Abstract
Objectives
This study was undertaken to determine the diagnostic and prognostic values of galectin‐3 (Gal‐3) in patients with chronic kidney disease (CKD).
Methods
Patients with CKD (n=150) were enrolled as the CKD group, which was divided into six groups according to glomerular filtration rates (GFR) indexes. At the same time, 50 healthy adults were chosen for the control group (NC). Measured data included the levels of serum Gal‐3, serum creatinine (SCr), β2‐microglobulin (β2‐MG), 24‐hour urinary protein, cystatin C (CysC), serum albumin (Alb) and other related indicators.
Results
There was no significant difference between CKD and NC group in age, gender and the level of Alb. CKD group had lower estimated glomerular filtration rate (eGFR) but higher Gal‐3, CysC, SCr, β2 MG and 24‐hour urinary protein excretion than control group (P<.001). Moreover, the receiver operating characteristic (ROC) analysis of Gal‐3, CysC and SCr revealed that the corresponding areas under the curve (AUC) were 0.89, 0.83 and 0.85, respectively, and the AUC value of joint ROC curve of Gal‐3, CysC and SCr was 0.96. In addition, the 6‐year kidney survival rates of low Gal‐3 group and high Gal‐3 group were 47.3% and 22.8% respectively (HR=2.65; P<.01).
Conclusions
Our study verified Gal‐3, CysC and SCr were negatively related to eGFR. Besides, it is suggested that Gal‐3 can be used as an indicating factor in the diagnosis of CKD; the joint analysis of Gal‐3, CysC and SCr for CKD may distinctly improve diagnostic accuracy.
Keywords: chronic kidney disease, diagnosis, Gal‐3, glomerular filtration rates, prognosis
1. Introduction
Chronic kidney disease (CKD) is a term used to describe heterogeneous disorders damaging the structure and function of the kidney. To be more precisely, CKD is defined as kidney damage or glomerular filtration rate (GFR)<60 mL/min/1.73 m2 for 3 months or longer.1, 2 The approximate prevalence of CKD is 8‐16% worldwide.2 Up to 25‐35% of those older than 65 years suffer from CKD according to current statistics.3 The etiology of CKD is complex, and it is generally accepted that diabetes and hypertension are the primary causes of CKD.2 There also are secondary causes contributing to the development of CKD, such as old age, obesity, cardiovascular infection and genetic factors.1, 2 Common effects of CKD include loss of renal function resulting in end‐stage renal disease, accelerating cardiovascular disease (CVD) and potentially death.4 Although early CKD is usually asymptomatic, it can be detected during the assessment of comorbid diseases and can be treated with success. However, the rapid development of CKD in some patients often leads to kidney failure within 1 month.5 Therefore, new diagnostic tools are necessary to identify CKD patients in order to slow or reverse kidney function decline.
Galectin‐3, a 29‐ to 35‐kDa protein, is a member of soluble β‐galactoside‐binding lectins. It presents as a ubiquitous localization in the cell, but it can also be secreted to the extracellular space in the kidney and in the heart.6, 7 The dual localization of Gal‐3 means that Gal‐3 exerts multiple functions, such as the pre‐mRNA splicing factor, regulating cell cycle, modulation of inflammatory/immune function and promoting fibrogenesis.8, 9 One previous study showed that Gal‐3 was an effective indicator of heart failure and mortality,8 and Gal‐3 expression is increased in models of inflammatory disease and cancer.10 Likewise, Gal‐3 functions similarly in patients with CKD. The development of progressive CKD will cause tubulointerstitial fibrosis.11 The expression and secretion of Gal‐3 have been proven to accelerate the development of tubulointerstitial fibrosis in mice.12 Previous studies showed that higher circulating Gal‐3 levels were related with the increased risk of incident CKD and the rapid loss of kidney function.13 Moreover, recent research also showed that circulating Gal‐3 concentrations are inversely associated with renal function, and are only related to clinical outcomes in patients with impaired renal function.10 Therefore, Gal‐3 has potential value as a diagnostic and prognostic marker of CKD.
Early diagnosis of CKD is essential to prevent the progression of CKD and reduce the risk of cardiovascular morbidity and mortality.2 Currently, the most effective tool to measure renal function and diagnose patients with CKD is GFR detection.14 A number of equations have been presented to estimate GFR, and the most common equations used in the general population are based on serum creatinine (SCr) or serum cystatin C (CysC).15 SCr is easy to measure, thus, it is commonly used in clinical diagnosis. However, this method is biased because creatinine is also generated from muscle mass and its levels are associated with other factors such as age and sex.16 CysC has more advantages compared with creatinine because its non‐GFR determinants are less than that of SCr, and CysC is a good predictor for subsequent CVD and mortality.17 However, the non‐GFR determinants of CysC are poorly understood.1 Thus, the further research in finding a new novel marker for CKD and clarification of the non‐GFR determinants of those markers is necessary.
Our study measures the SCr, CysC and serum Gal‐3 levels, and then compares those levels in CKD group (150 patients with CKD) and NC group (50 healthy adults) in order to explore the diagnostic value of GFR in patients and its relationship with clinical prognosis.
2. Materials and Methods
2.1. Study objects
Patients (n=150) diagnosed with CKD were collected from May 2009 to May 2010 during inpatient treatment, and functioned as the CKD group in this study. Inclusion criteria were as follows: (1) meet the “CKD assessment and management of clinical practice guidelines” released by KDIGO in 2009; (2) the patients had no other kidney diseases such as infection, kidney cancer or perinephritis which could affect test results; (3) exclude coinfection and other allergic diseases, cancer, pulmonary fibrosis, liver failure, heart failure, cerebrovascular and other serious systemic diseases.
According to patients’ condition of CKD, the patients were classified into five stages and those below grade 4 were followed up on for 72 months. In line with the classification criteria of GFR, the CKD group was further subdivided into G1 group (GFR>90 mL/(min*1.73 m2)), G2 group (GFR: 60‐89 mL/(min*1.73 m2)), G3 group (GFR: 30‐59 mL/(min*1.73 m2)), G4 group (GFR: 15‐29 mL/(min*1.73 m2)) and G5 group (GFR<15 mL/(min*1.73 m2)).18
Healthy adults (n=50) were selected as the healthy control group (NC) with the selection criteria of normal renal function and no hematuria or proteinuria. Patients were excluded to exclude other interference factors under any of the following circumstances: patients were diagnosed clearly with CKD, myocardial damage, pulmonary fibrosis, end‐stage cirrhosis with liver failure, liver neoplasms with liver failure, other system tumors and allergic diseases when admitted to hospital.
Procedures were in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Human Experimentation of Affiliated Hospital of Nantong University. All patients included in this study have signed handwritten consent documents.
2.2. Specimen collection
In the case of strict sterile operation, peripheral vein blood from all subjects under fasting conditions was collected in a volume of 3 mL with 10 minutes standing at room temperature. Then the blood sample was handled in a centrifuge at 4°C with a speed of 698.75 g for 10 minutes, and the serum was collected and kept in the refrigerator at −80°C. Meanwhile, urine samples were collected from each subject including CKD patients and the healthy control group. Thus, detection of the levels of Gal‐3, SCr, β2‐microglobulin (β2‐MG), 24‐hour‐urine protein, CysC, serum albumin (Alb) and other related indicators was possible, and these levels were recorded.
2.3. Detection method and reagent
Enzyme linked immunosorbent assay (ELISA) has been previously described in detail.19, 20 Briefly, whole blood samples were collected through venipuncture in non‐fasting subjects, processed to plasma (i.e., immediate centrifugation for 10 minutes at 1300 g), and stored at −80°C, then Gal‐3 and SCr levels were measured, according to the manufacturer's instructions, using the human Gal‐3 and creatinine ELISA (Bender MedSystem, Vienna, Austria). Immune turbidimetric method was taken to determine the levels of β2‐MG and urinary protein. The method was illustrated in previous studies.21, 22 Bromocresol green (BCG) method was used for the determination of Alb. The method was introduced in previous studies.23
2.4. Calculation formula of eGFR
According to the CKD‐epidemiology (CKD‐EPI) creatinine equation formula24 modified on basis of demographic data of the country in which the study took place, estimated glomerular filtration rate (eGFR) was calculated as below: eGFR=a×(serum creatinine/b)c×(0.993)age. The variable a takes on the following values on the basis of race and sex: in Asia, women=144, men=141; the variable b takes on the following values on the basis of sex: women=0.7, men=0.9; the variable c takes on the following values on the basis of sex and creatinine measurement: women serum creatinine≤0.7 mg/dL=−0.329, serum creatinine>0.7 mg/dL=−1.209; men serum creatinine≤0.7 mg/dL=−0.411, serum creatinine>0.7 mg/dL=−1.209.
2.5. Statistical analysis
Measurement data between two groups were processed using t‐test or rank sum test (Mann‐Whitney U‐test), while measurement data among multiple groups were done using one‐way ANOVA or non‐parametric Kruskal‐Wallis test. The chi‐square test was adopted to handle counting data. P<.05 indicates that the difference was significant. MedCalc 11 (Medcalc Software, Mariakerke, Belgium) was used to evaluate the diagnostic value of serum Gal‐3, SCr and CysC for CKD. Receiver operating characteristic curve (ROC curve) was utilized when an index was evaluated in the diagnosis of diseases. Area under curve (AUC) of ROC curve between 0.7 and 0.9 shows there is a certain accuracy for diagnosing, while there is a higher accuracy when AUC more than 0.9. Single factor analysis and multivariate analysis were performed for all the factors respectively, then the whole biological indicators were analyzed by logistic regression method. GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) was utilized to analyze the kidney survival rate of CKD group.
3. Results
3.1. Comparison of Gal‐3 and other clinical indicators between CKD and healthy control
Statistics results (as shown in Table 1) showed that the level of eGFR in CKD group was significantly lower than those in NC group (P<.001). The levels of Gal‐3, CysC, SCr, β2‐MG and 24‐hour urinary protein excretion in CKD group were higher than those in NC group (P<.001), while the level of Alb (P=.303) was not significantly different between two groups. Besides, age (P=.318), gender (P=.742) were not significantly different factors in the onset of CKD. Staging and grouping patients with CKD in line with the state of eGFR, the results showed that number of patients in stage G1, G2, G3, G4, and G5 were 16 (10.7%), 27 (18.0%), 35 (23.3%), 50 (33.3%), and 22 (14.7%), respectively.
Table 1.
Comparison of Gal‐3 and other clinical indicators between CKD and healthy control
| Group | CKD (n=150) | NC (n=50) | P value |
|---|---|---|---|
| Age (years) | 51.7±10.7 | 49.5±10.6 | .318a |
| Gender (male/female) | 83/67 | 29/21 | .742b |
| eGFR, mL/(min*1.73 m2) | 46.0±28.4 | 102.2±26.2 | <.001a |
| eGFR grade, n (%) | |||
| G1≥90 mL/(min*1.73 m2) | 16 (10.7%) | ||
| G2 60‐89 mL/(min*1.7 3m2) | 27 (18.0%) | ||
| G3 30‐59 mL/(min*1.73 m2) | 45 (23.3%) | ||
| G4 15‐29 mL/(min*1.73 m2) | 40 (33.3%) | ||
| G5<15 mL/(min*1.73 m2) | 22 (14.7%) | ||
| Gal‐3 (ng/mL) | 6.0±0.9 | 4.2±1.2 | <.001a |
| CysC (mg/L) | 1.33±0.41 | 0.85±0.28 | <.001a |
| SCr (mg/dL) | 1.54±0.49 | 0.99±0.22 | <.001a |
| β2MG (mg/dL) | 63.1±15.6 | 35.4±11.2 | <.001a |
| Alb (g/dL) | 3.91±0.72 | 4.05±0.56 | .303a |
| Urine protein (g/24 h) | 1.67±0.81 | 0.11±0.03 | <.001a |
Mann‐Whitney test.
Chi‐square test.
CKD, Chronic kidney diseases; NC, Normal control; eGFR, Estimated glomerular filtration rate; Gal‐3, Galectin‐3; CysC, Cystatin C; SCr, Serum creatinine; β2MG, β2 microglobulin; Alb, serum albumin.
3.2. Evaluation on the role of Gal‐3 in the diagnosis of CKD by ROC curve
The first step of this section was taking Gal‐3, CysC and SCr as test variables, and the clinical diagnosis results as state variables (0 means no CKD, 1 means extant CKD) to plot ROC curve (as shown in Figure 1). The ROC analysis results revealed the AUC of Gal‐3, CysC and SCr in diagnosis of CKD were 0.89, 0.83, and 0.85 with 95% CI 0.89‐0.93, 0.77‐0.88 and 0.79‐0.90 respectively. The differences were statistically significant which suggested the above three indicators can be utilized in the diagnosis of CKD. Wherein the sensitivity and specificity of Gal‐3 in diagnosis of CKD were 0.68 and 0.90, the sensitivity and specificity of CysC in diagnosis of CKD were 0.60 and 0.92, and the sensitivity and specificity of SCr were 0.71 and 0.92, respectively. In conclusion, the results revealed that all three indexes may be explored as a factor in the diagnosis of CKD. Multivariate logistic regression analysis (as shown in Table 2) showed that Gal‐3, CysC and SCr were correlated with CKD independently. Based on logistic regression coefficients, we combined the risk scores of Gal‐3, CysC, and SCr to establish the joint predictor model: logt (P)=−46.208×Gal‐3+11.707×CysC+20.499×SCr, including the variables and statistics. According to the above predictor model, the joint ROC curve of Gal‐3, CysC, and SCr was established to obtain the combined effect value as a new standard for the diagnosis of CKD (as shown in Figure 1). The sensitivity and specificity of the combined value were significantly increased to 0.99 and 0.90 with area under the curve of 0.96 and 95% CI of 0.93–0.99.
Figure 1.
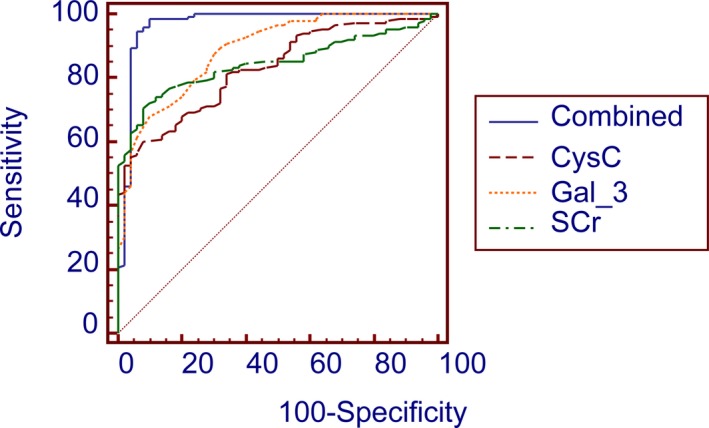
Receiver operating characteristic curves of Gal‐3, cystatin C, and SCr in the diagnosis of CKD
Table 2.
Multivariate logistic regression analysis among Gal‐3, CysC, and SCr
| Variable | Coefficient | SE | P value |
|---|---|---|---|
| Gal‐3 | −46.208 | 8.047 | <.001 |
| CysC | 11.707 | 1.828 | <.001 |
| SCr | 20.499 | 4.603 | <.001 |
Gal‐3, Galectin‐3; CysC, Cystatin C; SCr, Serum creatinine.
3.3. The diagnostic value of Gal‐3 in the 6‐year renal survival rate of patients with CKD
The survival curve began when patients received a definite diagnosis of CKD when admitted to the hospital, and the endpoint was taken as when the patient entered into the final period of CKD (G5 period) with GFR<15 mL/(min*1.73 m2). Collecting data of duration and 6‐year renal survival rate of CKD G5 patients whose follow‐up time was more than 2 years, results from retrospective analysis showed that the median duration from initial treatment to CKD G5 of all patients was 5.8 years; therefore 6‐year renal survival rate of patients with CKD was carried out.
On the basis of the level of CysC in CKD group, we took the level of 1.48 mg/L as the standard, and classified the level of CysC less than or equal to 1.48 mg/L in patients with CKD (n=97) as low CysC expression group, more than 1.48 mg/L in patients with CKD (n=53) as high CysC expression group. Similarly, according to the level of SCr in CKD group, 133 mol/L performed as the standard. For the low SCr expression group, the level of SCr was less than or equal to 133 mol/L in patients with CKD (n=100); for high SCr expression group, the level was more than 133 mol/L in patients with CKD (n=50). Meanwhile, 6 ng/mL was used as the standard in CKD group when concerned with the level of Gal‐3, low Gal‐3 expression group was considered when the level of Gal‐3 was less than 6 ng/mL in patients with CKD (n=93), and high Gal‐3 expression group was considered when the level of Gal‐3 was more than or equal to 6 ng/mL in patients with CKD (n=57). Therefore, we made assessment on diagnostic values of CysC, SCr and Gal‐3 expression levels in 6 years kidney survival rate of patients diagnosed with CKD.
The results (as shown in Figure 2) showed that the 6 years renal survival rate of low Gal‐3 expression group (n=93) and high Gal‐3 expression group (n=57) were 47.3% and 22.8% respectively with statistically significant difference (HR =2.66; 95% CI: 1.68‐4.23; P<.01). In terms of the precision of evaluation on survival rate in patients with CKD, the indicator of Gal‐3 was higher than CysC (HR =1.61; 95% CI: 1.03‐2.52; P=.038) and SCr (HR =1.56; 95% CI: 0.99‐2.47; P=.057).
Figure 2.
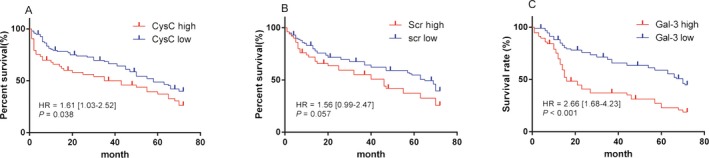
Six‐year renal survival rate curves of cystatin C (A), SCr (B) and Gal‐3 (C)
4. Discussion
Chronic kidney disease is a type of disease which occurs frequently in recent decades, and has become a public health problem because it can result in many other diseases such as CVD, heart failure and various infections.25 The causes of CKD are diverse and complex, and the development of CKD is a long process, during which eGFR declines. Early diagnosis of CKD is very important to enhance therapy efficacy and protect patients from pain at end stage, thus improve their life qualify. Although there are some potential diagnostic indicators, their accuracy is not satisfying and they cannot predict early stage CKD. As a result, the discovery and development of novel biomarkers which can diagnose early‐stage CKD is an urgent project.
In our study, we investigated the association between CKD and various indictors such as Gal‐3, SCr, β2‐MG, 24‐hour‐urine protein, CysC and Abl. The results showed that Gal‐3, SCr, β2‐MG, 24‐hour‐urine protein, CysC were negatively related with eGFR, while Abl was positively correlated with eGFR. Further analysis was performed to assess the diagnostic value of Gal‐3, CysC and SCr in CKD using ROC model. Based on the result of logistic regression analysis, all of the three indexes can be used as indicators of CKD diagnosis in consideration of specificity and sensitivity. Besides, this study demonstrated that compared to Gal‐3 alone, its combination with SCr and CysC is more accurate for CKD diagnosis.
Gal‐3 is a type of lectin binding to beta‐galactoside, and resulting in inflammation, fibrosis, and immune response. Tang et al.26 reported that elevated plasma Gal‐3 level is correlated with poor kidney function, including lower eGFR and higher CysC, whereas whether it can be used to diagnose CKD is not mentioned here. Consistent with this, Conall et al. also found that higher plasma Gal‐3 level is associated with decreased eGFR, and a higher risk of incident CKD based on the analysis report of renal outcome from 2450 samples with a mean follow‐up of 10 years.13 These reports are consistent with our results.
Creatinine has been used as a diagnostic indicator of CKD for a long time. Ratio of albumin‐to‐creatinine in urine was reported as a reliable predicator of CKD.27 In addition, creatinine level in saliva can also be used to diagnose CKD.28 Moreover, Silva et al.29 reported that creatinine in dry blood sample is a convenient marker for CKD screening. But some limitations exist for creatinine as a diagnostic indicator. For example, a study reported that estimated GFR through CKD‐EPI equation based on creatinine is overestimated compared to the exact GFR in patients with cirrhosis.30 To improve the diagnostic accuracy of current indicators in CKD, some scientists combine different biomarkers and investigate the correlation between this indicator cocktail and CKD, to find more exact biomarkers for CKD diagnosis. Coincidently, what we have done in this study offers potential to solve this problem and proves that combined use of three indicators enhance the accuracy of CKD diagnosis. In fact, other studies also reported similar outcomes.
CysC is an inhibitor of cysteine protease and has been suggested as a diagnostic indicator of kidney disease superior to serum creatinine.31 Ng et al.32 investigated the correlation of CKD diagnosis through CysC or creatinine and kidney disease in a clinical trial containing 1725 Indian adults, and the data showed that when compared with CKD diagnosed based on two markers alone, CKD defined by the combination of both are more strongly associated with retinopathy. To find an accurate biomarker of CKD in cirrhotic patients, Krones et al.30 compared measured GFR (gold standard) and eGFR according to the equation of CysC or creatinine, and the results showed that estimated GFR obtained by CKD‐ EPI equation based on the combination of CysC and creatinine is closer to exact GFR. The above reports are consistent with our results, and when taken together show that the combination of all three biomarkers is more accurate.
A further benefit of our study is that we have identified and delineated the prognosis value of Gal‐3. Although Zamora et al.33 reported that the prediction role of Gal‐3 in kidney disease influences its prognostic accuracy in heart failure, whether Gal‐3 can be effectively used in CKD diagnosis was not mentioned. There have been no related publications regarding this question until now. In this study, we have analyzed association between the level of Gal‐3, CysC, SCr, and 6‐year‐survival of kidney. The data showed that higher levels of Gal‐3, CysC, and SCr were correlated with shorter survival time of the kidney. This suggests that these three indicators could all be used in the diagnosis of patients with CKD, and the diagnostic value of Gal‐3 is significantly better than CysC or SCr. However, there are some limitations in our study. The sample size is not large enough, so further verification in larger populations is necessary. Besides, the detection of Gal‐3 is expensive and time‐consuming.
In this study, the use of a biomarker cocktail provided a more accurate prediction method for CKD diagnosis; the discovery of the diagnostic value of Gal‐3, CysC, and SCr offers to enrich the clinical applications of their use in CKD. We provide new avenues for clinical research and also supply an auxiliary reference for the diagnosis of patients with CKD.
Conflict of Interest
The authors have declared no conflict of interest.
Acknowledgments
It is funded by Nantong Health Bureau Youth Fund (Grant No. WQ2015026).
Fen Ji and Shuqin Zhang are both first authors and contributed equally to this work.
References
- 1. Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. [DOI] [PubMed] [Google Scholar]
- 2. Jha V, Garcia‐Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 3. James MT, Hemmelgarn BR, Tonelli M. Early recognition and prevention of chronic kidney disease. Lancet. 2010;375:1296–1309. [DOI] [PubMed] [Google Scholar]
- 4. Kiuchi MG, Mion D Jr. Chronic kidney disease and risk factors responsible for sudden cardiac death: a whiff of hope? Kidney Res Clin Pract. 2016;35:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Levin A, Bakris GL, Molitch M, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. [DOI] [PubMed] [Google Scholar]
- 6. Wang L, Guo XL. Molecular regulation of galectin‐3 expression and therapeutic implication in cancer progression. Biomed Pharmacother. 2016;78:165–171. [DOI] [PubMed] [Google Scholar]
- 7. Ravani P, Barrett BJ. Galectin‐3 and new‐onset CKD: marker or mediator? J Am Soc Nephrol. 2013;24:1342–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pugliese G, Iacobini C, Ricci C, Blasetti Fantauzzi C, Menini S. Galectin‐3 in diabetic patients. Clin Chem Lab Med. 2014;52:1413–1423. [DOI] [PubMed] [Google Scholar]
- 9. Thijssen VL, Heusschen R, Caers J, Griffioen AW. Galectin expression in cancer diagnosis and prognosis: a systematic review. Biochim Biophys Acta. 2015;1855:235–247. [DOI] [PubMed] [Google Scholar]
- 10. Drechsler C, Delgado G, Wanner C, et al. Galectin‐3, renal function, and clinical outcomes: results from the LURIC and 4D studies. J Am Soc Nephrol. 2015;26:2213–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez‐Iturbe B, Johnson RJ, Herrera‐Acosta J. Tubulointerstitial damage and progression of renal failure. Kidney Int Suppl. 2005;99:S82–S86. [DOI] [PubMed] [Google Scholar]
- 12. Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin‐3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Seaghdha CM, Hwang SJ, Ho JE, Vasan RS, Levy D, Fox CS. Elevated galectin‐3 precedes the development of CKD. J Am Soc Nephrol. 2013;24:1470–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou BO, Nie J, Yang W, Huang C, Huang YE, Zhao H. Effect of hydrothorax EGFR gene mutation and EGFR‐TKI targeted therapy on advanced non‐small cell lung cancer patients. Oncol Lett. 2016;11:1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Florkowski CM, Chew‐Harris JS. Methods of estimating GFR ‐ different equations including CKD‐EPI. Clin Biochem Rev. 2011;32:75–79. [PMC free article] [PubMed] [Google Scholar]
- 16. Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function–measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. [DOI] [PubMed] [Google Scholar]
- 17. Shlipak MG, Sarnak MJ, Katz R, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. New Engl J Med. 2005;352:2049–2060. [DOI] [PubMed] [Google Scholar]
- 18. Levey AS, Coresh J, Balk E, et al. , National Kidney F . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 19. Lee YJ, Kang SW, Song JK, et al. Serum galectin‐3 and galectin‐3 binding protein levels in Behcet's disease and their association with disease activity. Clin Exp Rheumatol. 2007;25(4 Suppl 45):S41–S45. [PubMed] [Google Scholar]
- 20. Filler G, Witt I, Priem F, Ehrich JH, Jung K. Are cystatin C and beta 2‐microglobulin better markers than serum creatinine for prediction of a normal glomerular filtration rate in pediatric subjects? Clin Chem. 1997;43(6 Pt 1):1077–1078. [PubMed] [Google Scholar]
- 21. Pezzilli R, Billi P, Fiocchi M, et al. Serum beta 2‐microglobulin in chronic diseases of the pancreas. Int J Pancreatol. 1995;17:161–166. [DOI] [PubMed] [Google Scholar]
- 22. Barratt J, Topham P. Urine proteomics: the present and future of measuring urinary protein components in disease. CMAJ. 2007;177:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971;31:87–96. [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hall JA, Yerramilli M, Obare E, Almes K, Jewell DE. Serum concentrations of symmetric dimethylarginine and creatinine in dogs with naturally occurring chronic kidney disease. J Vet Int Med. 2016;30:794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang WH, Shrestha K, Shao Z, et al. Usefulness of plasma galectin‐3 levels in systolic heart failure to predict renal insufficiency and survival. Am J Cardiol. 2011;108:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huan L, Yuezhong L, Chao W, HaiTao T. The urine albumin‐to‐creatinine ratio is a reliable indicator for evaluating complications of chronic kidney disease and progression in IgA nephropathy in China. Clinics (Sao Paulo). 2016;71:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lasisi TJ, Raji YR, Salako BL. Salivary creatinine and urea analysis in patients with chronic kidney disease: a case control study. BMC Nephrol. 2016;17:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva AC, Gomez JF, Lugon JR, Graciano ML. Creatinine measurement on dry blood spot sample for chronic kidney disease screening. Jornal brasileiro de nefrologia: ‘orgao oficial de Sociedades Brasileira e Latino‐Americana de. Nefrologia. 2016;38:15–21. [DOI] [PubMed] [Google Scholar]
- 30. Krones E, Fickert P, Zitta S, et al. The chronic kidney disease epidemiology collaboration equation combining creatinine and cystatin C accurately assesses renal function in patients with cirrhosis. BMC Nephrol. 2015;16:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tenstad O, Roald AB, Grubb A, Aukland K. Renal handling of radiolabelled human cystatin C in the rat. Scand J Clin Lab Invest. 1996;56:409–414. [DOI] [PubMed] [Google Scholar]
- 32. Ng WY, Teo BW, Tai ES, et al. Cystatin C, chronic kidney disease and retinopathy in adults without diabetes. Eur J Prev Cardiol. 2016;23:1413–1420. [DOI] [PubMed] [Google Scholar]
- 33. Zamora E, Lupon J, de Antonio M, et al. Renal function largely influences Galectin‐3 prognostic value in heart failure. Int J Cardiol. 2014;177:171–177. [DOI] [PubMed] [Google Scholar]


