Abstract
Background
Visceral adiposity index (VAI) and Lipid accumulation product (LAP) are novel visceral adiposity indexes, proposed for the evaluation of cardiometabolic risk in adult population. Considering contradictory results obtained from many studies so far, we aimed to examine the potential benefit of applicability of VAI and LAP, over simple anthropometric indices and traditional lipid parameters in individuals with type 2 diabetes mellitus (DM2).
Methods
A total of 180 DM2 (of them 50% females) and 119 controls who volunteered to participate in this cross‐sectional study were enrolled. Anthropometric and biochemical parameters, as well as blood pressure were obtained. VAI and LAP were calculated.
Results
Multivariate logistic regression analysis showed that high‐density lipoprotein cholesterol (HDL‐c), (P<.001), waist circumference (WC), (P=.027), age (P=.001), hypolipemic therapy (P=.024), and LAP (P=.005) were independent predictors of DM2 in adjusted models.
In Receiver Operating Characteristic curve analysis, used to discriminate subjects with DM2 from those who did not have it, good accuracy of the applied procedures was only achieved with models which were consisted of parameters used in VAI (Body mass index, WC, HDL‐c, triglycerides) and LAP (WC, triglycerides) indexes equations, respectively [Area under the curve (AUC)=0.819 and AUC=0.800, respectively], but not with VAI (AUC=0.781) and LAP (AUC=0.784) indexes themselves.
Conclusion
Visceral adiposity index and Lipid accumulation product may not be better than parameters that enter its equation in type 2 diabetes prediction.
Keywords: lipid accumulation product, obesity, type 2 diabetes mellitus, visceral adiposity index
1. INTRODUCTION
A growing proportion of individuals with type 2 diabetes mellitus (DM2) worldwide is attributed in large part to the increase in body fat mass, especially in intra‐abdominal (visceral) region.1 Visceral adipose tissue is regarded to be metabolically active endocrine organ, secreting a variety of pro‐inflammatory adipokines, thus further leading to many cardiometabolic disorders.1, 2
Simple anthropometric parameter of abdominal adiposity such as waist circumference (WC) is established and widely used in many investigations.3, 4, 5 As early recognition and treatment of individuals with abdominal adiposity is of utmost importance in reducing cardiometabolic risk, some novel visceral adiposity markers, such as visceral adiposity index (VAI) and the lipid accumulation product (LAP) have been validated so far.6, 7
The VAI is gender‐specific mathematical model that uses both anthropometric indices [e.g., body mass index (BMI) and WC] and lipid parameters [e.g., high‐density lipoprotein cholesterol (HDL‐c) and triglycerides (TG)] in its equation, and is highly correlated with visceral adiposity as measured with the gold standard method, such as magnetic resonance imaging.6 The LAP is a gender‐specific index based on a combination of WC and TG in its equation.7 Both of these indexes are proposed as simple, accurate, and low‐cost tools for the evaluation of visceral adipose tissue dysfunction and its associated cardiometabolic risk in adult population,6, 7, 8, 9, 10 even superior than simple anthropometric parameters (e.g., BMI, WC).
These indexes were proposed as markers of insulin resistance and metabolic‐associated disturbances in women with polycystic ovary syndrome (PCOS), implicating that VAI and LAP may be used as screening tool for young women who are susceptible to the development of diabetes and CVD.11, 12
Furthermore, higher VAI13 and LAP14 were associated with metabolic syndrome (MetS), with the presence of DM2,15, 16 and with chronic kidney disease (CKD),9 showing even better discriminative power in relation to cardiometabolic risk than anthropometric parameters alone.7, 12, 17
Even though a large number of studies in adult population indicate VAI and LAP as reliable markers of cardiometabolic risk advantageous over BMI and WC,6, 7, 8, 9, 10, 12, 13, 17 there are another ones that report the opposite.18, 19, 20, 21, 22
Considering contradictory results obtained from many studies so far, we aimed to examine the potential benefit of applicability of novel visceral adiposity indexes, such as VAI and LAP, over simple anthropometric indices and traditional lipid parameters in individuals with DM2.
2. MATERIALS AND METHODS
2.1. Study population
A total of 180 sedentary DM2 (of them 50% females), as well as 119 healthy controls (of them 71% females) who volunteered to participate in this cross‐sectional study were enrolled. Diabetic patients were consecutively recruited by the endocrinologist in the Center of Laboratory Diagnostics of the Primary Health Care Center in Podgorica, Montenegro, for their regular check‐up in a period from October 2015 to May 2016. All the participants completed a self‐administered questionnaire including demographic characteristics, somatic illnesses, medication use, and lifestyle habits (e.g., information about cigarette smoking, diabetes duration, and physical activity). Medical history and clinical examinations were carried out on the same day.
Inclusion criteria for group of diabetic participants were as follows: DM2, sedentary patients (<90 minutes of weekly exercise), without acute inflammatory disease, with no history or the presence of malignancy, hypo‐ and hyperthyroidism. Diabetes cases were defined as self‐reported diabetes, or with at least two elevated plasma glucose levels (fasting glucose ≥7.0 mmol/L, a random plasma glucose level of ≥11.1 mmol/L, or a plasma glucose level ≥11.1 mmol/L 2 h after an oral glucose tolerance test), or HBA1c ≥6.5% on two different occasions in the absence of symptoms; or treatment with hypoglycemia medication (insulin or oral hypoglycemic agents).23
For non‐diabetics group, inclusion criteria were fasting glucose <7.0 mmol/L and glycosylated hemoglobin (HbA1c) ≤6%. In addition, all participants with fasting glucose ≥5.6 mmol/L, but <7.0 mmol/L, underwent a 2‐h oral glucose tolerance test (OGTT). Participants with 2‐h postload glucose ≥11.1 mmol/L were excluded from the control group.23
Exclusion criteria for all participants were as follows: type 1 diabetes mellitus, ethanol consumption >20 g/day, high‐sensitivity C‐reactive protein levels (hsCRP) >10 mg/L, liver disease, hypothyroidism or hyperthyroidism, patients on chronic dialysis, renal disease other than diabetic nephropathy, with a recent (6 months) history of acute myocardial infarction or stroke, pregnancy, usage of anti‐inflammatory medications in the last 6 months, usage of insulin or hypoglycemic agents for controls, as well as participants who were unwilling to enter the study.
All participants who used lipid‐modifying drugs (55.5%) in our study used statins (100% of them), whereas the smaller number of them used fibrates, also (3.3%).
All the participants provided written informed consent. The study protocol was approved by the Ethical Committee of Primary Health Care Center in Podgorica, Montenegro and the research was carried out in compliance with the Declaration of Helsinki.
2.2. Anthropometric measurements
Basic anthropometric measurements: body height (cm), body weight (kg), and WC (cm) were obtained, and BMI was calculated, as described previously.2
Visceral adiposity index (VAI) was calculated using the formula: .6
where WC is expressed in cm, BMI in kg/m2, and TG and HDL‐c in mmol/L.
Lipid accumulation product (LAP) was calculated by the following equation: 7
where WC is expressed in cm, and TG in mmol/L.
2.3. Biochemical analyses
The blood samples were taken between 7 and 9 hours a.m., after 12‐14 hours of an overnight fast. Samples were left to clot for 30 minutes and then centrifuged at 3000 rpm for 10 minutes.
Serum samples were divided into aliquots and stored at −80°C, without prior thawing and re‐freezing before analyses, except for glucose, which was determined immediately after the blood was drawn. Another aliquot was collecting as a whole blood in K2EDTA for determination of HbA1c, and it was measured with immunoturbidimetric assay (Roche Cobas 400, Mannheim, Germany).
Serum levels of glucose, total cholesterol (TC), HDL‐c, low‐density lipoprotein cholesterol (LDL‐c), TG, uric acid, bilirubin, aspartat aminotransferase (AST), alanine aminotransferase (ALT), and gamma‐glutamil transferase (GGT) were measured using standardized enzymatic procedures, spectrophotometrically (Roche Cobas 400). HsCRP) levels were determined using a nephelometric assay (Behring Nephelometer Analyzer, Marburg, Germany).
Glomerular filtration rate was estimated by using creatinine in the Modification of Diet in Renal Disease Study equation (eGFRMDRD):24
Blood pressure was measured as described previously.2
2.4. Statistical analysis
Data are expressed as arithmetic mean±standard deviation for normally distributed variables, geometric mean [95% Confidence interval (CI) for the mean] for log‐normally distributed variables, and as median (interquartile range) for skewed distributed variables. Differences in continuous variables between DM2 patients and control population were tested with Student's t‐test for normally and log‐normally distributed variables, and Mann‐Whitney test for skewed distributed variables. Differences between categorical variables were examined with the Chi‐square test. The associations among VAI, LAP index, and variables which entered VAI and LAP equations with other clinical parameters were assessed by Spearman's correlation analysis and presented as correlation coefficient (ρ). Using logistic regression analysis, we examined the associations and VAI, LAP index, and each parameter of VAI and LAP equations with risk for DM2 development. Multivariate logistic regression analysis was used to identify factors associated with risk for DM2 development. Multivariate analysis adjustment was made for all parameters which showed significant differences between two tested populations, exhibit significant correlations with other independent parameters, and associated with DM2 occurrence and development. Data were presented as odds ratio (OR) and 95% CI for odds. The explained variation in dependent variable was given by Nagelkerke R 2 value. Receiver Operating Characteristic (ROC) curve analysis was used to test the additional values of VAI, LAP index, single parameter used in VAI and LAP calculations and models to distinguish subjects that suffered from DM2 from those that did not. Differences between curve areas were also tested. All statistical analysis was carried out in the PASW® Statistic version 18 (Chicago, IL, USA) and the MedCalc® (Mariakerke, Belgium) Version 15.8. A P value <.05 was considered statistically significant in all analyses.
3. RESULTS
In Table 1 are summarized demographic characteristics of studied groups. Diabetic patients were older (P<.001), had significantly higher BMI (P=.003) and WC (P<.001). Chi‐square analysis showed unequal distribution of gender, subjects who used hypolipemic and antihypertensive therapy (P<.001 for all).
Table 1.
Demographic characteristics of two study groups
| Control group | Diabetic group | P | |
|---|---|---|---|
| N (male/female) | 119 (34/85) | 180 (90/90) | <.001 |
| Age, years | 55.00 (36.25‐66.75) | 63.00 (55.00‐69.00) | <.001 |
| BMI, kg/m2 | 26.96 (23.26‐30.46) | 29.07 (26.80‐32.28) | .003 |
| WC, cm | 96.00 (86.00‐104.75) | 106.00 (98.50‐146.00) | <.001 |
| SBP, mmHg | 133.00 (127.00‐141.75) | 134.00 (126.00‐142.00) | .801 |
| DBP, mmHg | 77.00 (70.00‐80.00) | 80.00 (70.00‐85.00) | .102 |
| Smoking habits, (Smoker/Non‐smoker) | 28/91 | 42/138 | .969 |
| Hypolipemics (Yes/No) | 23/96 | 77/103 | <.001 |
| Statins (Controls/Diabetics, 23/77) | |||
| Fibrates (Controls/Diabetics, 0/6) | |||
| Antihypertensives (Yes/No) | 58/61 | 129/51 | <.001 |
| ACE inhibitors (Controls/Diabetics, 51/116) | |||
| Beta blockers (Controls/Diabetics, 36/31) | |||
| Diuretics (Controls/Diabetics, 19/68) | |||
| Antihyperglycemics (Yes/No) | ‐ | 0/157 | <.001 |
| Metformin (Controls/Diabetics, 0/149) | |||
| Sulfonylurea (Controls/Diabetics, 0/8) | |||
| Insulin (Controls/Diabetics, 0/31) | ‐ | 0/31 | ‐ |
| Duration of diabetes, years | ‐ | 4.00 (2.00‐9.00) | ‐ |
Data are presented as median (interquartile range) and compared by Mann‐Whitney test.
Categorical variables are presented as absolute frequencies and compared by Chi‐square test.
Statistical analysis showed that diabetic patients had significantly higher TG, LAP index, VAI, GGT, uric acid (P<.001 for all), ALT (P=.022), creatinine (P=.001), eGFRMDRD (P=.007), and hsCRP (P=.004) than controls. Also, diabetic patients had significantly lower HDL‐c and total bilirubin level than control group (P<.001, and P=.028, respectively) (Table 2).
Table 2.
Laboratory and clinical parameters of two study groups
| Control group | Diabetic group | P | |
|---|---|---|---|
| TC, mmol/La | 5.41 (5.20‐5.63) | 5.24 (5.08‐5.41) | .227 |
| HDL‐c, mmol/La | 1.45 (1.37‐1.53) | 1.14 (1.09‐1.23) | <.001 |
| LDL‐c, mmol/La | 3.16 (2.96‐3.36) | 3.10 (2.96‐3.25) | .605 |
| TG, mmol/La | 1.39 (1.27‐1.52) | 1.91 (1.77‐2.06) | <.001 |
| LAPa | 44.34 (38.14‐51.53) | 82.87 (76.69‐90.73) | <.001 |
| VAIa | 1.73 (1.53‐1.96) | 2.90 (2.61‐3.21) | <.001 |
| Glucose, mmol/Lb | 5.20 (4.90‐5.77) | 7.10 (6.20‐8.90) | <.001 |
| HBA1c, %b | 4.90 (4.70‐5.30) | 6.60 (5.80‐8.00) | <.001 |
| AST, U/Lb | 19.00 (17.00‐22.50) | 19.00 (16.00‐32.00) | .790 |
| ALT, U/Lb | 20.00 (14.00‐25.75) | 22.00 (16.00‐32.00) | .022 |
| GGT, U/Lb | 14.00 (11.00‐20.00) | 19.00 (15.00‐29.00) | <.001 |
| Total bilirubin, μmol/Lb | 7.10 (6.12‐9.90) | 6.00 (4.60‐8.15) | .028 |
| Creatinine, μmol/Lb | 66.50 (59.00‐78.00) | 73.00 (64.00‐86.00) | .001 |
| Uric acid, μmol/Lb | 259.00 (213.75‐330.50) | 310.00 (249.00‐357.00) | <.001 |
| eGFRMDRD, mL/min/1.73 m² | 90.04±22.20 | 82.82±22.42 | .007 |
| HsCRP, mg/Lb | 1.31 (0.49‐2.68) | 1.52 (0.90‐3.64) | .004 |
Data are presented as arithmetic mean±SD and compared with Student's t‐test.
Log‐normal distributed data are presented as geometric mean (95% CI) and compared with Student's t‐test after logarithmic transformation.
Skewed distributed data are presented as median (interquartile range) and compared with Mann‐Whitney test.
Results of Spearman's correlation analysis were presented in Table 3. BMI and WC were highly positively correlated with ages, SBP, DBP, TG, glucose HbA1c, AST, ALT, GGT, uric acid, and hsCRP (P<.01), and negatively correlated with HDL‐c (P<.01) and eGFRMDRD (P<.05). WC also showed significant correlation with creatinine (P<.05). HDL‐c highly negatively correlated with TG, glucose, HBA1c, ALT, AST, GGT, uric acid, hsCRP, and creatinine (P<.01 for all). TG showed significant positive correlations with ages (P<.05), TC, LDL‐c, glucose, HBA1c, ALT, AST, GGT, uric acid, hsCRP. and creatinine (P<.01 for all). Negative correlations were obtained between TG and eGFRMDRD (P<.05). LAP index was positively correlated with ages, BMI, WC, TC, TG, LDL‐c, glucose HbA1c, AST, ALT, GGT, uric acid, hsCRP (P<.01 for all), and creatinine (P<.05), and negatively correlated with HDL‐c (P<.01) and eGFRMDRD (P<.05). VAI was positively correlated with ages (P<.05), BMI, WC, TC, TG, LDL‐c, glucose HbA1c, AST, ALT, GGT, uric acid, hsCRP (P<.01), and creatinine (P<.05), and negatively correlated with HDL‐c, total bilirubin (P<.01 for both), and eGFRMDRD (P<.05).
Table 3.
Associations among anthropometric, lipid parameters, VAI, and LAP with other clinical parameters
| BMI, kg/m2 | WC, cm | HDL‐c, mmol/L | TG, mmol/L | LAP | VAI | |
|---|---|---|---|---|---|---|
| Age, years | 0.182b | 0.238b | −0.036 | 0.140a | 0.191b | 0.127a |
| BMI, kg/m2 | − | 0.829b | −0.297b | 0.394b | 0.659b | 0.422b |
| WC, cm | 0.289b | − | −0.405b | 0.387b | 0.696b | 0.454b |
| SBP, mmHg | 0.285b | 0.215b | −0.007 | 0.049 | 0.147a | 0.069 |
| DBP, mmHg | 0.275b | 0.268b | −0.017 | 0.031 | 0.135a | 0.031 |
| TC, mmol/L | 0.083 | 0.017 | 0.087 | 0.406b | 0.337b | 0.284b |
| HDL‐c, mmol/L | −0.297b | −0.405b | − | −0.575b | −0.578b | −0.747b |
| LDL‐c, mmol/L | 0.080 | 0.060 | −0.068 | 0.320b | 0.280b | 0.270b |
| TG, mmol/L | 0.394b | 0.387b | −0.575b | − | 0.900b | 0.932b |
| Glucose, mmol/L | 0.328b | 0.388b | −0.356b | 0.379b | 0.451b | 0.415b |
| HbA1c, % | 0.326b | 0.398b | −0.466b | 0.421b | 0.488b | 0.470b |
| AST, IU/L | 0.222b | 0.202b | −0.087 | 0.190b | 0.219b | 0.144b |
| ALT, IU/L | 0.325b | 0.340b | −0.254b | 0.323b | 0.370b | 0.294b |
| GGT, IU/L | 0.342b | 0.405b | −0.350b | 0.475b | 0.503b | 0.419b |
| Uric acid, μmol/L | 0.344b | 0.383b | −0.240b | 0.322b | 0.377b | 0.283b |
| Total bilirubin, μmol/L | −0.028 | −0.040 | 0.076 | −0.096 | −0.110 | −0.159b |
| HsCRP, mg/L | 0.509b | 0.468b | −0.240b | 0.267b | 0.425b | 0.320b |
| Creatinine, μmol/L | 0.086 | 0.186a | −0.249b | 0.171b | 0.159a | 0.120a |
| eGFRMDRD, mL/min/1.73 m² | −0.131a | −0.126a | 0.065 | −0.148a | −0.168a | −0.147a |
Data are presented as correlation coefficient Rho (ρ).
P<.05.
P<.01.
Logistic regression analysis was used to establish the associations of VAI, LAP index, and each parameter used in their equations with DM2 development (Table 4). Predictors were unadjusted and adjusted for other parameters significantly different between study groups (Table 2). Also, ages and hypolipemic therapy, which showed significant odds in univariate logistic regression analysis, were tested further in models to determine their independent predictions on DM2 development. WC kept independent predictions for DM2 development (Model 1 OR=1.050 P=.027; Model 3 OR=1.052, P<.001). As WC rose for 1 cm, risk for DM2 development got higher for 5% and 5.2%, respectively. HDL‐c was also shown to be the independent predictor of DM2 development (Model 2 OR=0.142, P<.001). Rise in HDL‐c by 1 mmol/L reduced the probability of DM2 development by 85.8%. VAI lost its independent predictive role (Model 2 OR=1.136, P=.053), but LAP kept its independent prediction of DM2 development (Model 4 OR=1.010, P=.005). TG was showed not to be the independent predictor of DM2 development (Model 1 OR=0.954, P=.730 and Model 3 OR=1.200, P=.155).
Table 4.
Logistic regression analysis for the associations among VAI, LAP indexes, parameters used for indexes calculations, and DM2 development
| Predictors | Unadjusted OR (95%CI) | P | Nagelkerke R 2 |
|---|---|---|---|
| BMI, kg/m2 | 1.140 (1.079‐1.205) | <.001 | .115 |
| WC, cm | 1.068 (1.046‐1.091) | <.001 | .198 |
| HDL‐c, mmol/L | 0.095 (0.046‐0.198) | <.001 | .209 |
| TG, mmol/L | 1.718 (1.295‐2.279) | <.001 | .083 |
| LAP | 1.016 (1.010‐1.021) | <.001 | .162 |
| VAI | 1.292 (1.133‐1.474) | <.001 | .092 |
| Age, years | 1.052 (1.033‐1.071) | <.001 | .142 |
| Hypolipemics | 3.120 (1.814‐5.367) | <.001 | .081 |
| Adjusted OR (95%CI) | P | Nagelkerke R 2 | |
|---|---|---|---|
| Model 1 | |||
| BMI, kg/m2 | 0.963 (0.863‐1.076) | .513 | .411 |
| WC, cm | 1.050 (1.006‐1.097) | .027 | |
| HDL‐c, mmol/L | 0.142 (0.053‐0.385) | <.001 | |
| TG, mmol/L | 0.954 (0.728‐1.249) | .730 | |
| Age, years | 1.050 (1.021‐1.086) | .001 | |
| Hypolipemics | 2.130 (1.150‐4.108) | .024 | |
| Model 2 | |||
| VAI | 1.136 (0.998‐1.295) | .053 | .318 |
| Age, years | 1.064 (1.034‐1.095) | <.001 | |
| Hypolipemics | 2.040 (1.020‐3.770) | .023 | |
| Model 3 | |||
| TG, mmol/L | 1.200 (0.934‐1.536) | .155 | .356 |
| WC, cm | 1.052 (1.022‐1.082) | <.001 | |
| Age, years | 1.054 (1.023‐1.086) | <.001 | |
| Hypolipemics | 2.041 (1.086‐3.840) | .027 | |
| Model 4 | |||
| LAP | 1.010 (1.003‐1.017) | .005 | .333 |
| Age, years | 1.061 (1.031‐1.092) | <.001 | |
| Hypolipemics | 1.985 (1.065‐3.698) | .031 | |
Model 1: age, BMI, WC, HDL‐c, TG, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
Model 2: age, VAI, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
Model 3: age, WC, TG, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
Model 4: age, LAP, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
In all models, ages and hypolipemic therapy were shown to be the independent predictors of DM2 development. Usage of hypolipemic therapy presented around two times higher risk of DM2 development (all Models, Table 4). Also, as person got older by 1 year, the probability for DM2 development rose for 5.0% (P=.001, Model 1), for 6.4% (P<.001, Model 2), for 5.4% (P<.001, Model 3), and for 6.1% (P<.001, Model 4).
According to R 2 value, 41.1% variation in DM2 development could be explained by Model 1, 31.8% by Model 2, 35.6% by Model 3, and 33.3% by Model 4 (Table 4). Model 1 correctly classified 77%, Model 2 correctly classified 71%, Model 3 correctly classified 74%, and Model 4 correctly classified 72% of cases in group having DM2 (data were not presented in tables).
ROC analysis was used to discriminate subjects with DM2 from those who did not have it (Tables 5 and 6). The calculated AUCs for the measurement of single clinical parameter (ranking from 0.617 to 0.729) indicated that the clinical accuracy of the applied procedures containing single parameter was low.
Table 5.
ROC analysis for single parameter discriminatory abilities regarding DM2 development
| Predictors | AUC (95%CI) | SE | Sensitivity (%) | Specificity (%) | P |
|---|---|---|---|---|---|
| BMI, kg/m2 | 0.667 (0.603‐0.732) | 0.033 | 90.56 | 38.66 | <.001 |
| WC, cm | 0.715 (0.653‐0.777) | 0.032 | 66.11 | 68.07 | <.001 |
| HDL‐c, mmol/L | 0.729 (0.670‐0.787) | 0.030 | 70.56 | 65.55 | <.001 |
| TG, mmol/L | 0.671 (0.609‐0.734) | 0.034 | 68.89 | 60.50 | <.001 |
| LAP | 0.716 (0.657‐0.776) | 0.031 | 86.11 | 50.42 | <.001 |
| VAI | 0.707 (0.647‐0.776) | 0.030 | 59.44 | 73.95 | <.001 |
| Age, years | 0.669 (0.605‐0.733) | 0.030 | 70.56 | 56.30 | <.001 |
| Hypolipemics | 0.617 (0.553‐0.681) | 0.033 | 42.78 | 80.67 | .001 |
SE, standard error.
Table 6.
ROC analysis for model discriminatory abilities regarding DM2 development
| Predictors | AUC (95%CI) | SE | Sensitivity (%) | Specificity (%) | AUC difference | P |
|---|---|---|---|---|---|---|
| Model 1 | 0.819 (0.771‐0.861) | 0.025 | 82.20 | 70.60 | ‐ | ‐ |
| Model 2 | 0.781 (0.730‐0.826) | 0.027 | 66.11 | 79.00 | 0.038 | .018 |
| Model 3 | 0.800 (0.746‐0.841) | 0.027 | 80.00 | 66.40 | 0.019 | .076 |
| Model 4 | 0.784 (0.733‐0.829) | 0.027 | 69.44 | 76.47 | 0.035 | .019 |
P, probability for AUC differences.
Model 1: age, BMI, WC, HDL‐c, TG, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
Model 2: age, VAI, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
Model 3: age, WC, TG, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
Model 4: age, LAP, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD (continuous variables); gender, smoking status, hypolipemics, and antihypertensive therapies (categorical variables).
The same models were used in ROC analysis as in logistic regression analysis. Model 1 which included parameters that entered VAI equation showed higher ability to discriminate DM2 patients from controls than Model 2 which included VAI itself (AUC=0.819 and AUC=0.781, respectively, and AUC difference=0.038, P=.018). Also, difference between areas was significant between Model 1 and Model 4 which included LAP itself (AUC difference=0.035, P=.019). Good accuracy of the applied procedures was only achieved with Models 1 and 3 (AUC=0.819 and AUC=0.800, respectively) which were consisted of parameters used in VAI (BMI, WC, HDL‐c, TG) and LAP (WC, TG) indexes equations, but not VAI and LAP indexes themselves (AUC=0.781 and AUC=0.784, respectively) (Figure 1). The highest sensitivity and specificity was achieved by Model 1 (82.20% and 70.60%, respectively) (Table 6).
Figure 1.
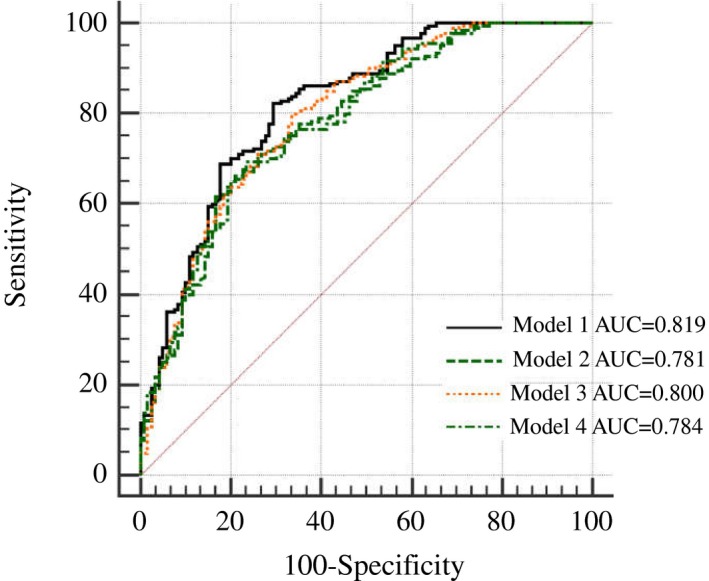
ROC curves for Models discriminative ability for DM2 development
4. DISCUSSION
The main finding of our study reveals that neither VAI nor LAP are superior to simple anthropometric indices (e.g., WC) and lipid parameters (e.g., HDL‐c) for DM2 prediction.
Logistic regression analysis showed that VAI lost its independent predictive role, but LAP kept its independent prediction of DM2 development, after adjustment for confounding factors (Table 4).
As more than 60%‐70% of DM2 patients are affected by non‐alcoholic fatty liver disease (NAFLD), and as that diabetic individuals with NAFLD have increased risk for long‐term diabetes complications such as chronic kidney disease and CVD,25 we determined liver enzymes activity in all participants. Moreover, we determined uric acid and total bilirubin levels, regarding that hyperuricemia and low total bilirubin levels are associated with increased risk of DM2 and its complications.26 In addition, we determined creatinine levels and calculated eGFR in all subjects, as studies report the association of high creatinine levels, and low eGFR levels with an increased risk of DM2, as well as its complications.27, 28
A ROC analysis was used to discriminate subjects with DM2 from those who did not have it and showed that good accuracy of the applied procedures was only achieved with models which were consisted of parameters used in VAI (BMI, WC, HDL‐c, TG) and LAP (WC, TG) indexes equations, respectively (AUC=0.819 and AUC=0.800, respectively), but not with VAI and LAP indexes themselves (Table 6, Figure 1).
The majority of previous studies reported VAI and LAP to be reliable indices in prediction of many metabolic disturbances.6, 7, 8, 9, 10, 12, 13, 17
Amato et al.8 reported superiority of the VAI, to other anthropometric indices (BMI, WC, hip circumference, and waist/hip ratio) in relation to cardiometabolic risk and adipose tissue dysfunction in individuals with DM2. In addition, in their another study,6 superiority of VAI than its individual components that entered equation (WC, BMI, HDL‐c, and TG) with regard to discriminating cardio‐ and cerebrovascular events was reported, too. On the contrary, our results show that the highest sensitivity and specificity was achieved by model consisted of parameters that enter VAI equation (82.2% and 70.6%, respectively) in discriminating subjects with DM2 from those who did not have it (Table 6).
Also, previous studies reported that LAP better than other variables (e.g., BMI and WC) predicted MetS,14 DM2,12, 17 and CVD.7, 10
However, our results are opposite, showing that visceral indexes, VAI and LAP, are not superior to WC and HDL‐c in predicting DM2 occurrence. Our findings are similar to results obtained from a recent study,19 which encompassed nearly 600 healthy Saudi children, showing superiority of BMI and WC relative to VAI in relation to cardiometabolic risk factors.
Similarly, some other investigations reported simple anthropometric measures, BMI and WC to be more accurate than VAI in prediction of the presence of MetS,20 hepatic steatosis,21 and incident CVD.22
In addition, it has been reported that although LAP was an independent predictor of cardiovascular events even in normal weight subjects, it had no advantageous capabilities over other anthropometrics indices.18 Similarly, Dai et al.9 showed that neither VAI nor LAP index was superior for identifying individuals with CKD over BMI in the male gender.
In our study, logistic regression analysis reported WC to be the independent predictor of DM2 development both in unadjusted and adjusted model. As WC rose for 1 cm, the risk for DM2 development got higher for 5% (Model 1) and 5.2% (Model 3), respectively (Table 4). Our results are in line with previous findings1, 29 suggesting that visceral adiposity is the independent risk factor for DM2. Visceral adipose tissue is significant source of variety of pro‐inflammatory adipo‐ and cytokines which reach the liver, thus impairing insulin signaling, with consequent insulin resistance occurrence, lipid accumulation, and metabolic disorders.1, 2
Although Computed Tomography (CT) represents the “gold standard method” for evaluating the quantification of visceral adipose tissue, it is not suitable diagnostic procedure in routine clinical practice due to its high cost and radiation exposure. Therefore, WC is regarded to be simple, easily obtained anthropometric parameter that can estimate visceral obesity in primary care settings.30
Furthermore, HDL‐c was also shown to be the independent predictor of DM2. Rise in HDL‐c by 1 mmol/L reduced the probability of DM2 development by 85.8% (Model 1) (Table 4).
Other studies also showed low HDL‐c to be the independent risk factor for DM2.31, 32
Gupta et al.31 found that increase in HDL‐c by 1 mmol/L decreased the probability of DM2 by 28%. Favorable properties of HDL‐c, such as antioxidative, anti‐inflammatory, and antiapoptotic actions, are well established, thus explaining its protective roles in DM2.33
Another important finding of our study was that in all models, ages and hypolipemic therapy were shown to be the independent predictors of DM2 development. Relationship between ages and DM2 was also reported previously.29 In our study, as person got older by 1 year, the probability for DM2 development rose for 5.0% in unadjusted and for 6.4% in adjusted model.
In this study usage of hypolipemic therapy presented around 3.12 time higher risk of DM2 development in unadjusted model, and 2 times higher risk of DM2 occurrence in adjusted model. It is important to note that all participants in our study who used hypolipemics, used statins. Our results are in line with recent studies which also showed an increased risk of DM2 with statins usage.34, 35 Cederberg et al.34 in a large study comprised of about 9,000 non‐diabetic participants, during a 6‐year follow‐up showed that statin treatment increased the risk of DM2 by 46%, mainly due to decrease in insulin sensitivity (by 24%) and insulin secretion (by 12%), compared with individuals without statin treatment.
Although this study has cross‐sectional design which precludes causal inferences, we speculate that novel visceral adiposity indexes, such as VAI and LAP, have no advantages over simple anthropometric indices or lipid parameters in prediction of DM2 risk. Large longitudinal studies are needed to further examine the potential benefits of VAI and LAP in DM2 prediction.
5. CONCLUSION
Visceral adiposity index and Lipid accumulation product may not be better than parameters that enter its equation in type 2 diabetes prediction.
ACKNOWLEDGMENT
This work was financially supported in part by a grant from the Ministry of Education, Science and Technological Development, Republic of Serbia (Project number 175035).
Kavaric N, Klisic A, Ninic A. Are visceral adiposity index and lipid accumulation product reliable indices for metabolic disturbances in patients with type 2 diabetes mellitus?. J Clin Lab Anal. 2018;32:e22283 10.1002/jcla.22283
REFERENCES
- 1. Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: exploring a pathway full of thorns. Arch Med Sci. 2015;11:463‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klisic A, Kotur‐Stevuljevic J, Kavaric N, Matic M. Relationship between cystatin C, retinol‐binding protein 4 and Framingham risk score in healthy postmenopausal women. Arch Iran Med. 2016;19:845851. [DOI] [PubMed] [Google Scholar]
- 3. Kavaric N, Klisic A, Soldatovic I, Bjelakovic B. Can waist circumference be a reliable anthropometric parameter in healthy normal weight and overweight adolescents? Iran J Public Health. 2015;44:883‐884. [PMC free article] [PubMed] [Google Scholar]
- 4. Klisic A, Kotur‐Stevuljevic J, Kavaric N, Martinovic M, Matic M. The association between follicle stimulating hormone and glutathione peroxidase activity is dependent on abdominal obesity in postmenopausal women. Eat Weight Disord. 2016. 10.1007/s40519-016-0325-1 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5. Klisic AN, Vasiljevic ND, Simic TP, Djukic TI, Maksimovic MZ, Matic MG. Association between C‐reactive protein, anthropometric and lipid parameters among healthy normal weight and overweight postmenopausal women in Montenegro. Lab Med. 2014;45:12‐16. [DOI] [PubMed] [Google Scholar]
- 6. Amato MC, Giordano C, Galia M, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33:920‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population‐based comparison. BMC Cardiovasc Disord. 2005;5:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amato MC, Giordano C. Visceral Adiposity Index: an indicator of adipose tissue dysfunction. Int J Endocrinol. 2014;2014:730827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai D, Chang Y, Chen Y, et al. Visceral Adiposity Index and Lipid Accumulation Product Index: two alternate body indices to identify chronic kidney disease among the rural population in Northeast China. Int J Environ Res Public Health. 2016;13:1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao X, Wang G, Wang A, Xu T, Tong W, Zhang Y. Comparison of lipid accumulation product with body mass index as an indicator of hypertension risk among Mongolians in China. Obes Res Clin Pract. 2013;7:e308‐e314. [DOI] [PubMed] [Google Scholar]
- 11. Abruzzese GA, Cerrrone GE, Gamez JM, et al. Lipid Accumulation Product (LAP) and Visceral Adiposity Index (VAI) as markers of insulin resistance and metabolic associated disturbances in young argentine women with polycystic ovary syndrome. Horm Metab Res. 2017;49:23‐29. [DOI] [PubMed] [Google Scholar]
- 12. Wiltgen D, Benedetto IG, Mastella LS, Spritzer PM. Lipid accumulation product index: a reliable marker of cardiovascular risk in polycystic ovary syndrome. Hum Reprod. 2009;24:1726‐1731. [DOI] [PubMed] [Google Scholar]
- 13. Chen GP, Qi JC, Wang BY, et al. Applicability of visceral adiposity index in predicting metabolic syndrome in adults with obstructive sleep apnea: a cross‐sectional study. BMC Pulm Med. 2016;16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng YL, Wang YJ, Lan KH, et al. Fatty liver index and lipid accumulation product can predict metabolic syndrome in subjects without fatty liver disease. Gastroenterol Res Pract. 2017;2017:9279836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu PJ, Ma F, Lou HP, Chen Y. Visceral Adiposity Index is associated with pre‐diabetes and type 2 diabetes mellitus in chinese adults aged 20‐50. Ann Nutr Metab. 2016;68:235‐243. [DOI] [PubMed] [Google Scholar]
- 16. Wakabayashi I, Daimon T. A strong association between lipid accumulation product and diabetes mellitus in Japanese women and men. J Atheroscler Thromb. 2014;21:282‐288. [DOI] [PubMed] [Google Scholar]
- 17. Bozorgmanesh M, Hadaegh F, Azizi F. Diabetes prediction, lipid accumulation product, and adiposity measures; 6‐year follow‐up: Tehran lipid and glucose study. Lipids Health Dis. 2010;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hosseinpanah F, Barzin M, Mirbolouk M, Abtahi H, Cheraghi L, Azizi F. Lipid accumulation product and incident cardiovascular events in a normal weight population: Tehran Lipid and Glucose Study. Eur J Prev Cardiol. 2016;23:187‐193. [DOI] [PubMed] [Google Scholar]
- 19. Al‐Daghri NM, Al‐Attas OS, Alokail M, et al. Does visceral adiposity index signify early metabolic risk in children and adolescents?: Association with insulin resistance, adipokines, and subclinical inflammation. Pediatr Res. 2014;75:459‐463. [DOI] [PubMed] [Google Scholar]
- 20. Elisha B, Messier V, Karelis A, et al. The Visceral Adiposity Index: relationship with cardiometabolic risk factors in obese and overweight postmenopausal women‐a MONET group study. Appl Physiol Nutr Metab. 2013;38:892‐899. [DOI] [PubMed] [Google Scholar]
- 21. Borruel S, Moltó JF, Alpañés M, et al. Surrogate markers of visceral adiposity in young adults: waist circumference and body mass index are more accurate than waist hip ratio, model of adipose distribution and visceral adiposity index. PLoS ONE. 2014;9:e114112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mohammadreza B, Farzad H, Davoud K, Fereidoun Prof AF. Prognostic significance of the complex “Visceral Adiposity Index” vs. simple anthropometric measures: Tehran lipid and glucose study. Cardiovasc Diabetol. 2012;11:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. American Diabetes Association . Classification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes‐2016. Diabetes Care. 2016;39(Suppl. 1):S13‐S22. [DOI] [PubMed] [Google Scholar]
- 24. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney diesease: evaluation, classification, and stratification. Kidney Disease Outcome Quality Initiative. Am J Kidney Dis. 2002;39:S1‐S246. [PubMed] [Google Scholar]
- 25. Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. Non‐alcoholic fatty liver disease and diabetes. Metabolism. 2016;65:1096‐1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ren Y, Jin N, Hong T, et al. Interactive effect of serum uric acid and total bilirubin for cardiovascular disease in Chinese patients with type 2 diabetes. Sci Rep. 2016;6:36437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schneider C, Coll B, Jick SS, Meier CR. Doubling of serum creatinine and the risk of cardiovascular outcomes in patients with chronic kidney disease and type 2 diabetes mellitus: a cohort study. Clin Epidemiol. 2016;8:177‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Looker HC, Colombo M, Agakov F, et al. Protein biomarkers for the prediction of cardiovascular disease in type 2 diabetes. Diabetologia. 2015;58:1363‐1371. [DOI] [PubMed] [Google Scholar]
- 29. Hajian‐Tilaki K, Heidari B. Is waist circumference a better predictor of diabetes than body mass index or waist‐to‐height ratio in Iranian adults? Int J Prev Med. 2015;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roriz AKC, Passos LCS, de Oliveira CC, Eickemberg M, Moreira PdeA, Sampaio LR. Evaluation of the accuracy of anthropometric clinical indicators of visceral fat in adults and elderly. PLoS ONE 2014;9:e103499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gupta AK, Dahlof B, Dobson J, Sever PS, Wedel H, Poulter NR. Anglo‐Scandinavian Cardiac Outcomes Trial Investigators. Determinants of new‐onset diabetes among 19,257 hypertensive patients randomized in the Anglo‐Scandinavian Cardiac Outcomes Trial–Blood Pressure Lowering Arm and the relative influence of antihypertensive medication. Diabetes Care. 2008;31:982‐988. [DOI] [PubMed] [Google Scholar]
- 32. Qi Q, Liang L, Doria A, Hu FB, Qi L. Genetic predisposition to dyslipidemia and type 2 diabetes risk in two prospective cohorts. Diabetes. 2012;61:745‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kruit JK, Brunham LR, Verchere CB, Hayden MR. HDL and LDL cholesterol significantly influence beta‐cell function in type 2 diabetes mellitus. Curr Opin Lipidol. 2010;21:178‐185. [DOI] [PubMed] [Google Scholar]
- 34. Cederberg H, Stančáková A, Yaluri N, Modi S, Kuusisto J, Laakso M. Increased risk of diabetes with statin treatment is associated with impaired insulin sensitivity and insulin secretion: a 6 year follow‐up study of the METSIM cohort. Diabetologia. 2015;58:1109‐1117. [DOI] [PubMed] [Google Scholar]
- 35. Carter AA, Gomes T, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]


