, culture and guinea pig inoculation in osteoarticular tuberculosis. Int Surg. 1982;67(4 Suppl):540‐542. [PubMed] [Google Scholar]
- 26. Mootoo A, Stylianou E, Arias MA, Reljic R. TNF‐alpha in tuberculosis: a cytokine with a split personality. Inflamm Allergy Drug Targets. 2009;8:53‐62. [DOI] [PubMed] [Google Scholar]
- 27. Kubota A, Hasegawa K, Suguro T, Koshihara Y. Tumor necrosis factor‐alpha promotes the expression of osteoprotegerin in rheumatoid synovial fibroblasts. J Rheumatol. 2004;31:426‐435. [PubMed] [Google Scholar]
- 28. Teng YT, Mahamed D, Singh B. Gamma interferon positively modulates Actinobacillus actinomycetemcomitans‐specific RANKL+ CD4+ Th‐cell‐mediated alveolar bone destruction in vivo. Infect Immun. 2005;73:3453‐3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Zande M, Walboomers XF, Briest A, Springer M, Alava JI, Jansen JA. The effect of combined application of TGFbeta‐1, BMP‐2, and COLLOSS E on the development of bone marrow derived osteoblast‐like cells in vitro. J Biomed Mater Res, Part A. 2008;86:788‐795. [DOI] [PubMed] [Google Scholar]
- 30. Gardner CR. Comparison of morphological effects of PGE2 and TGFbeta on osteoclastogenesis induced by RANKL in mouse bone marrow cell cultures. Cell Tissue Res. 2007;330:111‐121. [DOI] [PubMed] [Google Scholar]
- 31. Fox SW, Evans KE, Lovibond AC. Transforming growth factor‐beta enables NFATc1 expression during osteoclastogenesis. Biochem Biophys Res Commun. 2008;366:123‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mustafa T, Mogga SJ, Mfinanga SG, Morkve O, Sviland L. Immunohistochemical analysis of cytokines and apoptosis in tuberculous lymphadenitis. Immunology. 2006;117:454‐462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fiorenza G, Rateni L, Farroni MA, Bogue C, Dlugovitzky DG. TNF‐alpha, TGF‐beta and NO relationship in sera from tuberculosis (TB) patients of different severity. Immunol Lett. 2005;98:45‐48. [DOI] [PubMed] [Google Scholar]
- 34. Hirsch CS, Ellner JJ, Blinkhorn R, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926‐3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lv YJ, Liu SJ, Hu WN, et al. Association of tumor necrosis factor‐alpha gene polymorphism with osteoarticular tuberculosis prognosis in a Hebei population. Genet Mol Res 2016;15:gmr15048937. [DOI] [PubMed] [Google Scholar]
- 36. Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, Yorgancioglu R. Comparison of serum IL‐1 beta, sIL‐2R, IL‐6, and TNF‐alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol. 2007;26:211‐215. [DOI] [PubMed] [Google Scholar]
- 37. Wei ST, Sun YH, Zong SH, Xiang YB. Serum Levels of IL‐6 and TNF‐alpha May Correlate with Activity and Severity of Rheumatoid Arthritis. Med Sci Monit. 2015;21:4030‐4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kaminiarczyk‐Pyzalka D, Adamczak K, Mikos H, Klimecka I, Moczko J, Niedziela M. Serum TNF‐alpha levels and indicators of disease activity in children with oligoarticular juvenile idiopathic arthritis (oJIA) in the first year of the disease. Clin Lab. 2014;60:799‐807. [DOI] [PubMed] [Google Scholar]


