Abstract
Background
Left atrial appendage flow velocity (LAAFV) and presence of spontaneous echo contrast (SEC) have been reported to be predictors of thromboembolism in atrial fibrillation (AF) patients. Galectin‐3 is a biomarker reflecting pro‐inflammatory status, whose role in AF has recently drawn attention, particularly in persistent AF population.
Aim
In this study we aimed to investigate the association between serum galectin‐3 levels and echocardiographic predictors of thromboembolism in persistent AF patients.
Methods
We included 65 persistent AF patients (55.50±10.67 years, 46.15% male). Transesophageal echocardiography (TEE) was performed to assess LAAFV and presence of left atrial (LA)/LA appendage (LAA)‐located SEC and thrombus prior to direct current cardioversion or catheter ablation for AF.
Results
Median galectin‐3 level was 0.63 ng/mL. Serum galectin‐3 levels were significantly correlated with LAAFV (r=−.440, P<.001). Serum galectin‐3 levels were associated with presence of SEC (P<.001), and LA thrombus (P=.008). Receiver operating characteristic analysis revealed that a serum galectin‐3 greater or equal to the cut‐off value of 0.69 predicted presence of SEC with a sensitivity and specificity of 91.00% and 79.00%, respectively (P<.001).
Conclusion
In conclusion, in the setting of persistent AF, serum galectin‐3 levels are associated with presence of SEC and LAAFV on TEE. Our findings suggest that serum galectin‐3 level may have a place in thromboembolism risk stratification in persistent AF patients.
Keywords: atrial fibrillation, galectin‐3, left atrial appendage flow velocity, transesophageal echocardiography
1. Background
Atrial fibrillation (AF) is the most common sustained arrhythmia. It constitutes an increased risk of stroke due to formation of atrial thrombi, usually in the left atrial appendage (LAA). In AF patients, reduced LAA flow velocity (LAAFV) and the presence of spontaneous echo contrast (SEC), an echogenic swirling pattern of blood flow which indicates a low‐flow state in the left atrium (LA) have been shown to be markers of thromboembolic risk.1, 2, 3, 4, 5
Studies have suggested a possible association between inflammation, AF, and thrombosis. Levels of inflammatory markers, such as C‐ reactive protein (CRP) have been found to be related to LA/LAA‐located SEC and thrombus observed on transesophageal echocardiography (TEE).6, 7, 8 Another study has shown that thrombin‐ antithrombin III complex, plasmin‐α2‐plasmin inhibitor complex, and D‐dimer levels were significantly higher in patients with aortic SEC than in those without.9
Galectin‐3 is an α‐galactoside‐binding lectin that has been shown to mediate cell‐to‐cell and cell‐to‐extracellular matrix interactions and act as a novel chemoattractant for monocytes and macrophages.10, 11 Studies have supported an association between serum galectin‐3 levels and atrial fibrosis demonstrated with delayed‐ enhancement magnetic resonance imaging in AF patients.12, 13
In this study, we aimed to investigate the relation between serum galectin‐3 levels and echocardiographic markers of thromboembolism, including LAAFV, LA/LAA‐ located SEC and thrombus.
2. Methods
2.1. Study population
Sixty‐five patients with persistent AF who were scheduled for TEE prior to direct current cardioversion or catheter ablation for AF were enrolled in this observational study. AF episodes that lasted >7 days or required termination by cardioversion, either with drugs or by direct current cardioversion were defined as persistent AF.14
Patients who were pregnant and had moderate‐severe valvular disease, congenital heart disease, alcohol consumption, abnormal thyroid function, serum creatinine levels greater than 1.20 mg/dL, autoimmune disease, recent infection or attempted AF ablation were excluded from the study. Furthermore, patients who had systolic left ventricular dysfunction [left ventricular ejection fraction (LVEF) <40%] were not included in the study.
Baseline demographic and clinical characteristics; including age, gender, body mass index (BMI); history of hypertension, diabetes mellitus, coronary artery disease, peripheral artery disease, stroke, or transient ischemic attack and smoking were recorded. Data related to the diagnosis of AF including date of first diagnosis were also noted. Informed consent was taken from each patient before enrollment. The study was in compliance with the principles outlined in the Declaration of Helsinki and approved by the Institutional Ethics Committee.
2.2. Echocardiography
Complete transthoracic and transesophageal echocardiography were performed using commercially available equipment (Vivid S6; GE Healthcare, Horten, Norway). All echocardiographic parameters were measured according to the recommendations of the American Society of Echocardiography.15 Aerosolized topical anesthetic solution (10% lidocaine spray) was used to anesthetize the oropharynx before TEE. The presence of LA/LAA ‐ located thrombus was defined as an intracavitary echogenic mass, distinct from LA endocardium and pectinate muscles. The presence of SEC in LA/LAA was diagnosed by observation of dynamic, swirling smoke‐like echogenic material. The grading of SEC was evaluated as follows: 0, absence of echogenicity; 1+ (mild), minimal echogenicity, undetectable at normal gain setting, may be perceptible only transiently, and located in only the LAA or sparsely distributed in the LA; 2+ (mild to moderate), detectable without increased gain setting; 3+ (moderate), dense, swirling echogenic materials in the LAA and that in the LA was lesser in intensity than that in the LAA; and 4+ (severe), dense, swirling echogenic materials in the LAA and LA with similar intensity.16 In our study, dense SEC was defined as moderate or severe SEC. LAAFV profiles were obtained by pulsed‐wave Doppler echocardiographic interrogation at the orifice of the appendage. Peak outflow velocity signals within each R‐R interval were averaged over a minimum of six cardiac cycles.
Echocardiographic evaluations were performed by two experienced cardiologists who were blinded to the characteristics of the patients.
2.3. Measurement of serum galectin‐3 levels
After an overnight fast, blood samples were collected and immediately centrifuged and stored at −80°C. Immediately before the analysis, the frozen serum samples were rapidly thawed and brought to room temperature and then, assayed for human galectin‐3 by using enzyme linked immunosorbent assay (ELISA) kits (eBioscience, Europe/International, Austria), according to the manufacturer's instructions. Serial dilutions of known concentrations of human galectin‐3 were used to construct a standard curve of the analytes. The serum levels of galectin‐3 from the samples were estimated by extrapolation from a log:log linear regression curve determined from the serially diluted human recombinant galectin‐3 ranging from 25 to 0.39 ng/mL.
2.4. Statistical analysis
Normally distributed continuous parameters are presented as mean±standard deviation and skewed continuous parameters are expressed as median (interquartile range defined as minimum‐maximum). Categorical data are presented as frequencies and percentages and are compared using chi‐square test. Comparisons between baseline characteristics were performed by independent Student t, Mann–Whitney rank‐sum, Fisher exact or chi‐square tests where appropriate. Spearman correlation analysis was done to investigate the correlation between other parameters and LAAFV. Linear logistic regression analysis was performed to determine the independent predictors of LAAFV. Optimal cut‐off value for serum galectin‐3 levels for predicting presence of SEC on TEE was determined by the analysis of the sensitivity and specificity values derived from receiver operating characteristic (ROC) curve data. Statistical analyses were performed using SPSS statistical software (version 21.0; SPSS Inc., Chicago, IL, USA). A two‐tailed P<.05 is considered statistically significant.
3. Results
Sixty‐five patients with persistent AF (55.50±10.67 years, 46.15% male) were included in this study (Table 1). Median serum galectin‐3 level was 0.63 ng/mL (Table 2). Median CHADS2 and CHA2DS2‐VASc scores were 0 and 1 respectively (Table 1). The scores did not significantly differ between group of patients with low (<0.25 m/s) and high (≥0.25 m/s) LAAFV or patients with and without LA/LAA‐ located SEC or thrombus (Table 3). Median LAAFV was 0.30 (0.13‐0.45) m/s. 33.85% and 10.77% of patients had dense SEC and thrombus in LA/LAA respectively (Table 4). None of the patients were on anticoagulant therapy.
Table 1.
Baseline demographic and clinical parameters of the study population (n=65)
| Parameters | |
|---|---|
| Age (y) | 55.50±10.67 |
| Gender: male (n, %) | 30 (46.15) |
| Body mass index (kg/m2) | 24.12±2.03 |
| AF duration (mo) | 24 (1‐240) |
| Hypertension (n, %) | 24 (36.92) |
| Diabetes mellitus (n, %) | 9 (13.85) |
| Coronary artery disease (n, %) | 3 (4.62) |
| Previous TIA/stroke (n, %) | 1 (1.54) |
| Smoking (n, %) | 7 (10.77) |
| CHADS2 score | 0 (0‐3) |
| CHA2DS2‐ VASc score | 1 (0‐5) |
AF, atrial fibrillation; CHADS2 Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Prior Stroke or TIA or Thromboembolism [doubled]; CHA2DS2‐ VASc, Congestive heart failure, Hypertension, Age ≥75 years [doubled], Diabetes mellitus, Prior Stroke or TIA or thromboembolism [doubled], Vascular disease, Age 65 to 74 years, Sex category; TIA, transient ischemic attack.
Table 2.
Baseline laboratory and echocardiographic parameters of the study population (n=65)
| Parameters | |
|---|---|
| Platelet count (×103/μL) | 206.00±62.76 |
| Serum creatinine (mg/dL) | 0.83±0.19 |
| Serum galectin‐3 (ng/mL) | 0.63 (0.33‐1.28) |
| LAVI (mL/m2) | 28.29±3.35 |
| Left ventricular end‐diastolic volume (mL) | 84.28±10.58 |
| Left ventricular ejection fraction (%) | 62.12±3.68 |
| LAA flow velocity (m/s) | 0.30 (0.13‐0.45) |
| LA/LAA dense spontaneous echo contrast n (%) | 22 (33.85) |
| LA/LAA thrombus n (%) | 7 (10.77) |
LA, left atrial; LAA, left atrial appendage; LAVI, left atrial volume index.
Table 3.
Baseline demographic and clinical parameters of the study population regarding left atrial appendage flow velocity and presence of spontaneous echo contrast (n=65)
| Parameters | LAAFV (m/s) | LA/LAA dense SEC | LA/LAA thrombus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <0.25 (n=15) | ≥0.25 (n=50) | P value | − (n=43) | + (n=22) | P value | − (n=58) | + (n=7) | P value | |
| Age (y) | 68.47±7.09 | 51.53±8.14 | <.001a | 52.62±8.13 | 61.00±12.82 | .009a | 54.47±9.29 | 63.86±17.29 | .205 |
| Gender: male (n, %) | 8 (53.33) | 22 (44.00) | .567 | 19 (44.19) | 11 (50.00) | .794 | 28 (48.28) | 2 (28.57) | .437 |
| Body mass index (kg/m2) | 24.54±1.63 | 24.00±2.13 | .365 | 23.90±1.99 | 24.56±2.09 | .219 | 23.96±2.05 | 25.46±1.33 | .065 |
| AF duration (mo) | 24 (1‐84) | 24 (1‐240) | .663 | 15 (0‐240) | 24 (1‐60) | .207 | 36 (7‐48) | 24 (1‐240) | .785 |
| Hypertension (n, %) | 5 (33.33) | 19 (38.00) | 1.000 | 16 (37.21) | 8 (36.36) | 1.000 | 21 (36.21) | 3 (42.86) | .703 |
| Diabetes mellitus (n, %) | 2 (13.33) | 7 (14.00) | 1.000 | 6 (13.95) | 3 (13.64) | 1.000 | 8 (13.79) | 1 (14.29) | 1.000 |
| Coronary artery disease (n, %) | 1 (6.67) | 2 (4.00) | .551 | 2 (4.65) | 1 (4.55) | 1.000 | 3 (5.17) | 0 (0) | 1.000 |
| Previous TIA/stroke (n, %) | 1 (6.67) | 0 (0) | .234 | 0 (0) | 1 (4.55) | .344 | 1 (1.72) | 0 (0) | 1.000 |
| Smoking (n, %) | 2 (13.33) | 5 (10.00) | .658 | 4 (9.30) | 3 (13.64) | .681 | 7 (12.07) | 0 (0) | 1.000 |
| CHADS2 score | 0 (0‐3) | 0 (0‐3) | .836 | 0 (0‐3) | 0 (0‐3) | .594 | 0 (0‐2) | 0 (0‐3) | .975 |
| CHA2DS2‐ VASc score | 1 (0‐5) | 1 (0‐5) | .595 | 1 (0‐5) | 1 (0‐5) | .924 | 1 (0‐3) | 1 (0‐5) | .843 |
AF, atrial fibrillation; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Prior Stroke or TIA or Thromboembolism [doubled]; CHA2DS2‐ VASc, Congestive heart failure, Hypertension, Age ≥75 years [doubled], Diabetes mellitus, Prior Stroke or TIA or thromboembolism [doubled], Vascular disease, Age 65 to 74 years, Sex category; LA, left atrial; LAA, left atrial appendage; LAAFV, left atrial appendage flow velocity; SEC, spontaneous echo contrast; TIA, transient ischemic attack.
P<.05 denotes statistical significant difference.
Table 4.
Baseline laboratory and echocardiographic parameters of the study population regarding left atrial appendage flow velocity and presence of spontaneous echo contrast (n=65)
| Parameters | LAAFV (m/s) | LA/LAA dense SEC | LA/LAA thrombus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <0.25 (n=15) | ≥0.25 (n=50) | P value | − (n=43) | + (n=22) | P value | − (n=58) | + (n=7) | P value | |
| Platelet count (×103/μL) | 220.93±81.10 | 201.43±56.21 | .296 | 207.90±57.36 | 202.36±73.29 | .740 | 207.04±62.25 | 197.57±71.40 | .710 |
| Serum creatinine (mg/dL) | 0.83±0.23 | 0.83±0.18 | .965 | 0.83±0.20 | 0.83±0.19 | .940 | 0.84±0.19 | 0.73±0.17 | .136 |
| Serum galectin‐3 (ng/mL) | 0.77 (0.71‐0.91) | 0.59 (0.33‐1.28) | <.001a | 0.58 (0.33‐1.28) | 0.80 (0.63‐1.28) | <.001a | 0.60 (0.33‐1.28) | 0.86 (0.72‐0.91) | .008a |
| LAVI (mL/m2) | 28.10±2.92 | 28.34±3.49 | .804 | 28.22±3.53 | 28.41±3.04 | .827 | 28.29±3.44 | 28.23±2.75 | .962 |
| Left atrial appendage flow velocity (m/s) | — | — | — | 0.35 (0.17‐0.45) | 0.23 (0.13‐0.33) | <.001a | 0.32 (0.14‐0.45) | 0.17 (0.13‐0.32) | .001a |
| LA/LAA dense SEC (n, %) | 10 (66.67) | 12 (24.00) | <.001a | — | — | — | 15 (25.86) | 7 (100.00) | <.001a |
| LA/LAA thrombus (n, %) | 4 (26.67) | 3 (6.00) | .044a | 0 (0) | 7 (31.82) | <.001a | — | — | — |
| Left vntricular end‐diastolic volume (mL) | 81.33±9.61 | 85.17±10.78 | .221 | 85.61±12.32 | 81.68±5.14 | .157 | 84.37±11.09 | 83.57±4.81 | .852 |
| Left ventricular ejection fraction (%) | 61.53±3.70 | 62.30±3.69 | .484 | 62.02±3.55 | 62.32±3.99 | .762 | 62.22±3.81 | 61.28±2.36 | .528 |
LA, left atrial; LAA, left atrial appendage; LAAFV, left atrial appendage flow velocity; LAVI, left atrial volume index; SEC, spontaneous echo contrast.
P<.05 denotes statistical significance.
Patients with lower LAAFV (<0.25 m/s) were older (P<.001) and had higher serum galectin‐3 levels (P<.001) (Table 3). They also more frequently had dense SEC (P<.001) and LA/LAA‐ located thrombus (P=.044) on TEE (Table 3). Patients with dense SEC in LA/LAA were older (P=.009), had higher serum galectin‐3 levels (P<.001) and lower LAAFV (P<.001) (Table 4). They more frequently had LA/LAA‐ located thrombus on TEE (P<.001). Patients with LA/LAA‐ located thrombus on TEE had higher serum galectin‐3 levels (P=.008) and lower LAAFV (P=.001). All patients with LA/LAA‐ located thrombus had dense LA/LAA SEC on TEE (P<.001; Table 4).
Spearman's correlation analysis revealed a statistically significant negative correlation between LAAFV and serum galectin‐3 levels (r=−.440, P<.001). LAAFV was also significantly negatively correlated with age (r=−.552, P<.001) (Table 5). Multivariate linear regression analysis showed that age, and serum galectin‐3 levels were independently associated with LAAFV (Table 6).
Table 5.
Spearman's correlation analysis demonstrating the correlation between serum left atrial appendage flow velocity and baseline characteristics (n=65)
| Correlation coefficient (r) | P value | |
|---|---|---|
| Age (y) | −.552 | <.001a |
| Body mass index (kg/m2) | −.056 | .658 |
| Duration of AF (mo) | −.068 | .604 |
| LAVI (mL/m2) | −.052 | .680 |
| Left ventricular end‐diastolic volume (mL) | −.155 | .216 |
| Left ventricular ejection fraction (%) | .178 | .156 |
| Serum galectin‐3 (ng/mL) | −.440 | <.001a |
| Platelet count (×103/μL) | −.103 | .416 |
| Serum creatinine (mg/dL) | −.056 | .661 |
r, Spearman's correlation coefficient; AF, atrial fibrillation; CHADS2, Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, Prior Stroke or TIA or Thromboembolism [doubled]; CHA2DS2‐ VASc, Congestive heart failure, Hypertension, Age ≥75 years [doubled], Diabetes mellitus, Prior Stroke or TIA or thromboembolism [doubled], Vascular disease, Age 65‐74 years, Sex category; LAVI, left atrial volume index.
P<.05 denotes statistical significance.
Table 6.
Linear regression analysis for identifying independent associates of left atrial appendage flow velocity
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| B±SD | 95% CI | P value | B±SD | 95% CI | P value | |
| Age (y) | −0.005±0.001 | −0.006 to (‐) 0.003 | <.001a | −0.005±0.001 | −0.006±(−)0.003 | <.001a |
| Left ventricular end‐diastolic volume (mL) | 0.001±0.001 | −0.001 to 0.003 | .242 | — | — | — |
| Left ventricular ejection fraction (%) | 0.003±0.003 | −0.002 to 0.009 | .254 | — | — | — |
| Serum galectin‐3 (ng/mL) | −0.161±0.052 | −0.264 to (−) 0.057 | .003a | −0.101±0.043 | −0.186±(−)0.016 | .021a |
B, beta coefficient; CI, confidence interval; SD, standard deviation.
P<.05 denotes statistical significance.
Receiver operating characteristic analysis demonstrated that a serum galectin‐3 level ≥0.69 ng/mL predicted LA/LAA‐ located SEC presence with a sensitivity and specificity of 91% and 79% respectively (AUC: 0.909, 95% CI: 0.840‐0.977, P<.001; Figure 1). A serum galectin‐3 level ≥0.72 ng/mL predicted LAAFV <0.25 m/s with a sensitivity and specificity of 86% and 76% respectively (AUC: 0.851, 95% CI: 0.732‐0.971, P<.001; Figure 2).
Figure 1.
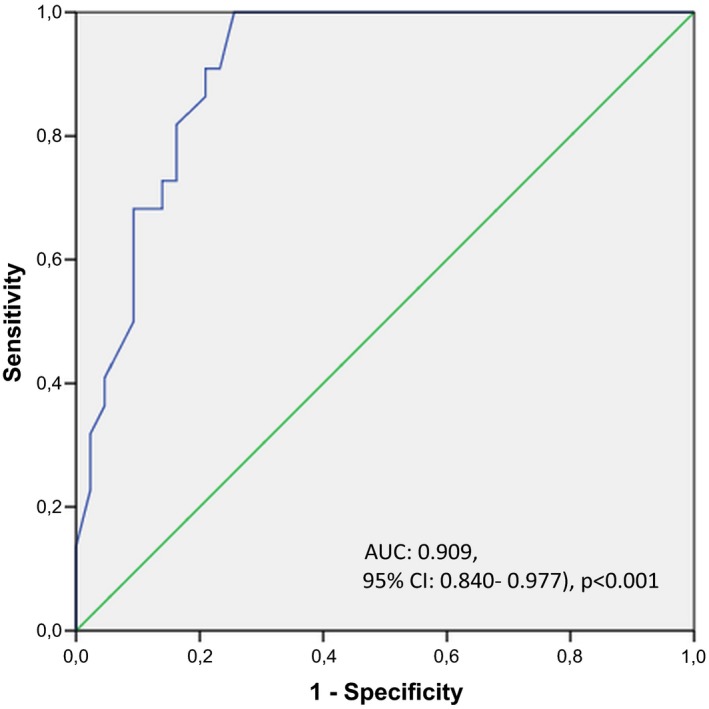
Figure showing receiver operating characteristic curve for determining the value of serum galectin‐3 levels for predicting left atrial/left atrial appendage‐ located spontaneous echo contrast presence
Figure 2.
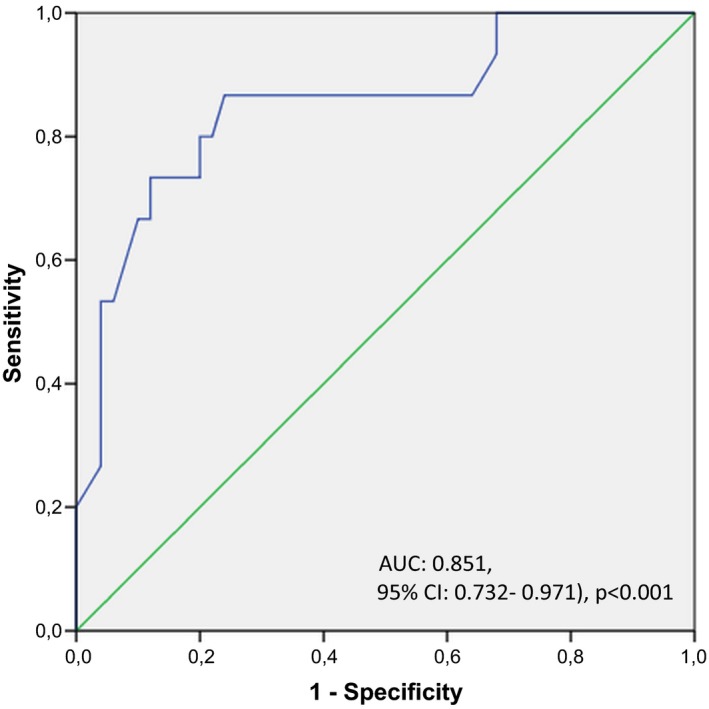
Figure showing receiver operating characteristic curve for determining the value of serum galectin‐3 levels for predicting left atrial‐appendage flow velocity <0.25 m/s
4. Discussion
This study demonstrates for the first time in the literature that, in the setting of persistent AF, serum galectin‐3 levels are associated with LAAFV, presence of LA/LAA‐ located SEC and thrombus on TEE.
Histological studies have suggested a possible association between inflammation, AF, and thrombosis.17 Levels of inflammation, hemostasis and coagulation markers in AF have been evaluated in many studies. Serum levels of CRP have been found to be associated with AF.18, 19 CRP has also been found to be related to fibrinogen, plasma viscosity, LA/LAA‐ located SEC and thrombus observed on TEE.6, 7, 8 The presence of AF has been shown to be an independent predictor of abnormal von Willebrand factor, fibrinogen and soluble P‐selectin levels.20 Another study has shown that thrombin‐ antithrombin III complex, plasmin‐α2‐plasmin inhibitor complex, and D‐dimer levels were significantly higher in patients with aortic SEC than in those without.9 However, major limitation of these studies were that most patients were on oral antithrombotic therapy.
Galectin‐3 is an α‐galactoside‐binding lectin that has been shown to mediate cell‐to‐cell and cell‐to‐extracellular matrix interactions and act as a novel chemoattractant for monocytes and macrophages.10 Studies have supported an association between serum galectin‐3 levels and AF and associated atrial fibrosis demonstrated with delayed‐ enhancement magnetic resonance imaging (DE‐ MRI).12, 13
It has also been reported that galectins play important roles in acute and chronic inflammatory responses.21 Galectin‐1, ‐3, and ‐8, either in a soluble or immobilized form, have been reported to be capable of triggering a wide spectrum of platelet responses,22, 23 including adhesion, aggregation, release of granule content and P‐selectin expression through the interaction with the carbohydrate backbone of the major platelet receptors involved in hemostasis (e.g., GPIb/IX/V complex and integrin αIIbβ3).24, 25, 26, 27, 28 Finding of our study suggest that serum galectin‐3 may be involved in thrombogenesis in LA/LAA.
In a study by Akoum et al.29 patients with LA/LAA‐ located thrombus on TEE were shown to have higher atrial fibrosis detected by DE‐ MRI compared to patients without thrombus. Atrial fibrosis was also higher in patients with LA/LAA‐ located SEC compared to those without. Multivariate logistic regression showed high fibrosis was a significant predictor of echocardiographic predictors of thromboembolism. A previous study by our group13 has demonstrated that serum galectin‐3 levels were independently correlated with extent of LA fibrosis detected with DE‐MRI in paroxysmal AF patients with preserved LV function. Therefore, it may proposed that the role of serum galectin‐3 in association with LA/LAA‐ located SEC and thrombus may involve the fibrogenic process in the atria.
4.1. Study limitations
There are some limitations of this study. First, a larger study population and a longer term follow‐up are required to confirm the predictive role of serum galectin‐3 levels for identifying thromboembolic risk in AF patients. Second, other markers of a thrombotic state or inflammation have not been evaluated in this study.
5. Conclusion
Serum galectin‐3 levels may be used in combination with established parameters of stroke risk stratification to determine thromboembolism risk in AF patients.
Kocyigit D, Gurses KM, Yalcin MU, et al. Serum galectin‐3 level as a marker of thrombogenicity in atrial fibrillation. J Clin Lab Anal. 2017;31:e22120 10.1002/jcla.22120
Funding information
This research was supported by Hacettepe University Scientific Research Projects Coordination Unit (project number: 1993).
References
- 1. Jones EF, Calafiore P, McNeil JJ, Tonkin AM, Donnan GA. Atrial fibrillation with left atrial spontaneous contrast detected by transesophageal echocardiography is a potent risk factor for stroke. Am J Cardiol. 1996;78:425–429. [DOI] [PubMed] [Google Scholar]
- 2. Kamp O, Verhorst PM, Welling RC, Visser CA. Importance of left atrial appendage flow as a predictor of thromboembolic events in patients with atrial fibrillation. Eur Heart J. 1999;20:979–985. [DOI] [PubMed] [Google Scholar]
- 3. Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31:1622–1626. [DOI] [PubMed] [Google Scholar]
- 4. Asinger RW, Koehler J, Pearce LA, et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: II. Dense spontaneous echocardiographic contrast (The Stroke Prevention in Atrial Fibrillation [SPAF‐III] study). J Am Soc Echocardiogr. 1999;12:1088–1096. [DOI] [PubMed] [Google Scholar]
- 5. Goldman ME, Pearce LA, Hart RG, et al. Pathophysiologic correlates of thromboembolism in nonvalvular atrial fibrillation: I. Reduced flow velocity in the left atrial appendage (The Stroke Prevention in Atrial Fibrillation [SPAF‐III] study). J Am Soc Echocardiogr. 1999;12:1080–1087. [DOI] [PubMed] [Google Scholar]
- 6. Conway DS, Buggins P, Hughes E, Lip GY. Relationship of interleukin‐6 and C‐reactive protein to the prothrombotic state in chronic atrial fibrillation. J Am Coll Cardiol. 2004;43:2075–2082. [DOI] [PubMed] [Google Scholar]
- 7. Conway DS, Buggins P, Hughes E, Lip GY. Relation of interleukin‐6, C‐reactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation. Am J Cardiol. 2004;93:1368–1373, A1366. [DOI] [PubMed] [Google Scholar]
- 8. Ederhy S, Di Angelantonio E, Dufaitre G, et al. C‐reactive protein and transesophageal echocardiographic markers of thromboembolism in patients with atrial fibrillation. Int J Cardiol. 2012;159:40–46. [DOI] [PubMed] [Google Scholar]
- 9. Nakagawa K, Hirai T, Shinokawa N, et al. Aortic spontaneous echocardiographic contrast and hemostatic markers in patients with nonrheumatic atrial fibrillation. Chest. 2002;121:500–505. [DOI] [PubMed] [Google Scholar]
- 10. McCullough PA, Olobatoke A, Vanhecke TE. Galectin‐3: a novel blood test for the evaluation and management of patients with heart failure. Rev Cardiovasc Med. 2011;12:200–210. [DOI] [PubMed] [Google Scholar]
- 11. Ji F, Zhang S, Jiang X, et al. Diagnostic and prognostic value of galectin‐3, serum creatinine, and cystatin C in chronic kidney diseases. J Clin Lab Anal. 2016; doi: 10.1002/jcla.22074. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gurses KM, Yalcin MU, Kocyigit D, et al. Effects of persistent atrial fibrillation on serum galectin‐3 levels. Am J Cardiol. 2015;115:647–651. [DOI] [PubMed] [Google Scholar]
- 13. Yalcin MU, Gurses KM, Kocyigit D, et al. The association of serum galectin‐3 levels with atrial electrical and structural remodeling. J Cardiovasc Electrophysiol. 2015;26:635–640. [DOI] [PubMed] [Google Scholar]
- 14. Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation–developed with the special contribution of the European Heart Rhythm Association. Europace. 2012;14:1385–1413. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 16. Fatkin D, Loupas T, Jacobs N, Feneley MP. Quantification of blood echogenicity: evaluation of a semiquantitative method of grading spontaneous echo contrast. Ultrasound Med Biol. 1995;21:1191–1198. [DOI] [PubMed] [Google Scholar]
- 17. Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. [DOI] [PubMed] [Google Scholar]
- 18. Aviles RJ, Martin DO, Apperson‐Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. [DOI] [PubMed] [Google Scholar]
- 19. Chung MK, Martin DO, Sprecher D, et al. C‐reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. [DOI] [PubMed] [Google Scholar]
- 20. Li‐Saw‐Hee FL, Blann AD, Gurney D, Lip GY. Plasma von Willebrand factor, fibrinogen and soluble P‐selectin levels in paroxysmal, persistent and permanent atrial fibrillation. Effects of cardioversion and return of left atrial function. Eur Heart J. 2001;22:1741–1747. [DOI] [PubMed] [Google Scholar]
- 21. Liu FT, Yang RY, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci. 2012;1253:80–91. [DOI] [PubMed] [Google Scholar]
- 22. Schattner M. Platelets and galectins. Ann Transl Med. 2014;2:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Romaniuk MA, Negrotto S, Campetella O, Rabinovich GA, Schattner M. Identification of galectins as novel regulators of platelet signaling and function. IUBMB Life. 2011;63:521–527. [DOI] [PubMed] [Google Scholar]
- 24. Pacienza N, Pozner RG, Bianco GA, et al. The immunoregulatory glycan‐binding protein galectin‐1 triggers human platelet activation. FASEB J. 2008;22:1113–1123. [DOI] [PubMed] [Google Scholar]
- 25. Romaniuk MA, Croci DO, Lapponi MJ, et al. Binding of galectin‐1 to alphaIIbbeta(3) integrin triggers “outside‐in” signals, stimulates platelet activation, and controls primary hemostasis. FASEB J. 2012;26:2788–2798. [DOI] [PubMed] [Google Scholar]
- 26. Romaniuk MA, Tribulatti MV, Cattaneo V, et al. Human platelets express and are activated by galectin‐8. Biochem J. 2010;432:535–547. [DOI] [PubMed] [Google Scholar]
- 27. Etulain J, Negrotto S, Tribulatti MV, et al. Control of angiogenesis by galectins involves the release of platelet‐derived proangiogenic factors. PLoS ONE. 2014;9:e96402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cattaneo V, Tribulatti MV, Carabelli J, Carestia A, Schattner M, Campetella O. Galectin‐8 elicits pro‐inflammatory activities in the endothelium. Glycobiology. 2014;24:966–973. [DOI] [PubMed] [Google Scholar]
- 29. Akoum N, Fernandez G, Wilson B, McGann C, Kholmovski E, Marrouche N. Association of atrial fibrosis quantified using LGE‐MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]


