Abstract
Background
Phosphorus and urea are measurable in saliva. Measurements of saliva phosphorus (S‐Pho) and saliva urea (S‐Urea) could be useful because of low invasivity. Data are limited to saliva tests methodology and to correlations between plasma and saliva compositions. S‐Pho and S‐Urea were investigated focusing on blind duplicates, differences between collection sites, differences between collection times, freezing‐thawing effects, and plasma‐saliva correlations.
Methods
Tests were performed using fresh saliva collected by synthetic swap early morning after overnight fast (standard). Methodology was investigated in fifteen healthy volunteers. Plasma‐saliva correlations were investigated in thirty nephropathic outpatients.
Results
S‐Pho and S‐Urea in all measurements ranged above detection limits (0.3 mmol/L). In healthy volunteers, S‐Pho and S‐Urea were similar in duplicates (results for S‐Pho and S‐Urea: % difference between samples ≤ 4.85%; R between samples ≥ .976, P < .001), in samples from different mouth sites (≤4.24%; R ≥ .887, P < .001), and in samples of different days (≤5.61%; R ≥ .606, P < .01) but, compared to standard, were substantially lower in after‐breakfast samples (−28.0% and −21.3%; R ≥ .786, P < .001) and slightly lower in frozen‐thawed samples (−12.4% and −5.92%; R ≥ .742, P < .001). In nephropathic patients, S‐Pho was higher than but correlated with plasma phosphorus (saliva/plasma ratio 4.80; R = .686, P < .001), whereas S‐Urea and plasma urea were similar and correlated with each other (saliva/plasma ratio 0.96; R = .944, P < .001). Post‐dialysis changes in S‐Pho and S‐Urea paralleled post‐dialysis changes in plasma phosphorus and urea.
Conclusion
S‐Pho and S‐Urea reflect plasma phosphorus and plasma urea. Early morning fasting fresh samples are advisable because collection time and freezing‐thawing affect saliva tests.
Keywords: plasma phosphorus, plasma urea, saliva phosphorus, saliva urea
1. INTRODUCTION
The use of saliva for laboratory tests has been investigated in various medical areas because saliva could be used as a noninvasive diagnostic tool.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 An important prerequisite for the use of saliva in diagnostic workup is that the salivary levels of the marker associated with a given disorder should mirror the profile of the marker in biological samples used as gold standard in medical practice. Previous observations indicated that correlations between plasma concentrations and saliva concentrations are detectable for some but not for all analytes.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 It is well established that small molecules as phosphorus and urea are measurable in saliva.20, 21 High plasma levels of phosphorus and urea were reported in astronauts during space missions and in terrestrial models of microgravity.22, 23, 24 In these particular settings, high plasma levels of phosphorus and urea are considered secondary to alterations in bone and muscle metabolism leading to loss of bone mass and muscle mass.22 As part of a project about the use of saliva tests in space medicine,25 this study investigated methodological aspects in saliva measurements (duplicate samples, saliva from different sites of collection, day‐to‐day variability, time of collection, effects of freezing/thawing) and correlations between plasma concentrations and saliva concentrations. Methodological aspects were investigated in healthy volunteers, whereas plasma‐saliva correlations were investigated in patients with kidney disease because low kidney function increases the levels of phosphorus and urea not only in plasma but also in saliva.1, 6, 10, 11, 12, 13, 14
2. METHODS
This observational study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the local institutional Ethics Committees (n. 5/2012 and 4/2013). The study was part of a project developed in collaboration between the Italian Space Agency (Agenzia Spaziale Italiana, ASI) and the National Aeronautics and Space Administration (NASA) to explore the use of saliva for monitoring metabolic changes associated with reduction in bone mass and muscle mass.25 Healthy volunteers were enrolled from the department staff for analyses on methodological aspects. Patients with chronic kidney disease (CKD) were targeted for analyses on correlations between plasma levels and salivary levels because kidney dysfunction generally increases plasma levels both of phosphorus and urea.26 CKD patients were enrolled from the outpatient clinic of the University Hospital. Exclusion criteria for the healthy group were the presence or the report of a recent acute disease or of a chronic disease at the medical examination, or the report of pharmacological treatment. The selection criterion for the CKD group was a confirmed diagnosis of CKD according to standard definitions.26 Given that CKD modifies plasma phosphorus and plasma urea proportionally to kidney dysfunction, the enrollment of CKD patients was stratified by CKD stage to have at least five patients in each CKD stage, that is, to have a continuum of kidney function ranging from normal levels to severe reduction. The study included also a small group of CKD patients on regular chronic hemodialysis to investigate the relationships of saliva phosphorus and saliva urea with dialysis‐induced changes in plasma phosphorus and plasma urea.27 Smoking was an exclusion criterion for the group of healthy volunteers and for the CKD group to exclude the effect of this confounding on saliva composition.28 All participants were enrolled after having signed an informed consent.
Unless otherwise indicated, blood and saliva samples were collected early in the morning after an overnight fast. Blood samples were taken by peripheral venipuncture and rapidly centrifuged for plasma separation. Blood withdrawal was part of the standard routine workup programmed in the outpatient clinic for CKD patients and, in dialysis patients, was repeated as programmed for good medical practice after the completion of the morning, 4‐hour, standard dialysis session.27 Saliva samples were collected 1‐2 minutes after blood withdrawal using a synthetic swap (Salivette, Sarstedt, Germany).29 The protocol was designed to comply with the restraints of space missions in which the use of a swap is the standard method for saliva collection in the absence of gravity. The collection of saliva was not timed nor preceded by salivary flow stimulation with paraffin or other agents given that the restraints of space missions include also strict limitations in the time availability of astronauts, in the administration of substances to the astronauts, and in the use of materials or disposables, etc. The swap was rapidly centrifuged for saliva separation and for mucin removal. Unless otherwise indicated, laboratory measurements were performed using fresh saliva samples collected early in the morning under fasting condition (standard samples). Laboratory procedures were performed using automated biochemistry and commercially available kits (Abbott, IL, USA). Saliva measurements were performed by the molybdate UV method for phosphorus and by the urease/NADH method for urea.30
Saliva samples of the healthy group were for investigation about the following five objectives: blind duplicates of the same standard sample; differences between two standard samples simultaneously collected in the right vestibulum oris and in the left vestibulum oris; differences between standard samples collected in different days (day‐to‐day variability); differences between standard samples and samples collected 3 hours after the completion of breakfast (mid‐morning samples); and differences between standard samples and frozen/thawed samples. The 3‐hours time point for measurements on mid‐morning sample was selected in accordance with the schedule programmed for experiments in astronauts during space mission.29 In experiments on frozen/thawed samples, a standard saliva sample was split into two identical aliquots: One aliquot was kept at 2‐4°C for one hour while the other aliquot was frozen and thawed at 2‐4°C within the same time. Saliva and blood samples of the CKD group were for investigations about correlations between plasma phosphorus and saliva phosphorus and between plasma urea and saliva urea. Results were analyzed using Student's t‐test for paired observations, simple correlation coefficient (R), and linear regression. The report of results included mean ± SEM and 95% confidence intervals (95% CI).
3. RESULTS
The healthy group was made of 9 men and 6 women with age 35 ± 5 years, whereas the CKD group was made of 17 men and 13 women with age 37 ± 3 years. The saliva concentrations of phosphorus and urea were in all measurements well above the detection limits of laboratory tests (0.3 mmol/L). Table 1 summarizes the results in the healthy group with the use of standard samples (saliva collected early in the morning after an overnight fast and processed as fresh sample) with regard to blind duplicates, site of collection, and day‐to‐day variability. Table 1 shows that saliva concentrations of phosphorus and urea of blind duplicates were almost identical and highly correlated. The difference of duplicate samples from the mean was 1.85% for phosphorus and 5.60% for urea (technical error). With regard to the possible effects of the site of saliva collection, data indicated that saliva concentrations of phosphorus and urea of samples from the right and the left vestibulum oris were almost identical and highly correlated. With regard to day‐to‐day variability, findings were similar also in the comparisons of saliva concentrations of phosphorus and urea between samples of different days, although the value of the R was apparently lower for saliva phosphorus as compared to saliva urea.
Table 1.
Saliva concentrations of phosphorus and urea in standard samples of healthy volunteers: blind duplicates, site of collection, and day‐to‐day variability (n = 15, mean ± SEM)
| Blind duplicates | P for difference between samples | R between samples | ||
|---|---|---|---|---|
| First duplicate | Second duplicate | |||
| Phosphorus, mmo/L | 5.59 ± 0.73 | 5.81 ± 0.75 | .526 | .994* |
| Urea, mmol/L | 5.15 ± 0.72 | 4.90 ± 0.58 | .615 | .976* |
| Site of collection | ||||
| Right vestibulum oris | Left vestibulum oris | |||
| Phosphorus, mmo/L | 5.42 ± 0.65 | 5.19 ± 0.48 | .467 | .896* |
| Urea, mmol/L | 5.10 ± 0.97 | 5.12 ± 0.79 | .965 | .887* |
| Day‐to‐day variability | ||||
| First day | Second day | |||
| Phosphorus, mmol/L | 5.44 ± 0.68 | 5.70 ± 0.74 | .965 | .606** |
| Urea, mmol/L | 5.17 ± 0.76 | 4.88 ± 0.98 | .371 | .906* |
Standard samples: non‐stimulated fresh saliva collected early in the morning after an overnight fast and with the use of a synthetic swap.
*P < .001, **P ≤ .01.
Table 2 summarizes the results in the healthy group for analyses of saliva concentrations of phosphorus and urea of standard samples (saliva collected early in the morning after an overnight fast and processed as fresh sample) compared to mid‐morning samples (saliva collected in the morning three hours after the completion of the breakfast and processed as fresh sample) and to frozen/thawed samples (saliva collected early in the morning after an overnight fast and processed after 1‐hour freezing/thawing cycle). Mid‐morning samples had lower saliva concentrations both for phosphorus and urea. The difference was significant for urea and borderline significant for phosphorus. The R between concentrations in standard samples and concentrations in mid‐morning samples were highly significant but of lower order in comparison with the R found between duplicates and between simultaneous samples from different sites of collection. Frozen/thawed samples had slightly lower saliva concentrations of phosphorus but not of urea. The R between concentrations in standard samples and concentrations in frozen/thawed samples were highly significant but of lower order in comparison with the R found between duplicates processed as fresh samples.
Table 2.
Saliva concentrations of phosphorus and urea in healthy volunteers: comparisons of standard samples with mid‐morning samples and frozen/thawed samples (n = 15, mean ± SEM)
| Standard samples | Mid‐morning samples | P for difference between samples | R between samples | |
|---|---|---|---|---|
| Phosphorus, mmol/L | 5.89 ± 0.73 | 4.24 ± 0.62 | .095 | .786* |
| Urea, mmol/L | 5.17 ± 0.81 | 4.07 ± 0.45 | .009 | .822* |
| Standard samples | Frozen/thawed samples | |||
| Phosphorus, mmol/L | 5.45 ± 0.56 | 4.77 ± 0.51 | .093 | .742* |
| Urea, mmol/L | 5.24 ± 0.71 | 4.93 ± 0.66 | .200 | .865* |
Standard samples: non‐stimulated fresh saliva collected with the use of a synthetic swap early in the morning after an overnight fast.
Mid‐morning samples: non‐stimulated fresh saliva collected with the use of a synthetic swap in the morning three hours after the completion of breakfast.
Frozen/thawed samples: non‐stimulated saliva collected with the use of a synthetic swap early in the morning after an overnight fast.
* P < .001, ** P < .01.
In the CKD group, standard saliva samples and plasma samples collected 1‐2 minutes before saliva were apparently different for phosphorus concentration that was 4.80‐time higher in saliva (mean ± SEM in saliva and plasma = 7.78 ± 0.79 and 1.62 ± 0.14 mmol/L, P < .001 by t test for paired data). Vice versa, saliva and plasma were almost identical for urea concentration (17.5 ± 2.2 and 18.2 ± 2.2 mmol/L, P = .322). Figure 1 shows that significant direct associations were found between plasma concentrations and saliva concentrations both of phosphorus and urea. The 95% CI of the coefficients indicated that the regression line between plasma urea and saliva urea did not differ from the identity line (y‐axis intercept = 0 and slope = 1). Figure 2 shows the changes of saliva and plasma concentrations of phosphorus and urea in five dialysis patients from pre‐dialysis to post‐dialysis. The changes in saliva concentrations paralleled the changes in plasma concentrations both for phosphorus and urea.
Figure 1.
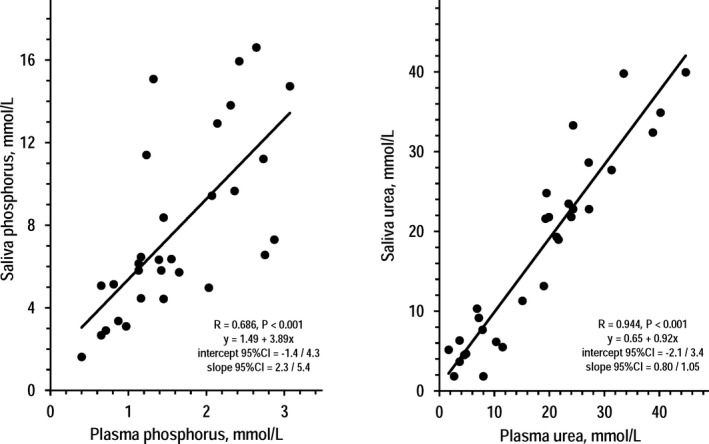
Individual values of saliva phosphorus over plasma phosphorus (left panel) and of saliva urea over plasma urea (right panel) in the group of 30 CKD patients. For each set of data, the insert reports the correlation coefficient (R), P value, and regression equation with 95% CI of the coefficients
Figure 2.
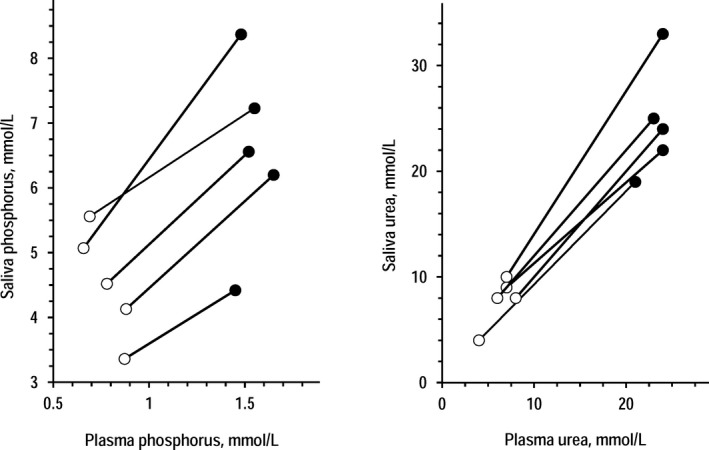
Individual changes from pre‐dialysis to post‐dialysis in saliva concentrations of phosphorus (left panel) and urea (right panel) in 5 patients undergoing a standard 4‐hour hemodialysis session. Closed circles = pre‐dialysis data; open circles = post‐dialysis data
4. DISCUSSION
The present study investigated methodological aspects in laboratory tests on saliva and correlations between plasma concentrations and saliva concentrations in patients with kidney dysfunction.
The measurements of saliva concentrations were viable with the use of automated biochemistry and commercially available kits for phosphorus and urea. Both these analytes ranged above the detection limits of the methods in all measurements. Data about blind duplicates indicated that the measurements of saliva phosphorus and saliva urea were accurate because the difference between duplicates ranged below 6%, whereas the correlation coefficients between duplicates ranged above 0.9. The site of the saliva collection did not add significant variability either for phosphorus, or for urea. The correlation coefficient between standard measurements performed in different days was >0.9 for saliva urea and ≈0.6 for saliva phosphorus indicating that, at least in the group of 15 healthy volunteers under analysis, the day‐to‐day variability was minor for saliva urea and larger for saliva phosphorus. It can be speculated that the day‐to‐day variability could reflect the influence of dietary factors, although other possibilities could not be excluded. The lack of previous data on blind duplicates, site of saliva collection, and day‐to‐day variability precluded the comparison with other studies. To comply with the restraints of space missions, the study protocol did not include any procedure to stimulate or to time the collection of saliva samples. Thus, it is unknown if the accuracy of results could differ in the case of stimulated or timed saliva collections.
The concentrations of phosphorus and urea were lower when saliva was collected mid‐morning after the breakfast as compared to standard samples collected early in the morning under fasting conditions. This datum was in agreement with the observation that saliva osmolality is lower in mid‐morning samples as compared to early morning samples.3 It is reasonable to propose that the lower concentrations in mid‐morning saliva reflected a higher dilution of saliva because an increase in plasma phosphorus and plasma urea was actually expected after the breakfast.31, 32 Other possibilities cannot be excluded. Although saliva concentrations in standard samples correlated with saliva concentrations in mid‐morning samples both for phosphorus and urea, the evidence of differences between early morning samples and mid‐morning samples pointed to the need of standardized conditions for saliva sampling to reduce the confounding of circadian rhythms.
Compared to fresh samples, frozen/thawed samples tended to have lower concentrations of phosphorus but not of urea. The lack of previous data on differences in laboratory tests between fresh saliva and frozen/thawed saliva precluded the comparison with other studies. The observation that the freezing/thawing effects differed between phosphorus and urea suggested that differences in the solubility of the analytes could be involved.
Data in the group of CKD patients together with the subset of pre‐dialysis and post‐dialysis data coherently indicated that saliva phosphorus was several times higher than plasma phosphorus and positively correlated with plasma phosphorus. The finding of a positive correlation between saliva phosphorus and plasma phosphorus was in accordance with the conclusions of the study by Savica et al where samples were collected after an overnight fast14 but was in contrast with the conclusions of the study by Rodrigues et al where samples were collected after 1‐hour fast.6 The past observation of absent correlation using samples collected after 1‐hour fast together with the present observation of reduced saliva phosphorus three hours after breakfast supported the interpretation that the lack of correlation in the study of Rodrigues et al could reflect the confounding of a short fasting before the collection of saliva samples. Regardless of the mechanism(s) accounting for the abundance of phosphorus in saliva, the evidence of a correlation between saliva phosphorus and plasma phosphorus could be explained hypothesizing that plasma phosphorus controls the secretion and/or the diffusion of phosphorus in saliva. With regard to urea, its saliva concentrations were almost identical and highly correlated with plasma urea in accordance with previous observations.10, 11, 13, 15 The high‐order correlation of saliva urea with plasma urea and the identity of urea concentrations in plasma and saliva reasonably reflected the chemical characteristics of urea, that is, extremely soluble and diffusive. The observations of similar post‐dialysis changes in plasma concentrations and in saliva concentrations of phosphorus and urea suggested that the correlations observed in non‐dialysis CKD patients reflected rapid cross‐talks between plasma and saliva rather than a long‐term equilibrium.
Study results have practical implications also. Besides the possible uses of saliva in aerospace medicine for monitoring the metabolic conditions of astronauts during space missions,22, 25 saliva laboratory tests could be useful noninvasive procedures for screening or follow‐up of kidney dysfunction and associate disorders. The noninvasivity of saliva sampling makes saliva particularly suitable in those cases in which the collection of blood samples would be difficult or undesirable, such as children, patients with arduous venipuncture, patients with the need of many repeated samples, and patients on peritoneal dialysis.
Briefly, the study reported new observations about phosphorus and urea measurements in saliva and about the correlation of saliva concentrations with plasma concentrations. The results indicated the saliva tests are accurate and well correlated with blood tests both for phosphorus and for urea. Moreover, the study results indicated also that the time of the saliva collection and the freezing/thawing procedure affect the results of the measurements in saliva.
ACKNOWLEDGMENTS
The study was made possible thanks to a grant of the Italian Space Agency (Agenzia Spaziale Italiana, ASI, contract n. 2013‐040‐I.0).
Bilancio G, Cavallo P, Lombardi C, et al. Salivary levels of phosphorus and urea as indices of their plasma levels in nephropathic patients. J Clin Lab Anal. 2018;32:e22449 10.1002/jcla.22449
REFERENCES
- 1. Peng CH, Xia YC, Wu Y, Zhou ZF, Cheng P, Xiao P. Influencing factors for saliva urea and its application in chronic kidney disease. Clin Biochem. 2013;46:275‐277. [DOI] [PubMed] [Google Scholar]
- 2. Gonçalves AC, de Lima Marson FA, de Holanda Mendonça RM, et al. Saliva as a potential tool for cystic fibrosis diagnosis. Diagn Pathol. 2013;8:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Perrier E, Demazieres A, Girard N, et al. Circadian variation and responsiveness of hydration biomarkers to changes in daily water intake. Eur J Appl Physiol. 2013;113:2143‐2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Venkatapathy R, Govindarajan V, Oza N, Parameswaran S, Dhanasekaran BP, Prashad KV. Salivary creatinine estimation as an alternative to serum creatinine in chronic kidney disease patients. Int J Nephrol. 2014;2014:742724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seethalakshmi C, Koteeswaran D, Chiranjeevi V. Correlation of serum and salivary biochemical parameters in end stage renal disease patients undergoing hemodialysis in pre and post‐dialysis state. J Clin Diag Res. 2014;8:C12‐CC14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrigues VP, Franco MM, Marques CPC, et al. Salivary levels of calcium, phosphorus, potassium, albumin and correlation with serum biomarkers in hemodialysis patients. Arch Oral Biol. 2016;62:58‐63. [DOI] [PubMed] [Google Scholar]
- 7. Zhao J, Huang Y. Salivary uric acid as a noninvasive biomarker for monitoring the efficacy of urate‐lowering therapy in a patient with chronic gouty arthropathy. Clin Chim Acta. 2015;450:115‐120. [DOI] [PubMed] [Google Scholar]
- 8. Fortes MB, Owen JA, Raymond‐Barker P, et al. Is this elderly patient dehydrated? Diagnostic accuracy of hydration assessment using physical signs, urine, and saliva markers. JAMDA. 2015;16:221‐228. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki M, Furuhashi M, Sesoko S, et al. Determination of creatinine‐related molecules in saliva by reversed‐phase liquid chromatography with tandem mass spectrophotometry and the evaluation of hemodialysis in chronic kidney disease patients. Anal Chim Acta. 2016;911:92‐99. [DOI] [PubMed] [Google Scholar]
- 10. Lasisi TJ, Raji YR, Salako BL. Salivary creatinine and urea analysis in patients with chronic kidney disease: a case control study. BMC Nephrol. 2016;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pandya D, Nagrajappa AK, Ravi KS. Assessment and correlation of urea and creatinine levels in saliva and serum of patients with chronic kidney disease, diabetes and hypertension– a research study. J Clin Diag Res. 2016;10:ZC58‐ZC62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yajamanam N, Vinapamula KS, Sivakumar V, Bitla AR, Srinivasa Rao PVLN. Utility of saliva as a sample to assess renal function and estimated glomerular filtration rate. Saudi J Kidney Dis Transpl. 2016;27:312‐319. [DOI] [PubMed] [Google Scholar]
- 13. Pham TAV. Validation of the salivary urea and creatinine tests as screening methods of chronic kidney disease in Vietnamese patients. Acta Odontol Scand. 2017;75:551‐556. [DOI] [PubMed] [Google Scholar]
- 14. Savica V, Calò LA, Granata A, et al. A new approach to the evaluation of hyperphosphatemia in chronic kidney disease. Clin Nephrol. 2007;68:216‐221. [PubMed] [Google Scholar]
- 15. Bagalad BS, Mohankumar KP, Madhushankari GS, Donoghue M, Kuberappa PH. Diagnostic accuracy of salivary creatinine, urea, and potassium levels to assess dialysis need in renal failure patients. Dent Res J (Isfahan). 2017;14:13‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutherfurd‐Markwick K, Starck C, Dulson DK, Ali A. Salivary diagnostic markers in males and females during rest exercise. J Int Soc Sports Nutr. 2017;14:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Javor A, Riedl R, Kindermann H, Brandstätter W, Ransmayr G, Gabriel M. Correlation of plasma and salivary oxytocin in healthy young men—experimental evidence. Neuro Endocrinol Lett. 2014;35:470‐473. [PubMed] [Google Scholar]
- 18. Patel BJ, Dave B, Dave D, Karmakar P, Shah M, Sarvaiya B. Comparison and correlation of glucose levels in serum and saliva of both diabetic and non‐diabetic patients. J Int Oral Health. 2015;7:70‐76. [PMC free article] [PubMed] [Google Scholar]
- 19. Rathnayake N, Buhlin K, Kjellström B, et al. Saliva and plasma levels of cardiac related biomarkers in post myocardial infarction patients. J Clin Periodontol. 2017;44:692‐699. [DOI] [PubMed] [Google Scholar]
- 20. Celec P, Tóthová L, Šebeková K, Podracká L, Boor P. Salivary markers of kidney function — potentials and limitations. Clin Chim Acta. 2016;453:28‐37. [DOI] [PubMed] [Google Scholar]
- 21. Kaczor‐Urbanowicz KE, Carreras‐Presas CM, Aro K, Tu M, Garcia‐Godoy F, Wong DTW. Saliva diagnostics – current views and directions. Exp Biol Med. 2017;242:459‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicogossian AE, Rummel JD, Leveton L, Teeter R. Development of countermeasures for medical problems encountered in space flight. Adv Space Res. 1992;12:323‐328. [DOI] [PubMed] [Google Scholar]
- 23. Bilancio G, Lombardi C, Pisot R, et al. Effects of prolonged immobilization on sequential changes in mineral and bone disease parameters. Am J Kidney Dis. 2013;61:845‐848. [DOI] [PubMed] [Google Scholar]
- 24. Bilancio G, Lombardi C, Pisot R, De Santo NG, Cavallo P, Cirillo M. Effects of bed‐rest on urea and creatinine: correlation with changes in fat‐free mass. PLoS ONE. 2014;9:e108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. NASA.gov [homepage on the internet] . Washington, DC: National Aeronautics and Space Administration. https://www.nasa.gov/missionpages/station/research/experiments/312.html. Accessed March 24, 2018.
- 26. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Int Med. 2003;139:137‐147. [DOI] [PubMed] [Google Scholar]
- 27. National Kidney Foundation . KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66:884‐930. [DOI] [PubMed] [Google Scholar]
- 28. Petrušić N, Posavac M, Sabol I, Mravak‐Stipetić M. The effect of tobacco smoking on salivation. Acta Stomatol Croat. 2015;49:309‐315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sarstedt.com [homepage on the internet] . Nümbrecht, DE. https://www.sarstedt.com/fileadmin/user_upload/99_Broschueren/Englisch/156_Salivette_GB_0813.pdf. Accessed March 24, 2018.
- 30. Pauli D, Seyfarth M, Dibbelt L. The Abbott Architect c8000: analytical performance and productivity characteristics of a new analyzer applied to general chemistry testing. Clin Lab. 2005;51:31‐41. [PubMed] [Google Scholar]
- 31. Portale AA, Halloran BP, Morris RC Jr. Dietary intake of phosphorus modulates the circadian rhythm in serum concentration of phosphorus. Implications for the renal production of 1,25‐dihydroxyvitamin D. J Clin Invest. 1987;80:1147‐1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bankir L. Urea and the kidney In: Brenner BM, ed. The kidney, 5th edn Philadelphia: WB Saunders; 1996:571‐606. [Google Scholar]


