Abstract
Background
The benefits of using serum markers to diagnose stages of liver disease in chronic hepatitis B (CHB) patients are controversial. We conducted a study to compare the clinical significance of four markers in evaluating liver inflammation and fibrosis in CHB patients.
Methods
A total of 323 treatment‐naive CHB patients who received a liver biopsy and routine laboratory testing were enrolled in our study. We used the Scheuer scoring system as a pathological standard for diagnosing liver inflammation and fibrosis. The diagnostic performance of the fibrosis index based on four factors (FIB‐4), the aspartate transaminase to platelet ratio index (APRI), the gamma‐glutamyl transpeptidase‐to‐platelet ratio (GPR), and the red cell distribution width‐platelet ratio (RPR) were analyzed with receiver‐operating characteristic curves (ROC).
Results
No significant differences among the four indexes for diagnosing significant fibrosis (S ≥ 2) was found, while APRI and GPR were superior to FIB‐4 and RPR in diagnosing moderate (G ≥ 2), severe (G ≥ 3) inflammation, and severe fibrosis (S ≥ 3). The AUROCs for diagnosing G ≥ 2 and G ≥ 3 were 0.732 and 0.861 for APRI, 0.726 and0.883 for GPR, 0.703 and0.705 for FIB‐4, and 0.660 and 0.747 for RPR, respectively. The AUROCs for diagnosing S ≥ 2 and S ≥ 3 were0.724 and 0.799 for APRI, 0.714 and0.801 for GPR, 0.683 and0.730 for FIB‐4, and 0.643 and 0.705 for RPR, respectively.
Conclusion
APRI and GPR were more effective than FIB‐4 and RPR at diagnosing liver inflammation and fibrosis.
Keywords: chronic hepatitis B, laboratory test, liver fibrosis, liver inflammation, noninvasive
1. INTRODUCTION
Hepatitis B virus (HBV) infection could result in different clinical outcomes that are determined by virological and immunological factors.1Use of antiviral treatment suppresses HBV replication and contributes to fibrosis and cirrhosis regression. Histological examination of liver tissue can determine the degree of fibrosis in patients with chronic hepatitis B. As such, fibrosis staging can assist patients in evaluating HBV disease progression and in deciding on treatment strategies.2, 3, 4 Therefore, early and accurate diagnosis of liver inflammation and fibrosis could benefit patients with HBV infection.
Liver biopsy with histopathology is the gold standard for diagnosing liver disease. However, liver biopsy is invasive and is coupled with the risk of bleeding or anesthetic complications and of fibrosis stage misclassification due to sampling errors. Staging fibrosis and inflammation using noninvasive methods have been designed to overcome the disadvantages and inconveniences of liver biopsy.5, 6, 7 Clinical practice needs a simple operation or a noninvasive and easy way to diagnose liver inflammation, injury, or fibrosis.7 Serologic tests and FibroScan have been recommended by the World Health Organization (WHO) as noninvasive tests for CHB patients.8 However, FibroScan is costly and has been shown to be unfavorable in patients with ascites.9 Serum markers have been proposed to evaluate liver disease including alanine aminotransferase (ALT), the aspartate aminotransferase (AST)‐to‐platelet ratio index (APRI), the fibrosis index based on the four factors (FIB‐4), the red cell volume distribution width (RDW)‐to‐platelet ratio (RPR), and the gamma‐glutamyl transpeptidase (GGT)‐to‐platelet ratio (GPR).
Alanine aminotransferase is the most common serum marker for evaluating liver injury. Recently, however, the role of ALT in predicting liver inflammation has been questioned. Some patients with moderate liver disease identified on histology can have a normal‐level ALT.10, 11 Studies showed that even when patients have a normal ALT, they can still have a significantly increased risk of liver disease or progression of liver disease.12
Increasingly, researchers focus on finding accurate formulas for predicting liver inflammation and fibrosis by combining laboratory tests. APRI and FIB‐4 have been used extensively to diagnose HBV‐related disease. The World Health Organization (WHO) recommended FIB‐4 and APRI as noninvasive tests for CHB patients. However, the sensitivity and specificity of these calculations are still controversial.13, 14
Recently, Lemoine et al proposed that GPR was a new and more accurate index than APRI or FIB‐4, which was supported by another study on a population of Chinese subjects.15, 16 In contrast, another group determined that GPR was not superior to APRI or FIB‐4.17 More research is necessary to determine whether GPR is better than APRI or FIB‐4 in diagnosing HBV‐related liver diseases. Chen et al18 proposed that RPR was superior to APRI and FIB‐4; however, in another study, the area under receiver‐operating characteristic curves (AUROC) was similar to FIB‐4 and APRI for diagnosing significant fibrosis.19
In addition, our previous studies focused on the genetic factors and the immune regulatory factors in patients with chronic infection. The association between immune cells and serum markers was found in our studies.20, 21, 22 Here, we are concerned about how serum markers represented the degree of inflammation in liver. Although numerous studies have attempted to compare different formulas in diagnosing fibrosis in patients with CHB, the studies on evaluating the application of APRI, FIB‐4, GPR, and RPR in diagnosing HBV‐related inflammation and fibrosis are still limited. The aim of our study was to evaluate the clinical significance of the above four formulas in diagnosing liver inflammation and fibrosis in patients with CHB.
2. MATERIALS AND METHODS
2.1. Patients
Our retrospective study included 323 patients with CHB from the West China Hospital of Sichuan University between June 2015 and December 2016. According to the Asian‐Pacific clinical practice guidelines on HBV23 and the American Association for the Study of Liver Diseases guidelines,24 chronic HBV infection is defined as having an HBsAg seropositive status for 6 months or beyond. All patients underwent liver biopsy and had routine laboratory assessments at the time of liver biopsy. The inclusion criteria were as follows: (i) age ≥ 18 years; (ii) HBsAg positivity for more than 6 months, no previous anti‐HBV treatment; (iii) no evidence related to other liver diseases; (iv) patients with reliable liver biopsy results and sufficient clinical information. The exclusion criteria were as follows: (i) co‐infection with any other viruses, including hepatitis A virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, human immunodeficiency virus, and syphilis; (ii) patients with liver cirrhosis, decompensated liver disease, hepatocellular carcinoma, or any other type of cancer; (iii) patients suffering from any other type of liver disease, such as autoimmune disease, primary biliary cirrhosis, toxic hepatitis, nonalcoholic steatohepatitis, or alcoholic liver disease; and (iv) patients who are pregnant, who are alcoholics or abusers, or who have hematological diseases or other diseases that could interfere with liver function tests. Written informed consent was obtained prior to enrollment from all subjects.
2.2. Liver biopsy
Liver biopsies were performed as described elsewhere.25 Histological grading of inflammation (G0‐G4) and staging of liver fibrosis (S0‐S4) were carried out following the Scheuer scoring system.7 None to mild inflammation was defined as G 0‐1; moderate inflammation was defined as G = 2; and severe inflammation was defined as G = 3. Patients with significant fibrosis were defined as S = 2; severe fibrosis was defined as S = 3; and early cirrhosis was defined as S = 4.
2.3. Serological tests
All patients had routine laboratory blood tests at the time of biopsy and based on manufacturer's instructions. Serum markers included ALT, AST, GGT, albumin (ALB), and glucose (GLU) were determined by auto‐analyzer (Roche, Germany). Blood routine test was performed using an automated hematology analyzer (Sysmex, Japan). Serological markers (HBsAg, anti‐HBs, HBeAg, anti‐HBe, anti‐HBc) were measured using commercially available kits (Roche, Germany). HBV‐DNA was measured using the real‐time polymerase chain reaction assay (Roche, Germany).
Formulas for FIB‐4, APRI, RPR, and GPR are as follows:
Note: ULN = upper limit of normal; the ULN of AST was 40 IU/L for females and 35 IU/L for males; the ULN of GGT was 45 IU/L for both females and males.
2.4. Statistical analyses
Data were analyzed by SPSS software (version 23.0). Comparison between groups was performed using Student's t test, ANOVA analysis, or the Kruskal‐Wallis test. Univariate logistic regression analysis and multiple logistic regression analysis were conducted to identify independent predictors of disease progression. Correlation analysis was carried out using Spearman's test.
The diagnostic performances of APRI, FIB‐4, RPR, GPR, ALT, and GGT to diagnose liver inflammation and fibrosis were estimated by the ROC curve, and optimal cut‐offs were determined using Youden's index. The AUROC, sensitivity, and specificity of each noninvasive test were analyzed using SPSS software (version 23.0); ROC curves were compared using MedCalc (version 11.4).
3. RESULTS
3.1. Baseline characteristics of patients
Three hundred twenty‐three CHB patients were enrolled in our study between June 2015 and December 2016. Of the 323 patients, the majority of patients had minimal histological changes on liver biopsy. Only 87 (27%) patients had moderate to severe inflammation (G ≥ 2), and 70 (21.7%) patients had significant to severe fibrosis (S ≥ 2). The majority of patients enrolled were male (65.3%) and HBeAg negative (58.8%). The median age for G 0‐1, G 2‐3, S 0‐1, and S 2‐4 were 34, 39, 35, and 39 years, respectively (Table 1).
Table 1.
The clinical information description of all patients
| Inflammatory activity | Fibrosis stage | |||||
|---|---|---|---|---|---|---|
| G0‐1 (n = 239) | G2‐3 (n = 84) | P value | S0‐1 (n = 252) | S2‐4 (n = 70) | P value | |
| Age (y) | 34.0 (27.0‐41.0) | 39.0 (30.0‐44.3) | >.05 | 35.0 (27.0‐41.0) | 39.0 (30.0‐43.8) | >.05 |
| ALT (IU/L) | 33.0 (21.0‐46.0) | 46.0 (30.0‐64.0) | <.05 | 35.0 (23.0‐49.0) | 46.0 (25.0‐68.5) | <.05 |
| AST (IU/L) | 28.0 (23.0‐34.5) | 35.0 (28.0‐47.0) | >.05 | 28.5 (23.0‐36.0) | 35.0 (27.5‐48.5) | >.05 |
| GGT (IU/L) | 16.0 (12.0‐24.0) | 23.0 (14.0‐36.0) | <.05 | 16.0 (12.0‐24.0) | 23.0 (14.0‐36.5) | <.05 |
| HBV DNA (log10 copies/mL) | 4.8 (3.5‐7.6) | 5.4 (3.8‐6.9) | >.05 | 4.9 (3.6‐7.7) | 5.3 (3.7‐6.6) | >.05 |
| WBC (109/L) | 5.4 (4.6‐6.7) | 5.3 (4.3‐6.4) | >.05 | 5.4 (4.7‐6.7) | 5.4 (4.4‐6.3) | >.05 |
| PLT (109/L) | 163 (130‐196) | 122 (97‐167) | <.05 | 162 (129‐195) | 120 (100‐163) | <.05 |
| Monocytes (%) | 6.4 (5.2‐7.5) | 6.8 (5.8‐8.0) | <.05 | 6.5 (5.3‐7.6) | 6.6 (5.5‐9.0) | >.05 |
| RDWCV | 13.0 (12.5‐13.7) | 13.1 (12.7‐13.8) | >.05 | 13.0 (12.5‐13.6) | 13.2 (12.6‐13.9) | >.05 |
| PT(s) | 11.6 (11.0‐12.1) | 11.7 (11.1‐12.4) | >.05 | 11.6 (11.0‐12.1) | 11.8 (11.2‐12.4) | >.05 |
| Inflammation grade (G0/G1/G2/G3) | 4 (1.2%)/235 (71.8%)/69 (21.4%)/18 (5.6%) | |||||
| Fibrosis stage (S0/S1/S2/S3/S4) | 35 (10.8%)/217 (67.2%)/39 (12.1%)/23 (7.1%)/8 (2.5%) | |||||
| HBeAg positive (%) | 133 (41.2%) | |||||
| Male (%) /Female (%) | 213 (65.3%)/110 (34.1%) | |||||
Data were expressed as median (quartile range) and compared with Kruskal‐Wallis H test, P < .05 considered statistical significance.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transpeptidase; PLT, platelet count; WBC, white‐blood cell; PT, prothrombin time; RDW, red cell distribution width.
Laboratory test results for the different groups are shown in Table 1. In general, compared with patients with G 0‐1 inflammation, those with G ≥ 2 inflammation had higher ALT and GGT levels but lower platelet (PLT) counts. Higher levels of ALT and GGT and lower PLT counts were also observed in patients with S ≥ 2 fibrosis (Table 1).
3.2. Correlations between serum markers or formulas and liver inflammation grades
Spearman's correlation analysis was performed to evaluate the correlation between serum markers and inflammatory grades. The levels of ALT (rs = 0.272, P < .05) and GGT (rs = 0.276, P < .05), the percentage of monocytes (rs = 0.172, P < .05), FIB‐4 (rs = 0.306, P < .05), APRI (rs = 0.397, P < .05), GPR (rs = 0.276, P < .05), and RPR (rs = 0.289, P < .05) were positively correlated with the liver inflammation grades. Increased inflammation correlated with a higher serum marker levels.
Of the patients with normal ALT levels (ALT < 40 IU/L), 31 (18.7%) of 166 patients had moderate to severe liver inflammation. In addition, 60 (24.2%) patients with G ≥ 2 had an ALT level less than 80 IU/L.
The Kruskal‐Wallis test showed that the markers mentioned above were significantly increased from G 0‐1 to G 3. GGT increased almost two‐fold in patients with severe inflammation (G = 3) over patients with none or mild inflammation. Multivariable analyses identified GGT (OR = 1.031, 95% CI = 1.015‐1.048; P < .05) and PLT (OR = 0.986, 95% CI = 0.980‐0.993; P < .05) as independent predictors of moderate to severe liver inflammation.
Interestingly, scores for predicting fibrosis, GPR and APRI, were also much higher in patients with G3 inflammation compared with patients with G0‐1 inflammation (Figure 1A, B).
Figure 1.
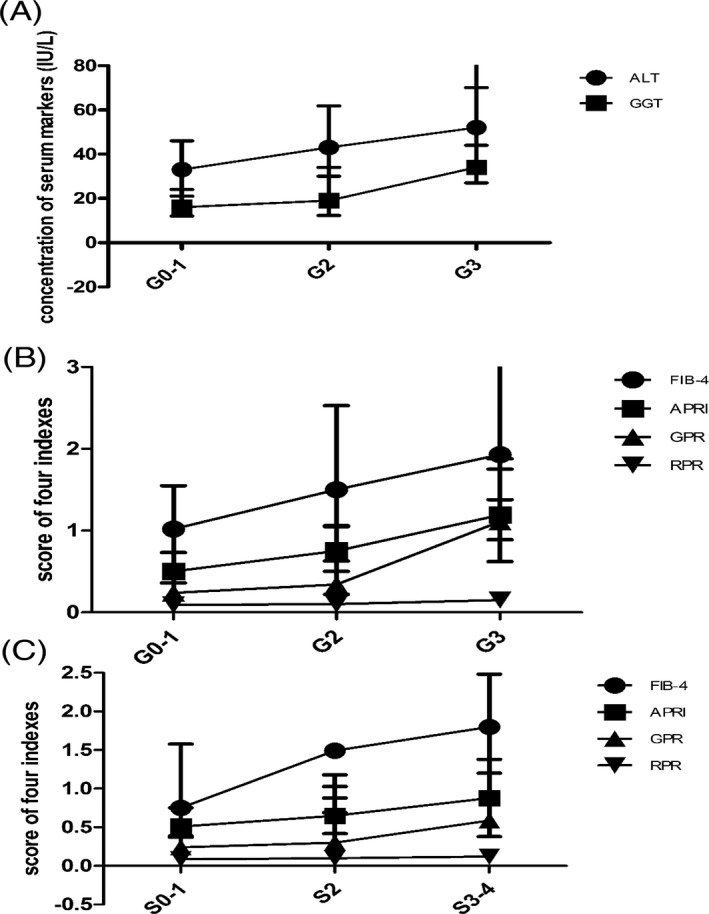
Discount graph of ALT, GGT, APRI, FIB‐4, GGT and RPR in different groups according to Scheuer scoring system. A, Concentration of ALT and GGT in liver inflammation grades. B, Scores of FIB‐4, APRI, GPR, and PRP in different inflammation grades. C, Scores of FIB‐4, APRI, GPR, and PRP in different fibrosis stages
3.3. Correlations between serum markers or formulas and liver fibrosis stages
Spearman's correlation analysis showed that ALT (rs = 0.168, P < .05), AST (rs = 0.234, P < .05), GGT (rs = 0.246, P < .05), and PLT (rs = ‐0.220, P < .05) were correlated with fibrosis stages.
Multivariable analysis showed GGT (OR = 1.027, 95% CI = 1.012‐1.043; P < .05) and PLT (OR = 0.986, 95% CI = 0.979‐0.993; P < .05) were independent predictors of significant to severe fibrosis.
Compared with S0‐1 patients, those with significant fibrosis to early cirrhosis (S ≥ 3) had higher FIB‐4, APRI, GPR, and RPR scores. The four markers increased with fibrosis progression. In other words, higher index scores were seen with increasing fibrosis stages (Figure 1C).
3.4. Performances of FIB‐4, APRI, GPR, and RPR for the diagnosis of liver inflammation
Alanine aminotransferase is the one of the most commonly used measures of liver inflammation. GGT is considered as an independent predictor of moderate to severe liver inflammation. FIB‐4, APRI, GPR, and RPR are noninvasive markers used in the diagnosis of liver fibrosis. We compared these indexes to assess their ability to correctly diagnosis liver inflammation.
All indexes could predict liver inflammation. The optimal cut‐off value was determined by Youden's index. The AUROCs, sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV), positive likelihood ratios (PLR), and negative likelihood ratios (NLR) are summarized in Table 2 and Figure 2.
Table 2.
The diagnostic performance of markers for predicting liver inflammatory
| AUROC | Cut‐off | Sensitivity (%) | Specificity (%) | PLR | NLR | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|
| FIB‐4 | ||||||||
| G ≥ 2 | 0.703 | 1.23 | 71.8 | 60.2 | 1.82 | 0.46 | 40.5 | 85.5 |
| G ≥ 3 | 0.705 | 1.34 | 80.0 | 60.7 | 2.01 | 0.33 | 10.6 | 98.1 |
| APRI | ||||||||
| G ≥ 2 | 0.732 | 0.77 | 56.3 | 79.8 | 3.04 | 0.50 | 53.0 | 84.5 |
| G ≥ 3 | 0.861 | 0.84 | 92.3 | 80.1 | 4.25 | 0.17 | 20.0 | 99.0 |
| GPR | ||||||||
| G ≥ 2 | 0.726 | 0.44 | 48.6 | 84.9 | 3.21 | 0.61 | 53.8 | 82.0 |
| G ≥ 3 | 0.883 | 0.48 | 80.0 | 81.6 | 4.34 | 0.25 | 20.3 | 98.6 |
| RPR | ||||||||
| G ≥ 2 | 0.660 | 0.090 | 66.7 | 61.5 | 1.73 | 0.54 | 37.4 | 84.6 |
| G ≥ 3 | 0.747 | 0.112 | 73.3 | 77.2 | 3.21 | 0.35 | 14.3 | 98.2 |
| GGT | ||||||||
| G ≥ 2 | 0.654 | 26.5 | 42.5 | 84.4 | 2.73 | 0.68 | 50.0 | 80.0 |
| G ≥ 3 | 0.871 | 33.5 | 66.7 | 87.1 | 5.04 | 0.38 | 22.7 | 97.8 |
| ALT | ||||||||
| G ≥ 2 | 0.671 | 23.5 | 90.3 | 31.2 | 1.32 | 0.30 | 32.8 | 89.9 |
| G ≥ 3 | 0.754 | 43.5 | 80.0 | 67.6 | 2.43 | 0.30 | 12.4 | 98.3 |
APRI, aspartate transaminase to platelet ratio index; FIB‐4, fibrosis index based on the 4 factors; GPR, gamma‐glutamyl transpeptidase to platelet ratio index; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; RPR, red cell volume distribution width‐to‐platelet ratio.
Figure 2.
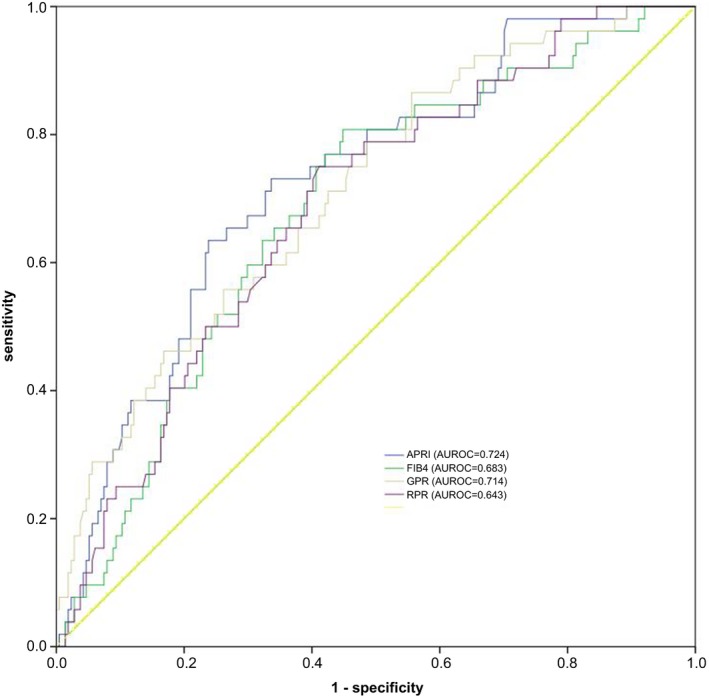
ROC curves of fibrosis scores (APRI, FIB‐4, GPR, and RPR) for diagnosing S ≥ 2
No significant differences were seen between the AUROCs of ALT and GGT for predicting G ≥ 2 inflammation. The cut‐off values for ALT and GGT for predictive G ≥ 2 were 23.5 IU/L and 26.5 IU/L, respectively, which were lower than the upper limit of normal level. Compared with ALT and GGT, the AUROC of APRI was superior in predicting patients with G ≥ 2 inflammation. The optimal cut‐off value of APRI for diagnosing G ≥ 2 inflammation was 0.77, the corresponding sensitivity, specificity, PLR, NLR, PPV, and NPV were 56.3%, 79.8%, 3.04, 0.50, 53.0, and 84.5, respectively.
To predict severe inflammation (G ≥ 3), the AUROCs for all the markers were increased. The AUROCs of GPR and APRI increased from 0.726 to 0.883 and from 0.732 to 0.861, respectively.
Among those markers, AUROCs of GPR and APRI were superior to those of FIB‐4, RPR, ALT, and GGT in predicting liver inflammation.
3.5. Performance of FIB‐4, APRI, GPR, and RPR for diagnosing moderate or severe fibrosis in CHB patients
To diagnose S ≥ 2 liver fibrosis, the AUROCs were 0.683 for FIB‐4, 0.727 for APRI, 0.711 for GPR, and 0.686 for RPR. However, the AUROC differences among all the markers were not significant. The cut‐off values derived from Youden's index for predicting significant fibrosis were 1.13 for FIB‐4, 0.52 for APRI, 0.217 for GPR, and 0.090 for RPR, respectively.
Similarly, to diagnose S ≥ 3 liver fibrosis, the AUROCs increased from 0.683 to 0.730 for FIB‐4, from 0.724 to 0.799 for APRI, from 0.714 to 0.801 for GPR, and from 0.643 to 0.705 for RPR. Among them, GPR and APRI were superior to FIB‐4 and RPR (Table 3 and Figure 3).
Table 3.
The diagnostic performance of markers for significant fibrosis and severe fibrosis in CHB
| AUROC | Cut‐off | Sensitivity (%) | Specificity (%) | PLR | NLR | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|
| FIB‐4 | ||||||||
| S ≥ 2 | 0.683 | 1.13 | 81.1 | 55.3 | 1.82 | 0.34 | 30.9 | 92.2 |
| 1.45 | 58.5 | 70.2 | 1.96 | 0.59 | 32.6 | 87.3 | ||
| 3.25 | 7.5 | 95.8 | 1.80 | 0.96 | 30.8 | 80.8 | ||
| S ≥ 3 | 0.730 | 1.15 | 95.7 | 53.1 | 2.04 | 0.082 | 16.1 | 99.2 |
| APRI | ||||||||
| S ≥ 2 | 0.724 | 0.52 | 80.8 | 52.8 | 1.67 | 0.37 | 29.1 | 91.7 |
| 1.5 | 13.2 | 94.9 | 2.59 | 0.91 | 38.9 | 81.7 | ||
| S ≥ 3 | 0.799 | 0.69 | 86.4 | 66.9 | 2.58 | 0.2 | 19.4 | 98.2 |
| GPR | ||||||||
| S ≥ 2 | 0.714 | 0.217 | 86.5 | 44.7 | 1.56 | 0.30 | 27.3 | 93.3 |
| S ≥ 3 | 0.801 | 0.374 | 73.9 | 72.8 | 2.71 | 0.36 | 20.2 | 96.8 |
| RPR | ||||||||
| S ≥ 2 | 0.643 | 0.090 | 70.7 | 60.4 | 1.79 | 0.49 | 29.7 | 89.7 |
| S ≥ 3 | 0.705 | 0.098 | 73.1 | 67.5 | 2.25 | 0.40 | 17.4 | 96.4 |
APRI, aspartate transaminase to platelet ratio index; FIB‐4, fibrosis index based on the 4 factors; GPR, gamma‐glutamyl transpeptidase to platelet ratio index; NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value; RPR, red cell volume distribution width‐to‐platelet ratio.
Figure 3.
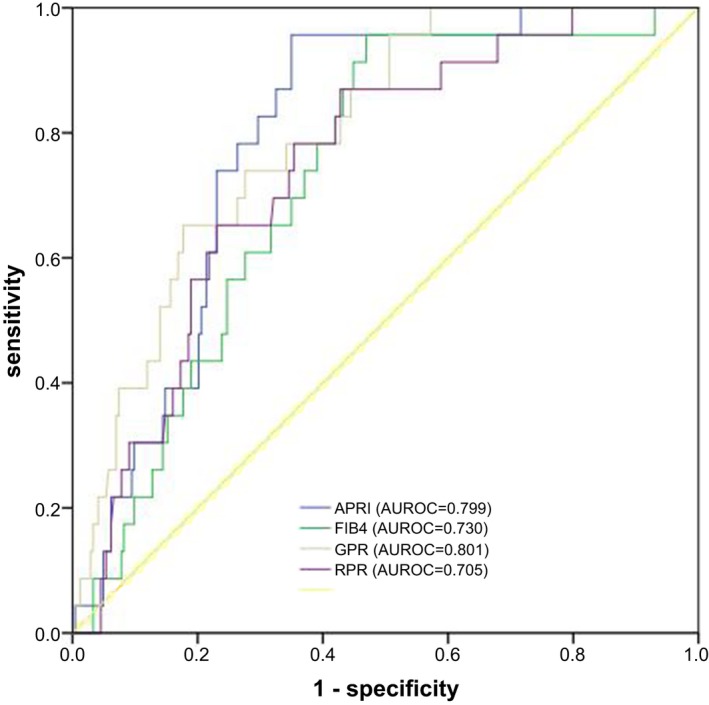
ROC curves of fibrosis scores (APRI, FIB‐4, GPR, and RPR) for diagnosing S ≥ 3
Using cut‐off value of FIB‐4 (1.45), recommended by WHO, for predicting significant to advance fibrosis, the sensitivity (8.70%) was lower but with higher specificity (95.5%). In our study, the optimal cut‐off value of APRI (0.5) to predict significant to severe fibrosis was similar to that recommended by WHO.
4. DISCUSSION
In our study, we validated the performance of FIB‐4, APRI, GPR, and RPR in diagnosing liver inflammation and fibrosis. We showed that these markers are potentially useful in predicting liver inflammation and fibrosis. APRI and GPR might be useful in diagnosing HBV‐related liver diseases. No significant differences were seen in the performance of APRI, GPR, FIB‐4, and RPR to diagnose S ≥ 2 liver fibrosis, but APRI and GPR were superior to the other markers in diagnosing S ≥ 3 liver fibrosis and in diagnosing G ≥ 2 and G ≥ 3 liver inflammation.
The risk of developing HCC is higher in patients with chronic hepatitis,29 and therefore, it is important to identify patients with liver inflammation and fibrosis so that treatment and HCC surveillance can be started. ALT has been used as a reliable marker for liver inflammation30; GGT has also been used for the diagnosis of HBV‐related inflammation. However, ALT is not always increased when liver inflammation is present.31, 32, 33 Michelle et al showed that patients with an HBV‐DNA > 10 000 copies/mL, age > 40 years, and ALT > 25 IU/L had an increased risk for HBV‐related liver disease progression.34
Our study found that both ALT and GGT increased gradually from G0 to G3. Nevertheless, some patients with normal ALT levels (ALT ≤ 40 IU/L) had moderate to severe liver inflammation. When patients had an ALT < 80 IU/L, 24.2% had histological changes in the liver biopsies, and a small proportion of CHB patients had normal ALT with chronic liver inflammation. Using ALT alone is likely not enough to predict disease progression. In addition, the AUROCs of ALT and GGT for predicting G ≥ 2 were only 0.671 and 0.654, respectively. We found the optimal cut‐off value of ALT for a G ≥ 2 inflammation diagnosis was 23.5 IU/L, and the sensitivity and specificity were 90.3% and 31.2%. Moreover, the optimal cut‐off value of GGT for a G ≥ 2 inflammation diagnosis was 26.5 IU/L, which was lower than the normal level of 45 IU/L in healthy people. At the same time, our results demonstrate that patients with increased GGT and decreased PLT might have an elevated risk of liver inflammation and fibrosis. Therefore, the indexes (APRI, FIB‐4, GRP, and RPR) related to GGT serum levels and PLT counts should be followed in patients with CHB, especially when the GGT or PLT counts persistently change.
GPR was calculated by GGT and PLT and first be reported in 2015. It was considered a novel and accurate index for liver fibrosis diagnosis compared with APRI and FIB‐4 in West Africa, but lower AUROCs in Chinese CHB patients.16, 35 We showed that GPR could be used to not only diagnose different stages of fibrosis but could also diagnosis different grades of inflammation. The AUROC of GPR was much higher than that of GGT and ALT in patients with G ≥ 2 inflammation, and using a cut‐off value of 0.44, the sensitivity and specificity were 48.6% and 84.9%, respectively. Moreover, the AUROC increased to even more when GPR was used to diagnose G ≥ 3 inflammation. In these cases, the sensitivity was 80.0% when we chose 0.48 as the optimal cut‐off value. To predict the stages of liver fibrosis in CHB patients, GPR was comparable with other indexes in the diagnosis of S ≥ 2 fibrosis. However, the AUROC of GPR was significantly higher than that of FIB‐4 and RPR in the diagnosis of S ≥ 3 fibrosis. The cut‐off values of GPR in our study (0.217 for S ≥ 2; 0.374 for S ≥ 3) were similar to a study by Lemoine et al16 (0.32 for S ≥ 2; 0.32 for S ≥ 3). Nevertheless, the cut‐off values in another Chinese study (0.61 for S ≥ 2; 0.65 for S ≥ 3) were higher than in our study.35 The cut‐off value of GPR was 0.217 to diagnose S ≥ 2 fibrosis, and the corresponding sensitivity, specificity, PPV, and NPV were 86.5%, 44.7%, 27.3%, and 93.3%, respectively. Evidently, GPR is likely useful at excluding moderate to severe liver inflammation and fibrosis.
Since APRI and FIB‐4 were successfully used in to diagnose patients with HCV,6, 26 the success of these indexes has also been demonstrated in HBV patients. However, inconsistencies lie in both the accuracy and cut‐off values for determining the different fibrosis stages. Bonnard et al found that there was no difference in predicting S ≥ 2 fibrosis using FIB‐4 and APRI. However, Liu et al suggested APRI had a lower diagnostic accuracy than FIB‐4 through meta‐analysis.36, 37 Our results indicated that the two formulas had similar diagnostic accuracy in predicting S ≥ 2 fibrosis, while APRI was superior to FIB‐4 in predicting S ≥ 3 fibrosis. The optimal cut‐off value of APRI for diagnosis of significant fibrosis was 0.52, which was much closer to the low cut‐off value recommended by WHO (0.5 for significant fibrosis). With a cut‐off value of 0.52 for the diagnosis S ≥ 2 fibrosis, the sensitivity and specificity were 80.8% and 52.8%. Another cut‐off value recommended by WHO was 1.5 for the diagnosis of S ≥ 2 fibrosis, which would result in a higher specificity and lower specificity results. In our cohort, changing the cut‐off value to 1.5 increased the specificity to 94.9%. We also found that APRI might be helpful in the diagnosis of liver inflammation; the AUROCs of APRI were 0.732 for G ≥ 2 and 0.861 for G ≥ 3 inflammation. Especially with the cut‐off value of 0.84 for diagnosis G ≥ 3 inflammation, the sensitivity and specificity were significantly higher than those of ALT and FIB‐4.
Chen et al proposed that RPR was superior to FIB‐4 and APRI in estimating liver fibrosis stages, and Lee et al19 reported RPR was comparable to FIB‐4 in the Korean population. We found that RPR did not have an advantage over FIB‐4, APRI, and GPR in the diagnosis of fibrosis. The AUROCs of RPR was 0.634 for diagnosis S ≥ 2 fibrosis and 0.705 for diagnosis S ≥ 3 fibrosis. With optimal cut‐off values for diagnosis of S ≥ 2 and S ≥ 3 fibrosis, we found that the NPV was 89.7% and 96.4%, and the PPV was 29.7%, 17.4%, respectively. RPR could also be used for diagnosis of inflammation; however, its performance was no better than that of the other indexes. Although the RPR was similar to the FIB‐4, researchers proposed a RPR‐HBV DNA algorithm to improve the diagnostic accuracy of the RPR for liver fibrosis.8 Therefore, a more accurate system for diagnosis of liver fibrosis could create by combining markers of liver disease.
The discrepancies among the four markers for the diagnosis of liver diseases could be due to the heterogeneity of patients enrolled, the prevalence of liver fibrosis stages, the different scoring systems used in pathological diagnosis of liver diseases, and genetic factors.
However, our study still has several limitations. First, our study included a small number of patients with S3‐4 and G3, which might not be enough to develop a novel predictive model for diagnosing liver inflammation and fibrosis. Second, our study was retrospective and patients were recruited from a single‐center, in which some selection bias could exist. Third, until now it has been demonstrated that liver stiffness measured by transient elastography (TE) or FibroScan could be used as a reliable marker for liver fibrosis diagnosis. Although FibroScan is not suitable for application in patients with ascites and elevated aminotransferase levels, the AUROCs for diagnosis of fibrosis were much higher than the serum markers.9, 38, 39 However, since there was insufficient information from the FibroScan, we did not make an analyze of FibroScan performance in diagnosis of liver fibrosis. In the future, we plan to expand the number of patients enrolled and develop a more accurate model for diagnosis liver inflammation and fibrosis by combining serum markers and FibroScan results. Furthermore, we will also verify the significance of serum markers dynamic change in liver diseases progression and regression.
In conclusion, our study showed that serum ALT is not robust enough to diagnose liver inflammation on its own. APRI and GPR appear to be better markers to diagnose HBV‐related liver inflammation and fibrosis. No difference of FIB‐4, APRI, GPR, and RPR to predict patients with S ≥ 2 fibrosis was observed. GPR and APRI were superior to RPR and FIB‐4 in being able to identify patients with S ≥ 3 fibrosis and patients with G ≥ 2 and G ≥ 3 inflammation. From this study, it appears that currently, the GPR and the APRI might be the most useful markers to monitor liver disease progression and to decide on treatment in patients with CHB.
Wu X, Cai B, Su Z, et al. Aspartate transaminase to platelet ratio index and gamma‐glutamyl transpeptidase‐to‐platelet ratio outweigh fibrosis index based on four factors and red cell distribution width‐platelet ratio in diagnosing liver fibrosis and inflammation in chronic hepatitis B. J Clin Lab Anal. 2018;32:e22341 10.1002/jcla.22341
Xiaojuan Wu and Bei Cai contributed equally to this work.
REFERENCES
- 1. Shin EC, Sung PS, Park SH. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat Rev Immunol. 2016;16:509‐523. [DOI] [PubMed] [Google Scholar]
- 2. Kim WR, Berg T, Asselah T, et al. Evaluation of APRI and FIB‐4 scoring systems for non‐invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773‐780. [DOI] [PubMed] [Google Scholar]
- 3. Marcellin P, Gane E, Buti M, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5‐year open‐label follow‐up study. Lancet. 2013;381:468‐475. [DOI] [PubMed] [Google Scholar]
- 4. Anonymous . EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370‐398 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 5. Singh S, Muir AJ, Dieterich DT, Falck‐Ytter YT. American Gastroenterological Association Institute Technical Review on the Role of Elastography in Chronic Liver Diseases. Gastroenterology. 2017;152:1544‐1577. [DOI] [PubMed] [Google Scholar]
- 6. Li J, Gordon SC, Rupp LB, et al. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930‐937. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Z, Wang G, Kang K, Wu G, Wang P. The Diagnostic Accuracy and Clinical Utility of Three Noninvasive Models for Predicting Liver Fibrosis in Patients with HBV Infection. PLoS ONE. 2016;11:e0152757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YP, Hu XM, Liang XE, et al. Stepwise Application of FIB‐4, Red cell distribution width‐Platelet Ratio and APRI for Compensated Hepatitis B Fibrosis Detection. J Gastroenterol Hepatol. 2017. 10.1111/jgh.13811. [DOI] [PubMed] [Google Scholar]
- 9. Shiha G, Ibrahim A, Helmy A, et al. Asian‐Pacific Association for the Study of the Liver (APASL) consensus guidelines on invasive and non‐invasive assessment of hepatic fibrosis: a 2016 update. Hepatol Int. 2017;11:1‐30. [DOI] [PubMed] [Google Scholar]
- 10. Nguyen MH, Garcia RT, Trinh HN, et al. Histological disease in Asian‐Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels. Am J Gastroenterol. 2009;104:2206‐2213. [DOI] [PubMed] [Google Scholar]
- 11. Kumar M, Sarin SK, Hissar S, et al. Virologic and histologic features of chronic hepatitis B virus‐infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376‐1384. [DOI] [PubMed] [Google Scholar]
- 12. Kim HC, Nam CM, Jee SH, et al. Normal serum aminotransferase concentration and risk of mortality from liver diseases: prospective cohort study. BMJ. 2004;328:983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shin WG, Park SH, Jang MK, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis. 2008;40:267‐274. [DOI] [PubMed] [Google Scholar]
- 14. Li Q, Ren X, Lu C, et al. Evaluation of APRI and FIB‐4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg‐negative CHB patients with ALT </= 2 ULN: a retrospective cohort study. Medicine (Baltimore). 2017;96:e6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li Q, Lu C, Li W, Huang Y, Chen L. The gamma‐glutamyl transpeptidase to platelet ratio for non‐invasive assessment of liver fibrosis in patients with chronic hepatitis B and non‐alcoholic fatty liver disease. Oncotarget. 2017;8:28641‐28649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lemoine M, Shimakawa Y, Nayagam S, et al. The gamma‐glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369‐1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schiavon LL, Narciso‐Schiavon JL, Ferraz MLG, Silva AEB, Carvalho‐Filho RJ. The gamma‐glutamyl transpeptidase to platelet ratio (GPR) in HBV patients: just adding up? Gut. 2017;66:1169‐1170. [DOI] [PubMed] [Google Scholar]
- 18. Chen B, Ye B, Zhang J, Ying L, Chen Y. RDW to platelet ratio: a novel noninvasive index for predicting hepatic fibrosis and cirrhosis in chronic hepatitis B. PLoS ONE. 2013;8:e68780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lee HW, Kang W, Kim BK, et al. Red cell volume distribution width‐to‐platelet ratio in assessment of liver fibrosis in patients with chronic hepatitis B. Liver Int. 2016;36:24‐30. [DOI] [PubMed] [Google Scholar]
- 20. Su Z, Li Y, Liao Y, et al. Polymorphisms in sodium taurocholate cotransporting polypeptide are not associated with hepatitis B virus clearance in Chinese Tibetans and Uygurs. Infect Genet Evol. 2016;41:128‐134. [DOI] [PubMed] [Google Scholar]
- 21. Zhang Q, Liao Y, Chen J, et al. Epidemiology study of HBV genotypes and antiviral drug resistance in multi‐ethnic regions from Western China. Sci Rep. 2015;5:17413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liao Y, Cai B, Li Y, et al. Association of HLA‐DP/DQ, STAT4 and IL‐28B variants with HBV viral clearance in Tibetans and Uygurs in China. Liver Int. 2015;35:886‐896. [DOI] [PubMed] [Google Scholar]
- 23. Sarin SK, Kumar M, Lau GK, et al. Asian‐Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu DB, Liu FW, Li J, et al. Intrahepatic IFN‐alpha expression in liver specimens from HBV‐infected patients with different outcomes. Eur Rev Med Pharmacol Sci. 2013;17:2474‐2480. [PubMed] [Google Scholar]
- 26. Vallet‐Pichard A, Mallet V, Nalpas B, et al. FIB‐4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32‐36. [DOI] [PubMed] [Google Scholar]
- 27. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518‐526. [DOI] [PubMed] [Google Scholar]
- 28. Wang J, Yan X, Yang Y, et al. A novel predictive model using routinely clinical parameters to predict liver fibrosis in patients with chronic hepatitis B. Oncotarget. 2017;8:59257‐59267. 10.18632/oncotarget.19501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varbobitis I, Papatheodoridis GV. The assessment of hepatocellular carcinoma risk in patients with chronic hepatitis B under antiviral therapy. Clin Mol Hepatol. 2016;22:319‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mohamadnejad M, Montazeri G, Fazlollahi A, et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B‐virus related liver disease. Am J Gastroenterol. 2006;101:2537‐2545. [DOI] [PubMed] [Google Scholar]
- 31. Huang H, Sun Z, Pan H, et al. Serum metabolomic signatures discriminate early liver inflammation and fibrosis stages in patients with chronic hepatitis B. Sci Rep. 2016;6:30853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760‐767. [DOI] [PubMed] [Google Scholar]
- 33. Wang H, Xue L, Yan R, et al. Comparison of histologic characteristics of Chinese chronic hepatitis B patients with persistently normal or mildly elevated ALT. PLoS ONE. 2013;8:e80585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Martinot‐Peignoux M, Lapalus M, Laouenan C, et al. Prediction of disease reactivation in asymptomatic hepatitis B e antigen‐negative chronic hepatitis B patients using baseline serum measurements of HBsAg and HBV‐DNA. J Clin Virol. 2013;58:401‐407. [DOI] [PubMed] [Google Scholar]
- 35. Li Q, Song J, Huang Y, et al. The Gamma‐Glutamyl‐Transpeptidase to Platelet Ratio Does not Show Advantages than APRI and Fib‐4 in Diagnosing Significant Fibrosis and Cirrhosis in Patients With Chronic Hepatitis B: A Retrospective Cohort Study in China. Medicine (Baltimore). 2016;95:e3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonnard P, Sombie R, Lescure FX, et al. Comparison of elastography, serum marker scores, and histology for the assessment of liver fibrosis in hepatitis B virus (HBV)‐infected patients in Burkina Faso. Am J Trop Med Hyg. 2010;82:454‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu XY, Kong H, Song RX, et al. The effectiveness of noninvasive biomarkers to predict hepatitis B‐related significant fibrosis and cirrhosis: a systematic review and meta‐analysis of diagnostic test accuracy. PLoS ONE. 2014;9:e100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ziol M, Handra‐Luca A, Kettaneh A, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48‐54. [DOI] [PubMed] [Google Scholar]
- 39. Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound‐based transient elastography for the detection of hepatic fibrosis: systematic review and meta‐analysis. Clin Gastroenterol Hepatol. 2007;5:1214‐1220. [DOI] [PubMed] [Google Scholar]


