Abstract
Background
The level of glycated albumin in circulation is an indicator of blood glucose control over the preceding 2 weeks. It is not known if the level of glycated albumin in circulation relates to an individual's postprandial blood glucose response.
Methods
Eighty‐four euglycemic young adults (21.1 [3.9] years, BMI 23.9 [4.0] kg/m2) primarily of European descent underwent a fasted meal test of 50 g carbohydrate from white bread. Capillary blood was then sampled at regular intervals over 2 hours. Correlations were sought between glycated albumin, fasted and 2‐hour post‐load blood glucose measurements, incremental area under the blood glucose curve, glycemic range, body mass index (BMI), and C‐reactive protein (CRP).
Results
When adjusted for age and sex, glycated albumin was inversely correlated with BMI (r=−.25, P=.027). No significant correlations existed for glycated albumin and postprandial markers of blood glucose control. BMI and CRP values correlate in this population (r=.30, P=.009).
Conclusions
Glycated albumin in circulation is not related to postprandial blood glucose response in young euglycemic adults. Glycated albumin is lower in euglycemic adults with higher BMI values. Contrary to research with older adults or those with impaired glucose control, glycated albumin did not correlate to CRP.
Keywords: biomarker, blood glucose control, body mass index, glycated plasma proteins, obesity
1. Introduction
The glycated hemoglobin and glycated albumin in circulation are indicators of blood glucose control. Glycated hemoglobin is widely used to monitor blood glucose over the past 6 weeks or more.1, 2, 3 In contrast, albumin has a half‐life in circulation of 17 days,4 suggesting it suitable to monitor blood glucose control over the past 2 weeks. Albumin undergoes permanent non‐enzymatic glycation inline with the surrounding glucose concentration,5, 6 and when degraded is resorbed by most organs in the body.7 The postprandial blood glucose response varies greatly between individuals8 and can be a driver of protein glycation.9 It is therefore feasible that the level of glycated albumin in circulation may relate to an individuals postprandial blood glucose response.
There has been very little published research to date that has considered the relationship between glycated albumin and measures of postprandial blood glucose.10, 11, 12 Previous comparisons have relied on spot 1‐ or 2‐hour post‐load values,10, 11 the sum of fasting and post‐load values,12 or self‐reported values10, 11 to represent postprandial blood glucose. The reliability of self‐reported values is unclear and the use of a single 1‐ or 2‐hour value may not fully characterize postprandial blood glucose response, contributing to the current uncertainty in the relationship between glycated albumin and postprandial response.
Previous studies have reported a weak‐to‐medium inverse correlation between glycated albumin and body mass index (BMI).13, 14, 15 It is hypothesized that increased adipocytokines concentration, due to greater fat mass, may decrease albumin synthesis or increase its turnover.13 This relationship has not been observed in a range of different populations and requires further consideration. The current analysis considers glycated albumin values with postprandial measures of blood glucose, BMI, and C‐reactive protein (CRP) in a novel population of young euglycemic adults.
2. Materials and Methods
Blood samples were collected between February and March of 2014 at the research clinic of the Department of Human Nutrition, University of Otago, Dunedin, New Zealand. The University of Otago Human Ethics Committee approved this study (09/012) with all participants providing written consent. Data were collected as part of a registered clinical trial ACTRN12614000264684.
2.1. Participants
Blood samples were obtained from adults aged over 18 years old recruited through an undergraduate science degree at the University of Otago. Diagnosed diabetes mellitus, cardiovascular disease, cancer, diseases of the digestive system, food allergies, and pregnancy excluded study participation.
2.2. Data collection
Participants were asked to fast overnight for a minimum 10 hours before providing capillary blood samples. Alcohol intake and physical activity were limited in the preceding 24 hours. The average of two capillary samples taken at rest determined a fasting glucose value for each participant. Participants were asked to eat white bread containing 50 g of carbohydrate within ten minutes. Capillary blood glucose was measured at 15, 30, 45, 60, 90, and 120 minutes from meal commencement, inline with published glycemic index assessment guidelines.16 All participants provided a second fasting blood glucose value within a week of the fasted meal test to confirm normal glucose tolerance. The same restrictions for alcohol intake and physical activity were applied in the 24 hours before this second blood collection.
2.3. Measurements
Anthropometric measurements were taken after the fasted meal test. Body Mass Index is the weight in kilograms divided by height in meters squared. Capillary blood glucose was measured by HemoCue 201+ systems (HemoCue, Ängelholm, Sweden), calibrated inline with manufacturer requirements. The coefficient of variation (CV) for the control ranged from 0.00 to 0.35% on the six HemoCue 201+ systems.
The blood glucose measurements derived from the fasted meal test were fasting plasma glucose, 2 hour post‐load blood glucose (2hPG), glycemic range (peak minus nadir), and shape of the blood glucose response as measured by incremental area under the blood glucose curve (iAUC). iAUC was calculated by the trapezoidal method ignoring the area below baseline.17
Capillary blood samples >250 μL were obtained from each participant for glycated albumin assessment. Whole blood were centrifuged at 1650 RCF for 5 minutes, with plasma then stored at −80°C for <2 months. Plasma samples were thawed then lightly mixed before assessment so to homogenize the plasma matrix. Glycated albumin values were determined by enzymatic assessment (Lucica GA‐L; Asahi Kasei, Tokyo, Japan) on a COBAS C‐311 centrifugal analyser (Roche Diagnostics, Risch‐Rotkreuz, Switzerland). The coefficient of variation (CV) for high and low controls were 1.3% and 1.2%. High sensitivity C‐reactive protein (CRP) values were determined 1 month after the fasted meal test by immunoturbidimetric assessment (Roche Diagnostics) undertaken by an external, nationally accredited laboratory.
2.4. Statistical analysis
Pearson correlations are presented both unadjusted, and adjusted. The model to assess adjusted correlations included terms for BMI, age, and sex where appropriate, using a backward selection procedure. CRP values were logarithmically transformed before analysis to address skew. A P value lower than .050 was considered significant. Data are presented as mean (SD) unless stated.
3. Results
Data from 78 adults were used in this cross‐sectional analysis. Samples were collected predominately from females (80%) with a mean age of 21.1 (3.9) years. Mean participant BMI was 23.9 (4.0) kg/m2. Mean blood glucose values (fasting plasma glucose 4.7(0.5) mmol/L, 2hPG 5.3(0.8) mmol/L, glycated albumin 11.7 (1.3) %) suggest normal glucose tolerance.18, 19 Mean iAUC was 132 (74.9) mmol/L.min, mean CRP was 2.9 (1.1) mg/L.
Both unadjusted and adjusted correlations are shown in Table 1. No association was observed between glycated albumin and any marker of postprandial blood glucose control. Once adjusted glycated albumin was inversely correlated with BMI (r=−.25, P=.027). A positive correlation between body mass and CRP (r=.30, P=.030) was observed. Glycated albumin did not correlate with CRP as previously observed. Between postprandial markers of blood glucose, iAUC correlated strongly with glycemic range (r=.83, P<.001). Scatterplots of glycated albumin (%) and FPG, iAUC, 2hPG, and BMI are shown in Figure 1.
Table 1.
Unadjusted and adjusted correlations between variables
| GA | FPG | 2hPG | iAUC | Range | BMI | CRP | |
|---|---|---|---|---|---|---|---|
| GA | 0.04 | −0.11 | −0.13 | −0.13 | −0.25 * | −0.14 | |
| FPG | −0.03 | −0.14 | 0.33 * | −0.20 | 0.30 * | −0.05 | |
| 2hPG | −0.12 | 0.28 | 0.38 * | 0.19 | 0.08 | 0.12 | |
| iAUC | −0.07 | −0.47 | 0.38 | 0.83 ** | −0.25 * | 0.09 | |
| Range | −0.07 | −0.27 | 0.17 | 0.83 | −0.26 * | −0.03 | |
| BMI | −0.29 | 0.28 | 0.05 | −0.23 | −0.23 | 0.30 * | |
| CRP | −0.18 | 0.00 | 0.18 | 0.38 | −0.11 | 0.22 |
GA, glycated albumin; FPG, fasting plasma glucose; 2hPG, 2‐hour post‐load blood glucose value; iAUC, incremental area under the blood glucose curve; Range is the peak blood glucose value minus the nadir; BMI, body mass index; CRP, C‐reactive protein.
Unadjusted correlations are italicized. Adjusted correlations are shown in bold.
*P<.05; **P<.001.
Figure 1.
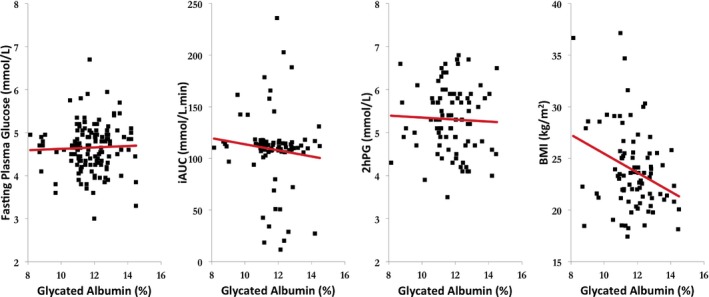
Scatterplot representation of glycated albumin (%) with blood glucose variables (FPG, 2hPG, iAUC), and BMI. FPG, fasting plasma glucose; 2hPG, 2‐hour post‐load blood glucose level; iAUC, incremental area under the curve; BMI, body mass index
4. Discussion
The findings of our study indicate that glycated albumin in circulation does not correlate with postprandial blood glucose response in euglycemic young adults. Given the role of an elevated postprandial response in protein glycation9, 20, 21 glycated albumin levels in euglycaemia is likely to reflect incidental glycation throughout the albumin lifespan. In contrast to euglycaemia, the elevated glycaemia of impaired glucose tolerance promotes albumin glycation, underpinning correlations between glycated albumin, glycated hemoglobin, and fasting plasma glucose.12
We observed a weak inverse association between glycated albumin and BMI, consistent with previous research.15 This has been hypothesized to be the result of weight‐mediated inflammatory cytokines reducing albumin synthesis and increasing its turnover. In both our study and that of Koga et al.15 a positive correlation between BMI and CRP was noted. However, glycated albumin did not correlate to CRP in our analysis. Both this analysis and that of Koga et al.15 were undertaken in adults without diabetes. The mean BMI and fasting glucose values between studies are comparable. CRP values from both studies were obtained with the same techniques.
However there were substantial differences between the participants of each study. Our participant mean age was considerably less (21.1 and 51.8 years) and our participants were of different ethnicity (European). Furthermore, the study of Koga et al. included blood glucose data from 158 normal glucose tolerance participants pooled with 54 impaired glucose tolerance19 participants.15 These differences between participants in age, ethnicity, or their inherent glycemic regulation may contribute to the differences in observed results. Older adults have higher levels of circulating CRP, potentially enabling the correlation with glycated albumin in the older participants of the study by Koga et al. A further speculation is CRP, as a general indicator of inflammation, may not adequately reflect adipocytokine levels when in lower amounts.
The primary strength of this study is use of young euglycemic participants to exclude many pharmacologic, impaired physiologic, and some lifestyle conditions that may confound previous observations. Furthermore, our calculation of iAUC and glycemic range enabled correlations between glycated albumin and a more definitive array of postprandial blood glucose markers than has been previously reported. While our findings enable comment on young euglycemic adults, a more generalized population would display higher inter variability in glycated albumin values, and a broader spectrum of risk of disease.22 Given this limitation, a relationship between glycated albumin and postprandial blood glucose response may exist in more diverse populations exhibiting a wider range of glucose tolerance.
A clear direction for further research has emerged from this cross‐sectional analysis. Our study and others have observed that glycated albumin in circulation is down regulated by higher BMI. Given that type 2 diabetes is associated with additional body fat,23, 24, 25 future research must consider if glycated albumin values should be adjusted relative to the weight of individuals. The precise nature of the relationship between glycated albumin and BMI is unknown, however, it is present independent of age, level of blood glucose control, and has now been observed in two distinct ethnic groups. A broader understanding of this topic may in the future warrant the adjustment of glycated albumin values when used as an indicator of blood glucose control.
5. Conclusions
Glycated albumin is a measure of blood glucose novel in its reflection of the previous 2 weeks control. Our findings support that albumin is glycated consistent with the prevailing glucose concentration, with no association observed between postprandial blood glucose response and glycated albumin in young euglycemic adults. Glycated albumin appears diminished in circulation due to body weight, although whether this is due to increased adipocytokines remains unclear.
Acknowledgments
We thank participants of this study and research nurse Glenna Paterson for drawing blood samples. This study was funded by the New Zealand Artificial Limb Service. Asahi Kasei provided the GA‐L assay kits. Sponsors did not play a role in the design, collection, analysis, or presentation and distribution of findings.
References
- 1. Turner R, Holman R, Cull C, et al. Intensive blood‐glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 2. Diabetes Control and Complications Trial Research Group . The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 3. Goldstein DE, Little RR, Lorenz RA, et al. Tests of glycemia in diabetes. Diabetes Care. 2004;27:1761–1773. [DOI] [PubMed] [Google Scholar]
- 4. Paroni R, Ceriotti F, Galanello R, et al. Performance characteristics and clinical utility of an enzymatic method for the measurement of glycated albumin in plasma. Clin Biochem. 2007;40:1398–1405. [DOI] [PubMed] [Google Scholar]
- 5. Garlick RL, Mazer JS. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983;258:6142–6146. [PubMed] [Google Scholar]
- 6. Guthrow CE, Morris MA, Day JF, Thorpe SR, Baynes JW. Enhanced nonenzymatic glucosylation of human serum albumin in diabetes mellitus. Proc Natl Acad Sci USA. 1979;76:4258–4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicholson J, Wolmarans M, Park G. The role of albumin in critical illness. Br J Anaesth. 2000;85:599–610. [DOI] [PubMed] [Google Scholar]
- 8. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163:1079–1094. [DOI] [PubMed] [Google Scholar]
- 9. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients variations with increasing levels of HbA1c. Diabetes Care. 2003;26:881–885. [DOI] [PubMed] [Google Scholar]
- 10. Yoshiuchi K, Matsuhisa M, Katakami N, et al. Glycated albumin is a better indicator for glucose excursion than glycated hemoglobin in type 1 and type 2 diabetes. Endocr J. 2008;55:503–507. [DOI] [PubMed] [Google Scholar]
- 11. Sakuma N, Omura M, Oda E, Saito T. Converse contributions of fasting and postprandial glucose to HbA1c and glycated albumin. Diabetol Int. 2011;2:162–171. [Google Scholar]
- 12. Shima K, Abe F, Chikakiyo H, Ito N. The relative value of glycated albumin, hemoglobin A1c and fructosamine when screening for diabetes mellitus. Diabetes Res Clin Pract. 1989;7:243–250. [DOI] [PubMed] [Google Scholar]
- 13. Koga M, Matsumoto S, Saito H, Kasayama S. Body mass index negatively influences glycated albumin, but not glycated hemoglobin, in diabetic patients. Endocr J. 2006;53:387–391. [DOI] [PubMed] [Google Scholar]
- 14. Nishimura R, Kanda A, Sano H, et al. Glycated albumin is low in obese, non‐diabetic children. Diabetes Res Clin Pract. 2006;71:334–338. [DOI] [PubMed] [Google Scholar]
- 15. Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007;378:48–52. [DOI] [PubMed] [Google Scholar]
- 16. Wolever TM, Jenkins DJ, Jenkins AL, Josse RG. The glycemic index: methodology and clinical implications. Am J Clin Nutr. 1991;54:846–854. [DOI] [PubMed] [Google Scholar]
- 17. Matthews J, Altman DG, Campbell M, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furusyo N, Koga T, Ai M, et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia. 2011;54:3028–3036. [DOI] [PubMed] [Google Scholar]
- 19. Alberti KGMM, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 20. Colagiuri S. Guideline for Management of Postmeal Glucose in Diabetes. Brussels, Belgium: International Diabetes Federation; 2011. [Google Scholar]
- 21. Woerle HJ, Neumann C, Zschau S, et al. Impact of fasting and postprandial glycemia on overall glycemic control in type 2 diabetes: importance of postprandial glycemia to achieve target HbA1c levels. Diabetes Res Clin Pract. 2007;77:280–285. [DOI] [PubMed] [Google Scholar]
- 22. Saydah SH, Loria CM, Eberhardt MS, Brancati FL. Subclinical states of glucose intolerance and risk of death in the US. Diabetes Care. 2001;24:447–453. [DOI] [PubMed] [Google Scholar]
- 23. Wadden TA, West DS, Neiberg RH, et al. One‐year weight losses in the look AHEAD study: factors associated with success. Obesity. 2009;17:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. [DOI] [PubMed] [Google Scholar]


