Abstract
Background
Accurate evaluation of hematology analyzers is recommended before these devices can be broadly introduced for the routine testing of continuous ambulatory peritoneal dialysis (CAPD), ascitic, and pleural fluids.
Methods
We evaluated the performance of Mindray BC‐6800 for white blood cell (WBC) and differential cell count in 50 CAPD, 60 ascitic and 40 pleural compared with manual microscopy. Within‐run precision, limit of blank (LoB), limit of detection (LoD), limit of quantitation (LoQ), and carryover were assessed.
Results
The Passing‐Bablok regression in all fluids showed the following equations: yWBC=1.05x+3.31 (95%CI slope 0.95 to 1.12; intercept −0.25 to 5.52); yMN=0.85x+15.63 (95%CI slope 0.72 to 1.05; intercept −24.18 to 84.47); and yPMN=1.21x+13.37 (95%CI slope 1.03 to 1.35; intercept 4.00 to 32.47) with bias 78 cells/μL. The AUC for clinical PMN cut‐off was 0.88 (95%CI: 0.77 to 0.98). In ascitic, pleural, and CAPD fluids the AUC for clinical PMN cut‐off were 0.88 (95%CI: 0.63 to 1.00), 0.83 (95%CI: 0.68 to 0.99), and 1.00 (95%CI: 1.00 to 1.00) respectively. CV ranged from 3%‐34%. LoB of 3 cell/μL was verified. LoD and LoQ reported the same result (8 cells/μL). Carry over never exceeded 0.05%.
Conclusion
The effectiveness of BC‐6800 to categorize cells from different body fluids was not compromised by the slight positive bias observed. This conclusion is supported by the high AUC and agreement between the automated method and the reference method. The results show that BC‐6800 offers rapid, accurate, and reproducible results for clinical management of CAPD, ascitic, and pleural fluids.
Keywords: automated hematology analyzer, cell count, differential count, serous fluid
1. Introduction
Body fluid analysis is a routine laboratory test that provides valuable information to clinicians for the diagnosis and management of several diseases, such as spontaneous bacterial peritonitis in patients with peritoneal dialysis or cirrhosis1 since peritonitis remains a leading complication of peritoneal dialysis.2 Moreover, it is the most rapid and cost‐effective method to investigate the probable cause of ascites3 and may be useful in pleural effusion classification.4, 5
Manual microscopy is still considered the gold standard for evaluation and classification of cells in body fluids,6 but may be a challenging and time consuming process in laboratories of referral hospitals, especially if a large number of biological fluids are received and shortage of skilled personnel occurs. Some drawbacks include manual performance along with inter‐observer variability, high imprecision, and high inaccuracy, especially if performed by less trained personnel. Therefore, different automated hematology analyzers have developed additional modes for body fluid cell count, which may be an alternative to microscopic examination to obtain faster and accurate results for clinical decision‐making. Some of these automated cell counters have been validated with different body fluid specimens to determine white blood cell (WBC) or total nucleated cell (TNC) count, but very few studies analyze differential cell count.7, 8, 9, 10, 11, 12 One of the most recently launched hematology analyzer with body fluid (BF) mode is Mindray BC‐6800 (Mindray Bio‐medical Electronics Co., Ltd., Shenzhen, China; hereinafter called BC‐6800). To our knowledge, there are no published articles focused in the analysis of continuous ambulatory peritoneal dialysis (CAPD) body fluids with BC‐6800. In addition, there is limited information regarding the performance evaluation of BC‐6800 with other body fluids.13, 14 Therefore, the aim of this study was to assess the BC‐6800 analyzer performance for WBC and differential cell count in CAPD, ascitic, and pleural body fluids compared with manual microscopy.
2. Materials and Methods
2.1. BC‐6800 BF mode
The BF mode provides WBC and differential cell count, RBC count and uses SF Cube technology to recognize and detect nucleated cells in body fluids. Targeted cells undergo 3D analysis by using information from the scatter of laser light at two angles and fluorescence flow cytometry signals. Body fluid WBC and TNC counts are directly generated in the DIFF channel and classified as mononuclear (MN), polymorphonuclear (PMN; neutrophils and eosinophils), and high fluorescence (HF) cell counts and percentages.
2.2. Patient samples
The study was carried out with 150 body fluid samples from 49 female and 101 male patients (median age: 57 years; range: 1‐97 years): 50 CAPD, 60 ascitic, and 40 pleural body fluids from hospital wards and peritoneal dialysis outpatients. Patients included in this study had a broad spectrum of diseases [e.g. CAPD fluids were recruited from patients with chronic kidney disease (n=50); ascitic body fluids were recruited from patients with hepatitis c virus cirrhosis (n=34), alcoholic cirrhosis (n=15), oncologic (n=6), and miscellanea (n=5) diseases; pleural body fluids were recruited from patients with pneumonia (n=12), congestive heart failure (n=8), oncologic (n=5), and miscellanea (n=15) diseases]. All samples were collected in 2.0 mL K2 EDTA tubes, sent to the laboratory for routine diagnostic purposes and microscopic cell count. The specimen leftover was mixed by gentle inversion 6‐8 times, and was further subjected to automated analysis by BC‐6800 and microscopic evaluation within 4 hours of sampling. The study was carried out in accordance with the Declaration of Helsinki and was in line with any relevant local legislation.
2.3. Method comparison analysis
The method comparison analysis of WBC and differential cell count was performed between the automated BF mode of BC‐6800 and microscopic examination. Manual microscopic WBC count was performed using the Fuchs‐Rosenthal chamber (surface area 16 mm2 and depth 0.2 mm). The Fuchs‐Rosenthal manual counting chamber was covered with a thin glass coverslide and body fluids were filled with no air bubbles into the chamber using a pipette. The cells were counted in the entire chamber at ×400 magnification and results were adjusted to cell/μL (16 mm2×0.2 mm/3.2 mm3). Microscopic evaluation of WBC count was performed directly and diluted with Türk`s solution. Mesothelial cells were not included in the WBC count.
Smears for PMN and MN differential cell count were performed after sample cytocentrifugation followed by May‐Grünwald‐Giemsa‐staining and only were reviewed by light microscopy under oil immersion at ×1000 magnification if the total WBC count was over 100 cells/μL. Lymphocytes, monocytes, and macrophages cells were included in the MN cell count. Mesothelial cells were excluded in the differential count. Samples were processed twice by both methods and microscopic WBC and differential count were performed by two trained experts and results were finally averaged.
2.4. BC‐6800 performance evaluation
The within‐run precision for WBC cell count was determined with BC‐6800 by measuring nine body fluid samples of different cell concentrations for a minimum of five times depending on the available volume. The coefficient of variation (CV) at the expected counts was calculated.15
The limit of blank (LoB) described by the BC‐6800 manufacturer was verified by running 20 measurements of cell‐free body fluids (n=3), which have been previously evaluated in the counting chamber.
The limit of detection (LoD) was determined by measuring 10 consecutive times low‐cell concentration body fluids (n=6), for a total of 60 results. All samples were within the range of four times the LoB. The LoD was determined according to the formula: LoD=LoB+1.645 *SD.16
The limit of quantitation (LoQ) was determined mathematically by the power regression equation, which was obtained from the plot between WBC count and the CV from the precision study. The LoQ was defined as the lowest cell concentration that can be measured with 95% confidence of a CV of 20%.
The WBC count carryover was verified by running a peritoneal fluid with a high count (A1, A2, A3) three consecutive times, followed by a peritoneal sample with a low cell count (B1, B2, B3). The results were calculated according to the formula15: carryover %=(B1‐B3)/(A2‐B3)×100.
2.5. Statistics
The results of the method comparison between the automated analysis by BC‐6800 and the manual microscopy for body fluid cell count were assessed by Passing and Bablok regression (interchambiability criteria were 95%CI of the slope including the 1 and the intercept including the 0), Wilcoxon signed‐rank paired test and Kappa agreement. Bias (and 95%CI) was calculated with the Bland‐Altman analysis. Sensibility, specificity, and diagnostic concordance with receiver operating characteristic curves (ROC) were calculated for BC‐6800 and then compared to manual microscopy. The statistical evaluation results were processed with the Statistical Package of Social Sciences (version 15.0; Chicago, IL, USA) for Windows.
3. Results
One hundred and fifty body fluids (50 CAPD, 60 ascitic, 40 pleural) were received over a 2‐month study period. Five ascitic body fluid samples with high cell count results outside analytical measuring range were excluded, so 145 samples were included in the statistical analysis. Differential cell count was performed in 72 samples with WBC count higher than 100 cells/μL.
3.1. Comparison results of the WBC, MN, and PMN count in all body fluids
The WBC count for all the body fluids ranged from 1 to 9360 cells/μL. The median WBC count values presented no significant statistical differences between both methods (Table 1). The comparison results of the WBC count between BC‐6800 and microscopic evaluation are shown in Figure 1 and the Passing‐Bablok regression sowed the following equation: y=1.05x+3.31 (95% CI of slope 0.95 to 1.12; and intercept −0.25 to 5.52). The Bland‐Altman analysis confirmed that there was no bias between both methods (Table 1 and Figure 1).
Table 1.
Median cell count, cell count agreement, and mean differences between the Mindray BC‐6800 analyzer and manual microscopy
| Body fluid | Median cells/μL (25%‐75% percentile) | Passing‐Bablock regression | Bland‐Altman | ||||
|---|---|---|---|---|---|---|---|
| Mindray BC‐6800 | Manual microscopy | P | Slope (95%CI) | Intercept (95%CI) | R | Mean difference cells/μL (95%CI) | |
| All (n=145) | |||||||
| WBC | 223 (37‐803) | 185 (28‐885) | .117 | 1.05 (0.95 to 1.12) | 3.31 (−0.25 to 5.52) | .95 | 2 (−66 to 70) |
| MN | 571 (328‐1423) | 659 (255‐1660) | .265 | 0.85 (0.72 to 1.05) | 15.63 (−24.18 to 84.47) | .88 | −95 (−240 to 50) |
| PMN | 149 (57‐451) | 101 (22‐284) | <.001 | 1.21 (1.03 to 1.35) | 13.37 (4.00 to 32.47) | .97 | 78 (31 to 126) |
| Ascitic (n=55) | |||||||
| WBC | 297 (135‐557) | 250 (120‐580) | .900 | 0.87 (0.75 to 1.01) | 29.90 (5.95 to 54.15) | .89 | −35 (−114 to 44) |
| CADP/Peritoneal (n=50) | |||||||
| WBC | 12 (7‐43) | 10 (3‐35) | .060 | 1.16 (0.95 to 1.38) | 1.73 (−0.37 to 3.37) | .99 | 16 (−3 to 35) |
| Pleural (n=40) | |||||||
| WBC | 1095 (523‐2625) | 1580 (471‐2200) | .640 | 1.01(0.81 to 1.24) | 45.02 (−95.64 to 187.85) | .94 | −21 (−246 to 204) |
CAPD, continuous ambulatory peritoneal dialysis; WBC, white blood cell count; MN, mononuclear cell count; PMN, polymorphonuclear cell count; CI, confidence interval.
Figure 1.
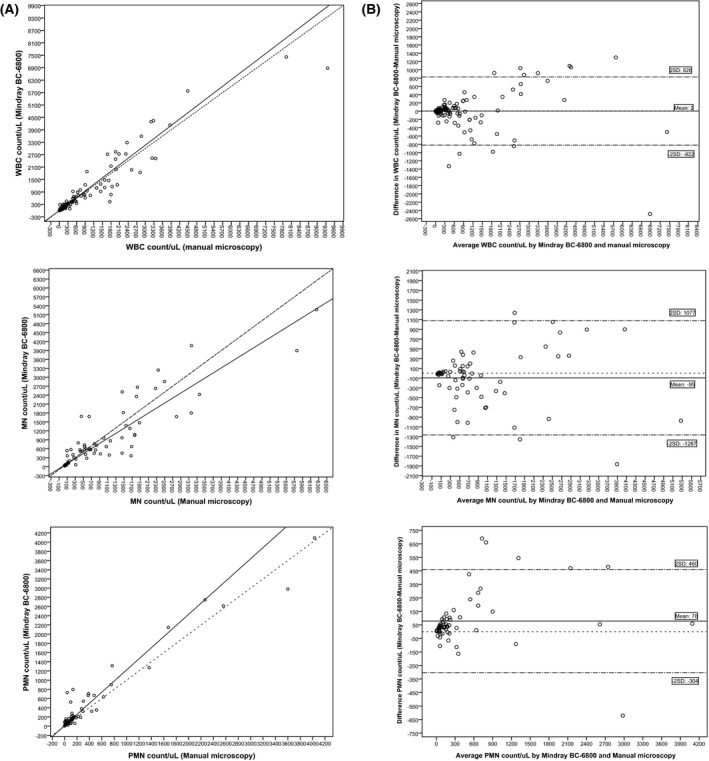
Passing‐Bablok agreement and Bland‐Altman difference plot between Mindray BC‐6800 and manual microscopy in serous fluids. (A). Passing‐Bablok regression for white blood cell (WBC), mononuclear (MN), and polymorphonuclear (PMN) cell count;  , regression line;
, regression line;  , Y=X. (B). Bland Altman plots for white blood cell (WBC), mononuclear (MN) and polymorphonuclear (PMN) cell count;
, Y=X. (B). Bland Altman plots for white blood cell (WBC), mononuclear (MN) and polymorphonuclear (PMN) cell count;  , mean bias;
, mean bias;  , zero bias;
, zero bias;  , 2 Standard deviation agreement
, 2 Standard deviation agreement
The MN differential cell count results were similar to the WBC results, with no significant statistical differences between both methods (Table 1 and Figure 1). However, the PMN result showed significant statistical differences in the median cell count and Passing‐Bablok regression: y=1.21x+13.37 (95% CI of slope 1.03 to 1.35; and intercept 4.00 to 32.47; Table 1 and Figure 1). The Bland‐Altman analysis confirmed that bias was present with a mean difference of 78 PMN/μL (Table 1). To evaluate the clinical relevance of the BIAS found we analyzed the diagnostic concordance of BC‐6800 compared to microscopic method. When a clinical PMN cutoff was applied for each body fluid type (ascitic >250 PMN, pleural >50% PMN and CAPD >100 WBC with PMN≥50%), the AUC was 0.88 (95%CI: 0.77 to 0.98) with a kappa index of 0.82 (95%CI: 0.67 to 0.97) and we found five discrepant results (Table 2). Two patients with pneumonia were misclassified as negative and an oncologic patient with pleural effusion as positive by the BC‐6800. On the other hand, two oncologic patients with pleural effusion and ascitic decompensation were classified as positive by the manual microscopy.
Table 2.
ROC analysis and agreement of the Mindray BC‐6800 analyzer compared with manual microscopy
| CUT‐OFF (cells/μL) | Sensitivity (95% CI) | Specificity (95% CI) | AUC (95% CI) | Kappa (95% CI) | |
|---|---|---|---|---|---|
| All fluids (n=145) | >1000 WBC | 91 (77‐100) | 93 (87‐98) | 0.91 (0.85‐0.98) | 0.74 (0.56‐0.88) |
| PMNa | 77 (53‐100) | 99 (97‐100) | 0.88 (0.77‐0.98) | 0.82 (0.67‐0.97) | |
| Ascitic fluids (n=55) | >1000 WBC | 100 (88‐100) | 94 (87‐100) | 0.97 (0.94‐1.00) | 0.70 (0.38‐1.00) |
| >250 PMN | 75 (20‐100) | 100 (99‐100) | 0.88 (0.63‐1.00) | 0.85 (0.56‐1.00) | |
| Pleural fluids (n=40) | >1000 WBC | 88 (68‐100) | 71 (50‐93) | 0.80 (0.67‐0.92) | 0.57 (0.32‐0.83) |
| >50% PMN | 70 (37‐100) | 96 (87‐100) | 0.83 (0.68‐0.99) | 0.71 (0.44‐0.97) | |
| CAPD fluids (n=50) | >100 WBC and 50% PMN | 100 (83‐100) | 100 (99‐100) | 1.00 (1.00‐1.00) | 1.00 (1.00‐1.00) |
CI, confidence interval; WBC, white blood cell count; PMN, polymorphonuclear cell count; CAPD, continuous ambulatory peritoneal dialysis.
Ascitic: >250 PMN, CAPD: >100 WBC and 50% PMN, Pleural: >50% PMN.
3.2. Comparison results of the WBC count in CAPD, ascitic, and pleural body fluids
Serous fluids were separately analyzed in three groups: CAPD, ascitic, and pleural fluids. The WBC count ranged from 1 to 730 cells/μL for CAPD, 30 to 3360 cells/μL for the ascitic and 20 to 9360 cells/μL for pleural fluids. The results showed no significant statistical differences in the median WBC counts (Table 1). The Passing‐Bablok results for CAPD and pleural fluids were: y=1.16x+1.73 (95% CI of slope 0.95 to 1.38; and intercept −0.37 to 3.37) and y=1.01x+45.02 (95% CI of slope 0.81 to 1.24; and intercept −95.64 to 187.85) respectively. The Bland‐Altman analysis showed that bias was not present for these two body fluids. However, ascitic fluid showed a constant bias for WBC: y=0.87x+29.90 (95% CI of slope 0.75 to 1.01; and intercept 5.95 to 54.15), which indicates a higher WBC count by BC‐6800 compared to the reference method (Table 1).
To evaluate the clinical relevance of the BIAS found for WBC count in ascitic fluids we analyzed the diagnostic concordance of BC‐6800 compared to microscopic method. When a cut‐off of 1000 WBC/uL was used, the AUC was 0.97 (95%CI: 0.94 to 1.00) with a kappa index of 0.70 (95%CI: 0.38 to 1.00) and we found three discrepant results (Table 2). One patient with spontaneous bacterial peritonitis was misclassified as negative by manual microscopy. The other two samples were classified as positive by the BC‐6800 and corresponded to ascitic decompensation in oncologic patients.
3.3. Performance results
The within‐ run precision was examinated for WBC count compressed between 2 and 2909 cells/μL. The CV obtained ranged between 3% and 34% (Table 3).
Table 3.
Imprecision results of the Mindray‐BC‐6800 analyzer for white blood cell count in serous fluids
| Cells/μL | CV (%) | |
|---|---|---|
| Very low | <10 | 34 |
| Low | 10‐100 | 7 |
| Borderline | 100‐250 | 5 |
| High | >250 | 3 |
CV, coefficient of variation.
The LoB claimed by the manufacturer (3 cell/μL) was confirmed. This result implies a LoD of 8 cells/μL. The LoQ was 8 cells/μL (Figure 2). Carryover was verified and never exceeded 0.05%, which was lower than the established by the manufacturer.
Figure 2.
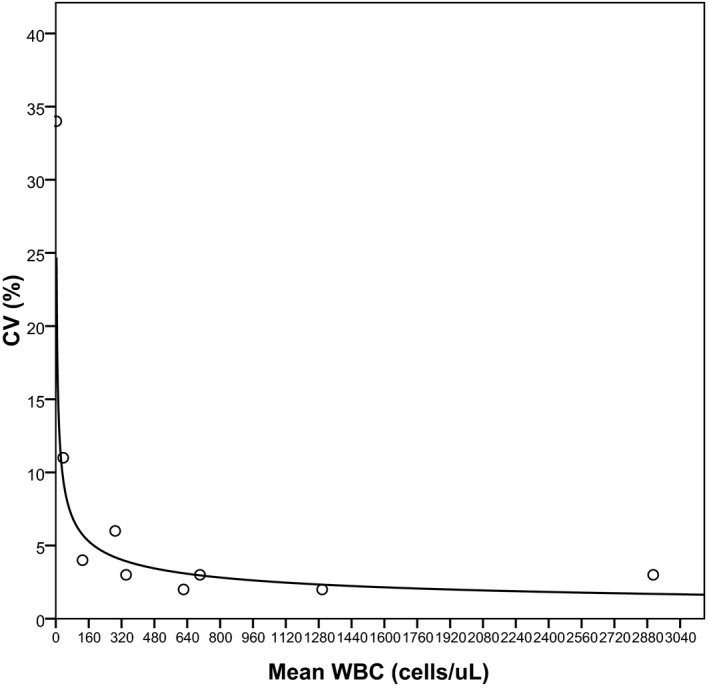
Imprecision results of the Mindray‐BC‐6800 analyzer for white blood cell count in serous fluids. The solid line represents the regression line obtained from the following equation: y=43.774x −0.3922 (r 2=.87)
4. Discussion
In the present study, we compared the automatic cellular analysis of different types of body fluids in a recently launched hematology analyzer (Mindray BC‐6800) with standard microscopic examination. The precision profile, LoB, LoD, LoQ, and carryover were also evaluated.
The analysis of body fluid provides essential information for the diagnostic approach of several medical conditions1, 2, 3: CAPD fluid with WBC above 100/μL and ≥ 50% neutrophil cells is indicative of peritonitis.2, 12 Similarly, ascitic fluids with a WBC count >1000/μL or a PMN cell count >250/μL, suggest spontaneous bacterial peritonitis.1, 3, 12, 17 Also in pleural effusions, cell count is helpful to determine the differential diagnoses between transudates (WBC<1000/μL) and exudates (WBC>1000/μL).4 Predominance of neutrophils indicates acute inflammation, while lymphocyte‐predominant effusion (>50%) suggests pleural malignancy or tuberculosis.4, 12, 18, 19
Manual method for cell count in a counting chamber using standard and alternative (e.g. Turk or Samson) staining solutions for microscopic evaluation are frequently used in clinical laboratory practice.20, 21 The main advantage of using alternative staining solutions with lysing reagent that destroys the RBC membrane is to prevent possible interference in the WBC count, leaving stained nucleated cells intact for microscopic evaluation. The main disadvantage is the possible error in the WBC count due to the dilution factor added. In any case, the quality and morphology of cells can be observed in all counting chamber methods subjected to the experience of the observer with the well known limitations described in the introduction of this paper. In this regard, our results suggest that BC‐6800 can be a suitable alternative to perform automated analysis for the WBC count as it showed interchangeable results with the microscopic method. Moreover, our experience shows that it is a device that displays a great practicability and this implies an advantage over microscopic counting for many reasons: fast, accurate, and reproducible results and fewer pre‐analytical sample treatments.8 In line with this, no sample preparation is needed prior to analysis in the BC‐6800. It takes about <2 minutes to switch from the blood mode to the BF mode (includes automatic rinse cycles and background check) and only takes 1 minute to process a sample. However, the positive bias that BC‐6800 presents in the PMN cell count implies that differential count should be carefully evaluated. The authors consider that automated differential analysis may be enough for ascitic fluid due to the elevated AUC with high sensitivity and specificity that we have obtained at the 250 PMN/μL threshold.1 We also obtained an elevated AUC with high sensitivity, specificity, and concordance between the automatic and microscopic methods in our series of peritoneal and pleural fluids. Nevertheless, we cannot assure the same result in other series of patients because the PMN/uL cut‐off could be different from that obtained in our series since it depends on the WBC count (PMN=50%WBC). As a result, in these types of fluids it might be of interest to complete the automatic WBC count with the smear review to ensure an accurate differential count.
Similarly to our result obtained by BC‐6800, a PMN positive bias count has been previously reported in other automated analyzers. One possible explanation for these results is loss of cells during centrifugation or deterioration at room temperature, particularly labile neutrophils, which may generate a different PMN enumeration.5, 8, 22
Another feature to consider when analyzing serous fluids in automated devices with the body fluid mode is the use of gating strategies to exclude tissue cells from WBC count.8, 22 According to previous reports hematology devices that use cell count fluorescent methods categorize macrophages as high fluorescent (HF) cells.5, 22 Nevertheless, these cells can be included in MN count if their fluorescence intensity is not so high.5 In this regard, our results indicated that BC‐6800 includes macrophages in the WBC count since we included macrophages in the chamber count to obtain the agreement data presented herein (Passing‐Bablok results showed proportional bias for BC‐6800 when we excluded macrophages from the manual method in all body fluid types. Data not shown).
We observed a slight bias for the ascitic WBC count according to a previous report.13 However our results showed a constant bias, while Lippi et al.13 indicated a proportional bias. The difference found between the manual and automatic methods for the ascitic WBC count and the discrepancy with the results of Lippi et al.13 may be due to different factors, such as biological matrix composition and presence of tissue cells. These cells may be misclassified between the WBC and the HF clusters produced by the Body Fluid channel. However, our significant statistical differences in the WBC count may not be clinically significant since the high Kappa Index value, AUC, sensitivity and specificity obtained between both methods at the abnormal threshold 1000 WBC/μL8, 17 indicated good agreement for clinical use.
Previously data reported in body fluids by Buoro et al.14 showed discordant bias compared to our data. This bias may also be explained by the different cell count range measurement assayed, different body fluid matrix (no CAPD fluids assayed) and the presence of tumor cells, which were not present in our samples. However, in line with our results, the slight bias observed by Buoro et al.14 did not compromise the ability of BC‐6800 to correctly categorize abnormal fluids in proper clinical category.
Performance data from the BC‐6800 suggest that this device is adequate for clinical management of serous and CAPD fluids, since the carryover (<0.05%) and LoB (3 cells/μL) were according to the manufacturer's specifications. LoQ (8 cells/μL) presented negligible differences with previous report that may be explained by the different power regression equation generated by using different samples14 and the CV at the 100 and 1000 cell/μL thresholds (i.e. 3%‐5%) were similar to previous report.14
In conclusion, although microscopic evaluation still remains the cornerstone in the workup of serous fluids, automated analysis is needed not only to obtain accurate results, but also to process samples within the required time. In line with this, the BC‐6800 analyzer provides acceptable results for the clinical management of serous fluids. In order to confirm our results, further studies should include a large number of samples for each body fluid type.
Fuster O, Andino B, Pardo A, Laiz B. Continuous ambulatory peritoneal dialysis, ascitic and pleural body fluids evaluation with the Mindray BC‐6800 hematology analyzer. J Clin Lab Anal. 2018;32:e22240 10.1002/jcla.22240
References
- 1. Moore KP, Aithal GP. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kam‐Tao P, Szeto I, Piraino B, et al. ISPD guidelines/recommendations peritoneal dialysis‐related infections recommendations: 2010 update. Perit Dial Int. 2010;30:393‐423. [DOI] [PubMed] [Google Scholar]
- 3. Runyon B. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49:2087‐2107. [DOI] [PubMed] [Google Scholar]
- 4. Conner B, Gary Lee Y, Branca P, et al. Variations in pleural fluid WBC count and differential counts with different sample containers and different methods. Chest. 2003;123:1181‐1187. [DOI] [PubMed] [Google Scholar]
- 5. Cho Y, Chi H, Park S, et al. Body fluid cellular analysis using the Sysmex XN‐2000 automatic hematology analyzer: focusing on malignant samples. Int J Lab Hem. 2015;37:346‐356. [DOI] [PubMed] [Google Scholar]
- 6. Clinical and Laboratory Standards Institute . Body Fluid Analysis for Cellular Composition; Approved Guideline. CLSI document H56‐A. Wayne, PA: Clinical and Laboratory Standards Institute; 2006. [Google Scholar]
- 7. Froom P, Diab A, Barak M. Automated evaluation of synovial and ascitic fluids with the Advia 2120 hematology analyzer. Am J Clin Pathol. 2013;140:828‐830. [DOI] [PubMed] [Google Scholar]
- 8. Fleming C, Brouwer R, van Alphen A, et al. UF‐1000i: validation of the body fluid mode for counting cells in body fluids. Clin Chem Lab Med. 2014;12:1781‐1790. [DOI] [PubMed] [Google Scholar]
- 9. Lippi G, Cattabiani C, Benegiamo A, et al. Evaluation of the fully automated hematological analyzer Sysmex XE‐5000 for flow cytometric analysis of peritoneal fluid. J Lab Autom. 2013;18:240‐244. [DOI] [PubMed] [Google Scholar]
- 10. De Smet D, Van Moer G, Martens G, et al. Use of the cell‐dyn sapphire hematology analyzer for automated counting of blood cells in body fluids. Am J Clin Pathol. 2010;133:291‐299. [DOI] [PubMed] [Google Scholar]
- 11. Paris A, Nhan T, Cornet E, et al. Performance evaluation of the body fluid mode on the platform sysmex XE‐5000 series automated hematology analyzer. Int J Lab Hem. 2010;32:539‐547. [DOI] [PubMed] [Google Scholar]
- 12. Fleming C, Russcher H, Lindemans J, et al. Clinical relevance and contemporary methods for counting blood cells in body fluids suspected of inflammatory disease. Clin Chem Lab Med. 2015;53:1689‐1706. [DOI] [PubMed] [Google Scholar]
- 13. Lippi G, Cattabiani C, Benegiamo A, et al. Evaluation of white blood cell count in peritoneal fluid with five different hemocytometers. Clin Biochem. 2013;46:173‐176. [DOI] [PubMed] [Google Scholar]
- 14. Buoro S, Mecca T, Azzarà G, et al. Mindray BC‐6800 body fluid mode, performance of nucleated cells, and differential count in ascitic and pleural fluids. Int J Lab Hematol. 2016;38:90‐101. [DOI] [PubMed] [Google Scholar]
- 15. Bourner G, de la Salle B, George T, et al. CSH guidelines for the verification and performance of automated cell counters for body fluids (ICSH). Int J Lab Hem. 2014;36:598‐612. [DOI] [PubMed] [Google Scholar]
- 16. Armbruster D, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29:49‐52. [PMC free article] [PubMed] [Google Scholar]
- 17. Wilkerson RG, Sinert R. The use of paracentesis in the assessment of the patient with ascites. Ann Emerg Med. 2009;54:465‐468. [DOI] [PubMed] [Google Scholar]
- 18. Hooper C, Gary Lee Y, Maskell N, On Behalf of the BTS Pleural Guideline Group . Investigation of a unilateral pleural effusion in adults: British Thoracic Society pleural disease guideline 2010. Thorax. 2010;65:4‐17. [DOI] [PubMed] [Google Scholar]
- 19. de Jonge R, Brouwer R, van Rijn M, et al. Automated analysis of pleural fluid total and differential leukocyte counts with the Sysmex XE‐2100. Clin Chem Lab Med. 2006;44:1367‐1371. [DOI] [PubMed] [Google Scholar]
- 20. Walker TJ, Nelson LD, Dunphy BW, et al. Comparative evaluation of the Iris iQ200 body fluid module with manual hemacytometer count. Am J Clin Pathol. 2009;131:333‐338. [DOI] [PubMed] [Google Scholar]
- 21. Fleming C, Brouwer R, Lindemans J, et al. Validation of the body fluid module on the new Sysmex XN‐1000 for counting blood cells in cerebrospinal fluid and other body fluids. Clin Chem Lab Med. 2012;50:1791‐1798. [DOI] [PubMed] [Google Scholar]
- 22. Sandhaus L. Body fluid cell counts by automated methods. Clin Lab Med. 2015;35:93‐103. [DOI] [PubMed] [Google Scholar]


