Abstract
Background
In this study, the pathologies of acute meningitis and encephalopathy were investigated, and biomarkers useful as prognostic indices were searched for.
Methods
The subjects were 31 children with meningitis, 30 with encephalopathy, and 12 with convulsions following gastroenteritis. Control group consisted of 24 children with non‐central nervous system infection. Cerebrospinal fluid cytokine analysis was performed.
Results
Chemokines significantly increased in the bacterial meningitis group compared with those in viral meningitis and encephalopathy groups. On comparison of interleukin(IL)‐17, it increased in cases with status epilepticus in influenza‐associated encephalopathy group. In the rotavirus encephalopathy and convulsions following gastroenteritis groups, IL‐17 particularly increased in the convulsions following gastroenteritis group. IL‐8 increased in all cases irrespective of the causative virus.
Conclusions
In the encephalopathy group, IL‐8 may serve as a neurological prognostic index. IL‐17 was increased in the convulsions following gastroenteritis group, particularly in cases with status epilepticus, suggesting its involvement as a convulsion‐related factor.
Keywords: chemokine, encephalopathy, interleukin‐17, meningitis
1. Introduction
Acute encephalopathy in children is a central nervous system (CNS) infection with a poor prognosis. In Japan, about 1000 cases of acute encephalopathy occur each year, and influenza virus is the most frequent cause among all viral encephalopathies.1 Over time, brain dysfunction may occur, although there is no sequela during the acute phase.
For acute encephalopathy in children, treatment methods are selected based upon the pathological classification.2, 3 In all types, a neural factor excessively produced in the whole body or brain induces inflammatory cytokines, mitochondrial disorder, induction of apoptosis, and vascular endothelial dysfunction.4, 5 However, currently there are no useful biomarkers for the diagnosis of this disease. Previously, we reported encephalopathy‐related changes in IL‐6, IL‐1β, neurotrophin, and nitrogen, which is an oxidation marker.6, 7 Additionally, several studies have reported chemokines to be increased during epileptic seizures,8 as well as in acute encephalopathy with febrile convulsive status epilepticus (AEFCSE).9 However, no exhaustive investigation of acute encephalopathy by causative virus has been performed.
In this study, the pathology and prognostic biomarkers of viral infection‐associated encephalopathy was investigated, focusing on chemokines and the IL‐17 family in cytokine profiling.
2. Measurement and Methods
The backgrounds of the subject groups are presented in Table 1. The duration of the study period was 5 years (2010‐2014). Subjects were 30 children (21 boys and nine girls, aged 21.2±15.9 months) with virus‐associated encephalopathy, hospitalized in the Tokyo Medical University Hospital and related institutions (Tokyo, Japan). The causative viruses in the patients with acute encephalopathy were: influenza virus (9 patients), respiratory syncytial virus; RS (10 patients), rotavirus (8 patients), and human herpes virus type 6; HHV6 (3 patients). Thirty‐one children with acute meningitis (bacterial: three boys and one girl, viral: 12 boys and 15 girls) were evaluated as a CNS disease comparative control group. Of the patients with acute bacterial meningitis, all CSF cultures were positive for a bacterial organism: two were Haemophilus influenzae, one Streptococcus pneumoniae, and one Group B Streptococcus.
Table 1.
Patient characteristics. Subjects were 30 children (21 boys and nine girls, aged 21.2 ± 15.9 months) hospitalized with virus‐associated encephalopathy. The causative viruses in the patients with acute encephalopathy were: influenza virus, respiratory syncytial virus, rotavirus, and human herpes virus type 6. For control groups, cerebrospinal fluid (CSF) samples were collected from 24 in‐patients (13 boys and 11 girls, aged 37.5 ± 27.2 months) with non‐CNS diseases (acute upper respiratory infection, urinary tract infection)
| Group | The causative viruses and bacterial infections | Cases | Male:female | Months |
|---|---|---|---|---|
| Acute encephalopathy: 30 cases | Influenza virus | 9 | 5:4 | 27.2±18.1 |
| Respiratory syncynial virus | 10 | 7:3 | 14.3±10.6 | |
| Human herpes virus 6 | 3 | 2:1 | 10.3±6.5 | |
| Rotavirus | 8 | 7:1 | 27.1±23.6 | |
| Convulsions following mild gastroenteritis: 12 cases | — | 12 | 6:6 | 21.5±5.5 |
| Acute meningitis: 31 cases | Identified causative viruses: five cases (mumps: three cases, entero: two cases) | 27 | 12:15 | 42.8±28.1 |
| Haemophilus influenzae: two cases | 4 | 3:1 | 18.2±6.8 | |
| Streptococcus pneumoniae: one case | ||||
| Group B Streptococcus: one case | ||||
| Control: 24 cases | Non‐central nervous system infection | 24 | 13:11 | 37.5±27.2 |
Twelve patients with convulsions following gastroenteritis were also evaluated for comparison with patients with rotavirus encephalopathy. After ruling out the presence of co‐infections, prematurity, cardiovascular disease, and immunosuppression, all patients were continued on the treatment. For control groups, cerebrospinal fluid (CSF) samples were collected from 24 in‐patients (13 boys and 11 girls, aged 37.5±27.2 months) with a non‐CNS disease (acute upper respiratory infection, urinary tract infection).
Experimental samples were collected by lumbar puncture on the first day that central nerve symptoms appeared. For the diagnosis of acute encephalopathy, patients in whom the possibility of central neuropathy resulting from other diseases had been ruled out. Exclusion criteria included history of a complex febrile seizure, history of an afebrile seizure, trauma, or severe underlying neurological disorder. And patients were assessed to have virus‐associated encephalopathy, as well as patients with neurological symptoms in whom consciousness disorder (Glasgow Coma Scale <8) was protracted for 12 hours or more, causative viruses were detected using rapid diagnosis, virus isolation, and polymerase chain reaction (PCR). We used rapid testing and virus isolation to diagnose influenza, rotavirus, and RS virus. On the other hand, HHV‐6 was diagnosed by PCR and virus isolation. The clinical course of patients with HHV‐6 infection is typical of exanthem subitum, and so we focused on patients that showed abnormal brain magnetic resonance imaging (MRI) and electroencephalogram (EEG) results.
Cytokine profiling was assessed in CSF samples using the Bio‐Plex suspension array system and cytokine Human 17‐Plex Panel (Bio‐Rad Laboratories, Tokyo, Japan) according to the manufacturers’ instructions. This method utilizes a magnetic bead‐based multiplex assay designed to measure multiple cytokines. We analyzed eight cytokines (interleukin (IL)‐1β, IL‐6, IL‐8, IL‐10, IL‐17, basic granulocyte‐colony stimulating factor (G‐CSF), CCL2 (monocyte chemoattractant protein‐1/MCAF), CCL4 (macrophage inflammatory protein‐1b).
The study protocol was approved by the Ethics Committee of Tokyo Medical University (NO.3001). Informed consent was obtained from the patients’ legal representatives.
Statistical analysis of the data was performed using SPSS version 14.0 for windows (SPSS Inc., Chicago, IL, USA). The non‐parametric Mann‐Whitney two‐tailed two‐sample test was used to perform statistical comparisons between the CNS disease groups and the control group. P values ≤.05 were considered significant. As the study population was small, we did not perform multivariate analyses to adjust for the effect of each parameter in the presence of others.
3. Results
The results of cytokine analysis are as follows: Changes in inflammatory and anti‐inflammatory cytokine levels were comparable among the groups. IL‐1β, IL‐6, and IL‐10 were significantly increased in the encephalopathy (IL‐1β: 31.8±27.7 pg/mL, IL‐6: 254.5±104.9 pg/mL, and IL‐10: 30.8±24.6 pg/mL) and bacterial meningitis (IL‐1β: 41.4±40.6 pg/mL, IL‐6: 1327.1±535.2 pg/mL, and IL‐10: 276.6±269.1 pg/mL) groups compared with the control group (IL‐1β: 0.27±0.19 pg/mL, IL‐6: 6.6±3.2 pg/mL, and IL‐10: 1.49±0.62 pg/mL). IL‐17 was increased in the bacterial meningitis group (260.1±226.2 pg/mL) compared with the control group (3.96±2.56 pg/mL). In the meningitis group, a significant increase in IL‐17 was noted in the bacterial meningitis group compared with the viral meningitis group (IL‐1β: 4.35±3.91 pg/mL, IL‐6: 38.5±37.3 pg/mL, IL‐17: 16.4±15.9 pg/mL, and IL‐10: 16.2±13.8 pg/mL) (Figure 1). Changes in cytokines in the encephalopathy group were compared among the causative viruses (Figure 2). Compared with the control group, IL‐17 was increased in viral encephalopathy group. Specifically, IL‐7 was significantly increased in the influenza‐associated encephalopathy (IAE) group (IL‐1β: 53.9±35.6 pg/mL, IL‐6: 435.7±281.2 pg/mL, IL‐17: 13.6±11.2 pg/mL, and L‐10: 23.9±11.0 pg/mL), and an increase was also noted in patients with status epilepticus. IL‐17 was significantly increased in the convulsions following gastroenteritis group (26.5±14.6 pg/mL) compared with the rotavirus encephalopathy group (5.1±4.9 pg/mL), particularly in cases of seizure clustering. Additionally, changes in chemokines were compared (Figure 3). In the meningitis groups, chemokines were increased in the bacterial meningitis group (IL‐8: 1975.7±1178.5 pg/mL, G‐CSF: 1274.9±585.7 pg/mL, CCL2: 28 791.6±9962.1 pg/mL, and CCL4: 863.5±231.8 pg/mL) compared with viral the meningitis group (IL‐8: 234.2±203.6 pg/mL, G‐CSF: 247.3±184.4 pg/mL, CCL2: 4017.3±2975.8 pg/mL, and CCL4: 32.3±25.3 pg/mL). Compared with control group (IL‐8: 14.6±9.8 pg/mL, G‐CSF: 161.3±104.1 pg/mL, CCL2: 1974.2±1092.5 pg/mL, and CCL4: 10.7±9.1 pg/mL), chemokines other than G‐CSF were significantly increased in the encephalopathy group (IL‐8: 242.7±182.8 pg/mL, G‐CSF: 178.8±104.9 pg/mL, CCL2: 6618.4±4618.1 pg/mL, and CCL4: 167.4±148.1 pg/mL). Among encephalopathy groups, IL‐8 was significantly increased in patients, irrespective of the causative virus (influenza virus: 168.5±84.5 pg/mL, RS virus: 138.8±104.9 pg/mL, HHV6: 320.4±213.3 pg/mL, and rotavirus: 164.5±120.9 pg/mL), and was marked increased in patients with a poor neurological prognosis (Pediatric Cerebral Performance Category: PCPC >2)10 (Figure 4). CCL2 was significantly increased in the influenza (10 116.6±5373.5 pg/mL) and HHV6 encephalopathy (19 205.1±7386.8 pg/mL) groups compared with the control group (1974.2±1092.5 pg/mL). G‐CSF (129.7±117.6 pg/mL) and CCL4 (263.7±255.2 pg/mL) were significantly increased in the rotavirus encephalopathy group compared with the convulsions following gastroenteritis group, and the increase in CCL4 in the rotavirus encephalopathy group was significantly higher than those of the other viral encephalopathy groups (influenza virus: 17.3±10.5 pg/mL, RS virus: 65.7±53.9 pg/mL, and HHV6: 32.9±23.2 pg/mL).
Figure 1.
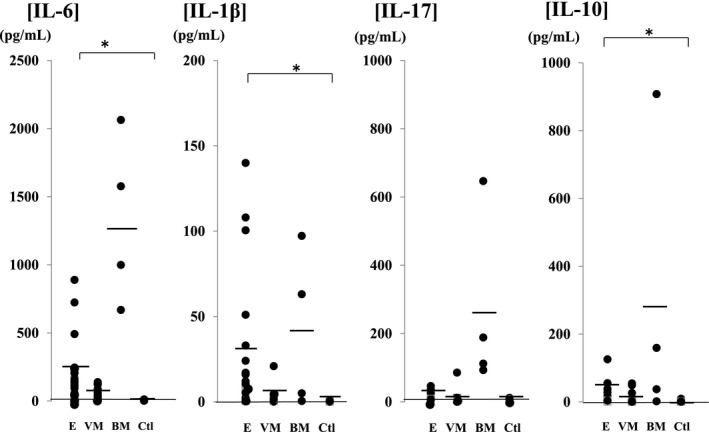
Inflammatory and anti‐inflammatory cytokine levels in cerebrospinal fluid of patients with central nervous system infections compared with the control group. IL‐1β, IL‐6, and IL‐10 were significantly increased in the encephalopathy (IL‐1β: 31.8±27.7 pg/mL, IL‐6: 254.5±104.9 pg/mL, and IL‐10: 30.8±24.6 pg/mL) and bacterial meningitis (IL‐1β: 41.4±40.6 pg/mL, IL‐6: 1327.1±535.2 pg/mL, and IL‐10: 276.6±269.1 pg/mL) groups compared with the control group (IL‐1β: 0.27±0.19 pg/mL, IL‐6: 6.6±3.2 pg/mL, and IL‐10: 1.49±0.62 pg/mL). IL‐17 was increased in the bacterial meningitis group (260.1±226.2 pg/mL) compared with the control group (3.96±2.56 pg/mL). In the meningitis group, a significant increase in IL‐17 was noted in the bacterial meningitis group compared with the viral meningitis group (IL‐1β: 4.35±3.91 pg/mL, IL‐6: 38.5±37.3 pg/mL, IL‐17: 16.4±15.9 pg/mL, and IL‐10: 16.2±13.8 pg/mL). Abbreviations: E (n=30), encephalopathy; VM (n=27), viral meningitis; BM (n=4), bacterial meningitis; Ctl (n=24), control; IL, interleukin. *P<.05
Figure 2.
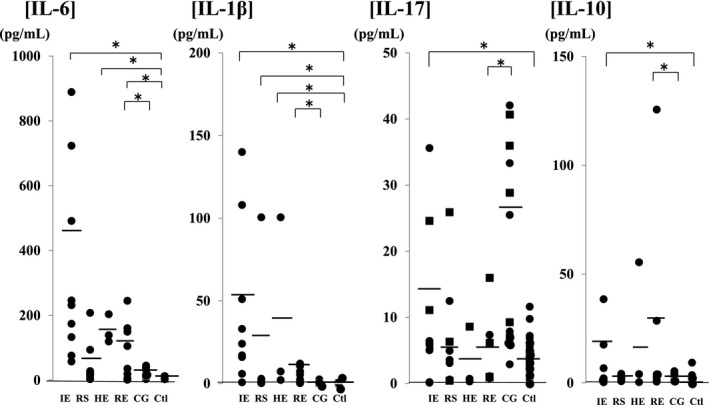
Comparison of cerebrospinal fluid inflammatory and anti‐inflammatory cytokine levels in encephalopathy groups according to the causative virus. Compared with the control group, IL‐17 was increased in viral encephalopathy group. Specifically, IL‐7 was significantly increased in the influenza‐associated encephalopathy group (IL‐1β: 53.9±35.6 pg/mL, IL‐6: 435.7±281.2 pg/mL, IL‐17: 13.6±11.2 pg/mL, and L‐10: 23.9±11.0 pg/mL), and an increase was also noted in patients with status epilepticus. IL‐17 was significantly increased in the convulsions following gastroenteritis group (26.5±14.6 pg/mL) compared with the rotavirus encephalopathy group (5.1±4.9 pg/mL), particularly in cases of seizure clustering (square mark). Abbreviations: IE (n=9), influenza virus; RS (n=10), respiratory syncytial virus; HE (n=3), human herpes virus type 6; RE (n=8), rotavirus; CG (n=12), convulsion following gastroenteritis; Ctl (n=24), control; IL, interleukin. *P<.05
Figure 3.
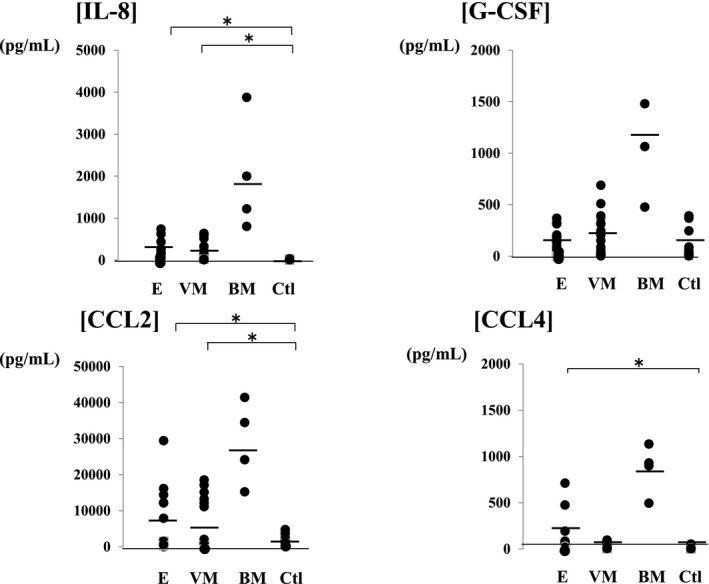
Chemokine levels in the cerebrospinal fluid of patients with central nervous system infections compared with the control group. In the meningitis groups, chemokines were increased in the bacterial meningitis group (IL‐8: 1975.7±1178.5 pg/mL, G‐CSF: 1274.9±585.7 pg/mL, CCL2: 28 791.6±9962.1 pg/mL, and CCL4: 863.5±231.8 pg/mL) compared with viral the meningitis group (IL‐8: 234.2±203.6 pg/mL, G‐CSF: 247.3±184.4 pg/mL, CCL2: 4017.3±2975.8 pg/mL, and CCL4: 32.3±25.3 pg/mL). Compared with control group (IL‐8: 14.6±9.8 pg/mL, G‐CSF: 161.3±104.1 pg/mL, CCL2: 1974.2±1092.5 pg/mL, and CCL4: 10.7±9.1 pg/mL), chemokines other than G‐CSF were significantly increased in the encephalopathy group (IL‐8: 242.7±182.8 pg/mL, G‐CSF: 178.8±104.9 pg/mL, CCL2: 6618.4±4618.1 pg/mL, and CCL4: 167.4±148.1 pg/mL). Abbreviations: E (n=30), encephalopathy; VM (n=27), viral meningitis; BM (n=4), bacterial meningitis; Ctl (n=24), control; IL, interleukin; G‐CSF, granulocyte‐colony stimulating factor; CCL2, monocyte chemoattractant protein‐1/MCAF; CCL4, macrophage inflammatory protein‐1b.
Figure 4.
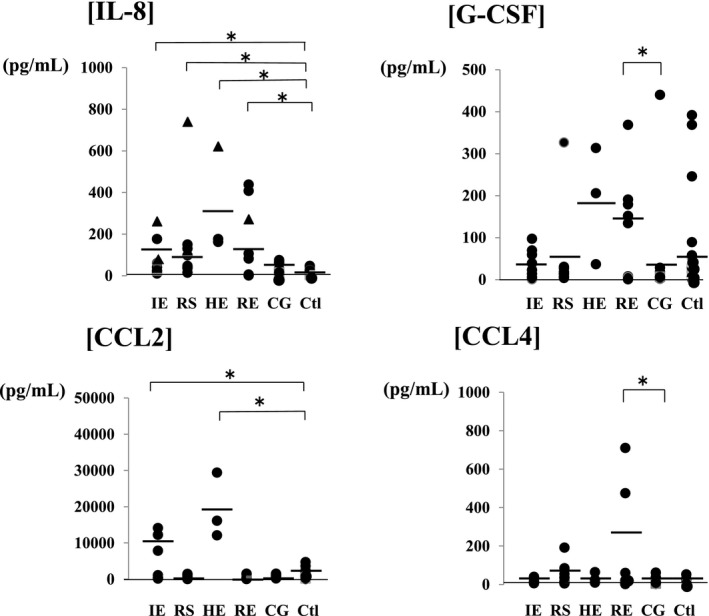
Comparison of the cerebrospinal fluid chemokine levels in encephalopathy groups according to the causative virus. Among encephalopathy groups, IL‐8 was significantly increased in patients, irrespective of the causative virus (influenza virus: 168.5±84.5 pg/mL, RS virus: 138.8±104.9 pg/mL, HHV6: 320.4±213.3 pg/mL, and rotavirus: 164.5±120.9 pg/mL), and the increase was marked with a triangle in patients with poor neurological prognosis (Pediatric Cerebral Performance Category: PCPC >2) [19]. CCL2 was significantly increased in the influenza (10 116.6±5373.5 pg/mL) and HHV6 encephalopathy (19 205.1±7386.8 pg/mL) groups compared with the control group (1974.2±1092.5 pg/mL). G‐CSF (129.7±117.6 pg/mL) and CCL4 (263.7±255.2 pg/mL) were significantly increased in the rotavirus encephalopathy group compared with the convulsions following gastroenteritis group, and the increase in CCL4 in the rotavirus encephalopathy group was significantly higher than those of the other viral encephalopathy groups (influenza virus: 17.3±10.5 pg/mL, RS virus: 65.7±53.9 pg/mL, and HHV6: 32.9±23.2 pg/mL). Abbreviations: IE (n=9), influenza virus; RS (n=10), respiratory syncytial virus; HE (n=3), human herpes virus type 6; RE (n=8), rotavirus; CG (n=12), convulsion following gastroenteritis; Ctl (n=24), control; IL, interleukin; G‐CSF, granulocyte‐colony stimulating factor; CCL2, monocyte chemoattractant protein‐1/MCAF; CCL4, macrophage inflammatory protein‐1b. *P<.05
4. Discussion
During the initial immune response to CNS infections, including acute encephalopathy, specific cells that produce interferon‐α and β and acts on the activated macrophages on antigen‐presenting cells, inducing chemokine production. IL‐8 is involved in neutrophil migration and adhesion, and CCL2 and CCL4 are involved in neutrophil accumulation in infected regions.
In this study, chemokine production, particularly IL‐8, was increased in several groups of CNS disease patients. IL‐8 mediates the activation and migration of neutrophils and plays a pivotal role in the initiation of inflammation. IL‐8 is an amino acid polypeptide that induces various effects on immune system cells, including neutrophil activation, chemotaxis, and T‐lymphocyte chemotaxis. In bacterial meningitis, IL‐8 has been shown to recruit neutrophils to the brain parenchyma, and IL‐8 induces a degranulation of neutrophils that releases chemoattractants for T lymphocytes and primes superoxide along with other potentially neurotoxic molecules.11 Monocytic chemoattraction into the CNS by chemokines, such as CCL2 and CCL4, also contributes to cellular damage through the recruitment of macrophages, and most chemokines have been found at elevated levels in the CSF of patients with bacterial meningitis.12 However, the correlation of chemokines with neurodamage is not yet fully understood in viral neuroinfections. A correlation was reported between IL‐8 levels and neutrophile counts in the CSF of patients with viral meningitis, and anti‐IL‐8 antibodies inhibited chemotactic activity.13 In this study, there was no correlation between chemokine levels and neutrophile counts in the CSF of patients with acute encephalopathy. To investigate the possible influence of the timing of specimen collection on our results, we are planning to perform a prospective investigation.
IL‐8 was increased in the CSF of patients with a poor neurological prognosis, suggesting that the action of IL‐8 on nerves varies depending on its concentration in HHV6 encephalopathy, and that IL‐8 may promote nervous system tissue damage through the same mechanism in other viral encephalopathies.
CCL4 induced cell adhesion with increased intracellular reactive oxygen species.14 The physiologic activity indicates that elevated chemokines produced in virus‐associated encephalopathy play an important role in the pathophysiology.
It has been reported that large amounts of proinflammatory cytokines, such as IL‐6 and TNF‐α, are produced in IAE patients.5, 15 In our study, comparing the cytokine profiles of children with IAE or CSF with HHV6 and Rota‐associated CNS symptoms revealed the production of CCL2 and CCL4 cytokines. These findings indicate a similar pathophysiology to that of patients with IAE in these CNS symptoms.
Since IL‐17 increased in the cytokine network activated in CNS infection in many cases with concomitant epilepticus and based on previous reports,8 IL‐17 is suggested to be involved in the pathology of encephalopathy as a convulsion‐related factor. Many previous studies reported an association between autoimmune disease, such as multiple sclerosis and Guillain‐Barre syndrome,16, 17 and IL‐17, and the marked increase in convulsions following gastroenteritis group compared with that in the rotavirus encephalopathy group suggested the involvement of an autoimmune mechanism in the development of convulsions following gastroenteritis. Regarding the pathology in which chemokines increase in encephalopathy, Arima et al.18 reported that a markedly high expression of chemokines enhances blood‐brain barrier permeability and promotes neutrophil infiltration, which may result in immune cell infiltration into the CNS induced by chemokines produced by activated macrophages.
5. Conclusion
In acute encephalopathy group, enhanced blood‐brain barrier penetration by neutrophils induced by chemokines is involved in the development of encephalopathy, and of chemokines, IL‐8 may serve as a neurological prognostic index. IL‐17 increased in convulsions following gastroenteritis group, particularly in cases with concomitant epilepticus, suggesting its involvement as a clinical convulsion‐related factor in encephalopathy. Since only a limited number of cases were investigated, it is necessary to accumulate more cases.
Morichi S, Urabe T, Morishita N, et al. Pathological analysis of children with childhood central nervous system infection based on changes in chemokines and interleukin‐17 family cytokines in cerebrospinal fluid. J Clin Lab Anal. 2018;32:e22162 10.1002/jcla.22162
References
- 1. Morishima T, Togashi T, Yokota S, et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002;35:512–517. [DOI] [PubMed] [Google Scholar]
- 2. Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenxa and other viral infections. Acta Neurol Scand. 2007;115:45–56. [DOI] [PubMed] [Google Scholar]
- 3. Kawashima H, Morichi S, Okumara A, Nakagawa S, Morishima T; Collaborating study group on influenza‐associated encephalopathy in Japan . National survey of pandemic influenza A (H1N1) 2009‐associated encephalopathy in Japanese children. J Med Virol. 2012;84:1151–1156. [DOI] [PubMed] [Google Scholar]
- 4. Ichiyama T, Suenaga N, Kajimoto M, et al. Serum and CSF levels of cytokines in acute encephalopathy following prolonged febrile seizures. Brain Dev. 2008;30:47–52. [DOI] [PubMed] [Google Scholar]
- 5. Aiba H, Mochizuki M, Kimura M, Hojo H. Predictive value of serum interleukin‐6 level in influenza virus‐associated encephalopathy. Neurology. 2001;57:295–299. [DOI] [PubMed] [Google Scholar]
- 6. Morichi S, Yamanaka G, Ishida Y, Oana S, Kashiwagi Y, Kawashima H. Brain‐derived neurotrophic factor and interleukin‐6 levels in the serum and cerebrospinal fluid of children withviral infection‐induced encephalopathy. Neurochem Res. 2014;39:2143–2149. [DOI] [PubMed] [Google Scholar]
- 7. Watanabe C, Kawashima H, Takekuma K, Hoshika A, Watanabe Y. Increased nitric oxide production and GFAP expression in the brains of influenza A/NWS virus infected mice. Neurochem Res. 2008;33:1017–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mao LY, Ding J, Peng WF, et al. Interictal interleukin‐17A levels are elevated and correlate with seizure severity of epilepsy patients. Epilepsia. 2013;54:e142–e145. [DOI] [PubMed] [Google Scholar]
- 9. Kawamura Y, Yamazaki Y, Ohashi M, Ihira M, Yoshikawa T. Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B‐associated acute encephalopathy with biphasic seizures and late reduced diffusion. J Med Virol. 2014;86:512–518. [DOI] [PubMed] [Google Scholar]
- 10. Kawano G, Iwata O, Iwata S, et al. Determinants of outcomes following acute child encephalopathy and encephalitis: pivotal effect of early and delayed cooling. Arch Dis Child. 2011;96:936–941. [DOI] [PubMed] [Google Scholar]
- 11. Ehrlich LC, Hu S, Sheng WS, et al. Cytokine regulation of human microglial cell IL‐8 production. J Immunol. 1998;160:1944–1948. [PubMed] [Google Scholar]
- 12. Lahrtz F, Piali L, Spanaus KS, Seebach J, Fontana A. Chemokines and chemotaxis of leukocytes in infectious meningitis. J Neuroimmunol. 1998;85:33–43. [DOI] [PubMed] [Google Scholar]
- 13. Ishiguro A, Suzuki Y, Inaba Y, et al. The production of IL‐8 in cerebrospinal fluid in aseptic meningitis of children. Clin Exp Immunol. 1997;109:426–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tatara Y, Ohishi M, Yamamoto K, et al. Macrophage inflammatory protein‐1beta induced cell adhesion with increased intracellular reactive oxygen species. J Mol Cell Cardiol. 2009;47:104–111. [DOI] [PubMed] [Google Scholar]
- 15. Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S. Tumor necrosis factor‐alpha, interleukin‐1 beta, and interleukin‐6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology. 1998;50:407–411. [DOI] [PubMed] [Google Scholar]
- 16. Raveney BJ, Oki S, Yamamura T. Nuclear receptor NR4A2 orchestrates Th17 cell‐mediated autoimmune inflammation via IL‐21 signalling. PLoS ONE. 2013;8:e56595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li S, Yu M, Li H, Zhang H, Jiang Y. IL‐17 and IL‐22 in cerebrospinal fluid and plasma are elevated in Guillain‐Barré syndrome. Mediators Inflamm. 2012;2012:260473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arima Y, Harada M, Kamimura D, et al. Regional neural activation defines a gateway for autoreactive T cells to cross the blood‐brain barrier. Cell. 2012;148:447–457. [DOI] [PubMed] [Google Scholar]


