Abstract
Background
This study was aimed to investigate the correlation between CYP2C19 and ABCB1 polymorphisms and the recurrence of ischemic cardiovascular adverse events in patients with coronary artery disease treated with clopidogrel.
Methods
A total of 168 patients with coronary heart disease who underwent PCI operation and received clopidogrel treatment were enrolled. Dual antiplatelet therapy was applied to the treatment of patients for 2 years. Thromboelastography was used to test the efficiency of blood coagulation. Polymerase chain reaction (PCR) was used to detect CYP2C19 and ABCB1 3435CT polymorphisms. One‐year follow‐up visit was carried out to record the incidence of cardiovascular adverse events after drug‐eluting stent implantation was inset.
Results
Follow‐up visit results suggested that the patients with high on‐treatment platelet reactivity (HPR) had a higher recurrence rate of cardiovascular adverse events after PCI operation and clopidogrel treatment. Gene polymorphism testing results indicated that patients with CYP2C19*3 had a significantly higher incidence of HPR, whereas CYP2C19*2 and ABCB1 3435CT were not significantly correlated with HPR. Multivariable logistic regression analysis showed that CYP2C19*3 might be an independent predictive factor of post‐PCI HPR. In addition, CYP2C19*3 as well as post‐PCI HPR could function as independent predictive factors of cardiovascular adverse events.
Conclusion
CYP2C19*3 polymorphism could be an important predictive factor of HPR and ischemic cardiovascular adverse events after clopidogrel treatment.
Keywords: ABCB1 3435CT, cardiovascular, clopidogrel, CYP2C19*3
Abbreviations
- ADP
adenosine diphosphate;
- ANOVA
analysis of variance;
- CAD
coronary artery disease;
- DAPT
dual antiplatelet therapy;
- DES
drug‐eluting stent implantation;
- DES
drug‐eluting stents;
- GOF
gain‐of‐function;
- HLP
hyperlipemia;
- HPR
high on‐treatment platelet reactivity;
- KS test
Kolmogorov‐Smirnov test;
- LOF
loss‐of‐function;
- MA
maximum amplitude;
- PCI
percutaneous coronary intervention;
- PCR
polymerase chain reaction.
1. INTRODUCTION
Coronary artery disease (CAD) is a leading disease incurred death worldwide.1, 2 CAD is of high recurrence rate of cardiovascular adverse events in those under 75 years old.3 Percutaneous coronary intervention (PCI) is one of the most prevalent therapeutic interventions in practice,4 and stent implantation has become the standard care for myocardial revascularization, especially in unstable CAD cases.5 However, the successful implementation of PCI therapy requires induction of dual antiplatelet therapy (DAPT).6 The advantages and disadvantages of DAPT continuing treatment after PCI had been accurately predicted by large DAPT trial's prediction rule during individualized therapy duration.8 A previous study showed that DAPT treatment for more than 1 year reduced late stent thrombosis and ischemic events after drug‐eluting stents (DES), but increased the risk of bleeding.7
Dual antiplatelet therapy, together with aspirin and a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor), is a standard follow‐up therapy for CAD patients after PCI treatment.9 As the most serious complication associated with ischemic events after DES, stent thrombosis often leads to myocardial infarction.10 Compared with aspirin treatment, the combined treatment of aspirin with clopidogrel can reduce the risk of cardiovascular death.11 Rudolph et al12 revealed that use of clopidogrel for stable CAD patients with long‐term aspirin treatment might lead to short‐term increase in endothelial function. Besides of that, about 40% of clopidogrel‐treated patients displayed a high on‐treatment platelet reactivity (HPR).13 Platelet activation and aggregation were the pathophysiological basis of various atherosclerotic thrombosis and coronary stent thrombosis,14 which might induce the recurrence of ischemic cardiovascular adverse events. Numerous clinical trials found that DAPT could reduce the higher risk of major adverse cardiovascular events in CAD patients. However, there are still some CAD patients suffering from thrombotic events, which might be related to HPR.14, 15
The function of CYP2C19 polymorphisms was inconsistent with different types of diseases. The CYP2C19*17 gain‐of‐function (GOF) variant was found to be related to ultrarapid enzymatic activity. Patients with CYP2C19*17 gene had considerably lower adenosine diphosphate‐induced platelet aggregation and higher risk of bleedings.15 However, in CHANCE trial (clopidogrel in high‐risk patients with acute nondisabling cerebrovascular events, a genetic study), reduced stroke recurrence could not be found in patients with clopidogrel‐aspirin with CYP2C19 loss‐of‐function (LOF) allele (*2 and *3),16 indicating that CYP2C19 LOF negatively influenced the effect of clopidogrel on CAD patients. To get an in‐depth insight into the influence of CYP2C19 on clopidogrel treatment of CAD, further studies were needed to explore more specific properties of CYP2C19 polymorphisms. Previous studies have indicated that the ABCB1 gene could affect clopidogrel absorption and encode an intestinal efflux pump.17, 18 In addition, patients with ABCB1 3435CT (rs1045642) genotype had an increased propensity for ischemic outcomes after acute coronary syndrome when treated with PCI and clopidogrel.19 Therefore, we speculated that ABCB1 exerted an influence on the recurrence of ischemic cardiovascular adverse events.
We have long paid attention to CYP2C19 and ABCB1 because of their close association with coagulation and clopidogrel absorption. Thus, in this study, we intended to investigate the influence of CYP2C19 and ABCB1 polymorphisms on the recurrence of ischemic cardiovascular adverse events in patients with coronary artery disease treated with clopidogrel.
2. MATERIALS AND METHODS
2.1. Study population and design
A total of 168 patients undergoing PCI operation and clopidogrel treatment from August 2012 to May 2013 at Shanghai Chest Hospital (Shanghai, China) were enrolled in this study. A 1‐year follow‐up visit was conducted for all participants. The general information of patients (age, gender, height, weight, and body mass index) and their CAD risk factors (smoking, drinking, high blood pressure, hyperlipemia [HLP], and diabetic history) were recorded in this study. Written informed consents were obtained from all participants.
All patients were given 100‐300 mg/d aspirin (Bayer China, Shanghai, China) according to their body conditions. Three hundred milligram of loading clopidogrel (Sanofi China, Shanghai, China) was taken at a dosage of 75 mg/d since the second day. Follow‐up visits were carried out within 24 months after PCI operation. Cardiovascular adverse events were observed and documented. Common adverse events included cardiovascular ischemic events (cardiac death, recurrent thrombosis in stent confirmed by coronary angiography, recurrent unstable angina, and recurrent acute myocardial infarction) and cardiovascular bleeding events (hemorrhagic apoplexy, secondary anemia, and hemorrhage of digestive tract).
2.2. Platelet agglutination function testing
Venous blood was extracted from patients on an empty stomach in the morning 3 days after PCI operation. Extracted venous blood was placed in blood collection tube with 31.3% sodium citrate, and then activator adenosine diphosphate (ADP) was added. The blood samples were detected by Thromboelastography 5000 coagulometer (Haemoscope, Niles, Illinois, USA) within 2 hours after extraction. The maximum amplitude (MA) of thromboelastography test was depicted in gram, indicating the maximum strength or hardness of forming blood clots, which were affected mainly by platelet and fibrinogen. According to the activator added, MA was constituted of MAThrombin (fibrinogen and thrombin), MAADP (fibrinogen and platelet uninhibited by ADP inhibitor), and MATFibrin (fibrinogen only). Platelet inhibition ratio (PIR%) = (MAADP − MATFibrin)/(MAThrombin − MATFibrin) × 100%. Patients with PIR ≥30% were in normal group. Patients with PIR <30% were in HPR group.
2.3. CYP2C19 and ABCB1 genotyping
Two millilitre venous blood of every patient was extracted and collected into an EDTA anticoagulant tube (Biotend, Shanghai, China). The samples were preserved at 20°C before detection. Blood genomic DNA isolation kit (DP318, TIANGEN Biotech, Beijing, China) was used to extract DNA samples. Q3000 ultraviolet spectrophotometer (Quawell, San Jose, CA, USA) was employed to detect the concentration of DNA. Extracted DNA was amplified by qRT‐PCR and crossbred. Primer sequences were indicated in Table 1. CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893), and ABCB1 3435CT (rs1045642) were mainly examined. The polymorphism of all patients was categorized into CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893) and ABCB1 3435CT (rs1045642). CYP2C19 was subgrouped into three genotypes: wild type (G/G), heterozygous mutant type (G/A), and homozygous mutant type (A/A). ABCB1 was divided into three genotypes: wild type (C/C), heterozygous mutant type (T/C), and homozygous mutant type (T/T). The representative results of sequence‐based typing were depicted in Figure 1.
Table 1.
The primers for conduction of qRT‐polymerase chain reaction
| Gene | Forward primer 5′‐3′ | Reverse primer 5′‐3′ |
|---|---|---|
| CYP2C19*2 | CATGTACAATAAAAATTTCCCCATC | AAGTCCCGAGGGTTGTTGAT |
| CYP2C19*3 | CTAGACAGCCATGGGGTGAAT | ACTCCAAAGTGCCTGGATGT |
| ABCB1 3435CT | CTGAAGTTGATCTGTGAACTCTTG | CTTACATTAGGCAGTGACTCGAT |
| β‐actin | AGAGCTACGAGCTGCCTGAC | AGCACTGTGTTGGCGTACAG |
Figure 1.
Gene sequencing map. Sequencing map of the following genotypes was shown: (A) wild type (G/G), heterozygous type (G/A), and homozygous type (A/A) of CYP2C19*2 (rs4244285); (B) wild type (G/G) and heterozygous type (G/A) of CYP2C19*3 (rs4986893); (C) wild type (C/C), heterozygous type (T/C), and homozygous type (T/T) of ABCB1 3435CT (rs1045642)
2.4. Statistical analysis
Clinical statistical analyses were carried out using SPSS 20.0 software (SPSS, Chicago, IL, USA). All variables were tested for normal distribution using Kolmogorov‐Smirnov test (KS test). Measurement data were analyzed using t test, and enumeration data were analyzed using chi‐square test. One‐way analysis of variance (ANOVA) was applied to intergroup comparisons. Multivariable logistic regression analysis was employed to analyze the correlation between genetic polymorphisms and HPR as well as that between genetic polymorphisms, HPR, and cardiovascular adverse events. P value <.01 denoted a statistically significant difference.
3. RESULTS
3.1. The general information and platelet function of patients
A total of 168 CAD patients after clopidogrel treatment were followed up, and clinical data were recorded. The number of patients in HPR group was 50 in total (50/168). The other 118 patients were assigned to the normal group. As shown in Table 2, there was no significant difference between the general demographic features (age, gender, height, weight, and body mass index) and HPR. Also, no difference was seen between CAD risk factors (smoking, drinking, high blood pressure, HLP, and diabetes) and HPR.
Table 2.
Characteristics of patients in the study
| Characteristics | Normal (n = 118) | HPR (n = 50) | P value |
|---|---|---|---|
| Gender (male) | 87 (73.73%) | 35 (70.00%) | .706 |
| Age (y) | 65.94 | 65.88 | .920 |
| Height (cm) | 166.25 | 162.00 | .653 |
| Weight (kg) | 63.21 | 59.25 | .588 |
| BMI | 22.87 | 22.58 | .476 |
| Smoking | 38 (32.20%) | 14 (28.00%) | .716 |
| Drinking | 78 (66.10%) | 31 (62.00%) | .724 |
| High blood pressure | 101 (85.59%) | 42 (84.00%) | .815 |
| HLP | 79 (66.95%) | 35 (70.00%) | .722 |
| Diabetes | 35 (29.66%) | 10 (20.00%) | .254 |
BMI, body mass index; HLP, hyperlipemia.
3.2. Incidence of cardiovascular adverse events
All patients were follow‐up visited within 24 months after PCI operation. The incidence of cardiovascular ischemic events and cardiovascular bleeding events was documented. In normal group (n = 118), there were 46 patients (38.98%) with ischemic events, including 1 case of cardiac death, 3 cases of myocardial infarction, 29 cases of angina recurrence, and 13 cases of stent thrombosis. Seventeen patients (14.41%) were found to have bleeding events, including 2 cases of hemorrhagic apoplexy, 9 cases of secondary anemia, and 6 cases of hemorrhage of digestive tract. In HPR group (n = 50), 47 patients showed ischemic events (94.00%), including 3 cases of cardiac death, 4 cases of myocardial infarction, 17 cases of angina recurrence, and 23 cases of stent thrombosis. However, no bleeding events were found in HPR group. All the above results indicated that patients in HPR group had a significantly higher incidence of cardiovascular ischemic events (Table 3). Therefore, HPR might be an independent predictive factor of cardiovascular ischemic adverse events.
Table 3.
Incidence of cardiovascular adverse events
| Events | Overall (n = 168) | Normal (n = 118) | HPR (n = 50) | P value |
|---|---|---|---|---|
| Cardiovascular adverse events | 110 (65.48%) | 63 (53.39%) | 47 (94.00%) | <.001* |
| Ischemic events | 93 (55.36%) | 46 (38.98%) | 47 (94.00%) | <.001* |
| Cardiac death | 4 (2.38%) | 1 (0.85%) | 3 (6.00%) | .079 |
| Myocardial infarction | 7 (4.17%) | 3 (1.79%) | 4 (8.00%) | .198 |
| Angina recurrence | 46 (27.38%) | 29 (24.58%) | 17 (34.00%) | .257 |
| Stent thrombosis | 36 (21.43%) | 13 (11.02%) | 23 (46.00%) | <.001* |
| Bleeding events | 17 (10.12%) | 17 (14.41%) | 0 (0%) | <.001* |
| Hemorrhagic apoplexy | 2 (1.19%) | 2 (1.69%) | 0 (0%) | 1.000 |
| Secondary anemia | 9 (5.36%) | 9 (7.63%) | 0 (0%) | .059 |
| Hemorrhage of digestive tract | 6 (3.57%) | 6 (5.08%) | 0 (0%) | .107 |
3.3. CYP2C19 and ABCB1 genotypes distribution
The specific distribution of CYP2C19 and ABCB1 genotypes was shown in Table 4. CYP2C19*2 G/G was found in 74 patients with 44.05% mutation incidence. CYP2C19 *2 G/A was detected in 70 patients with 41.67% mutation frequency. CYP2C19 *2 A/A was examined in 24 patients with 14.28% mutation frequency. In addition, 135 patients were found to have CYP2C19*3 G/G genotype with 80.36% mutation frequency. Thirty‐three patients were examined to have CYP2C19*3 G/A genotype with 19.64% mutation frequency. However, CYP2C19*3 A/A was not found in any patients. In terms of ABCB1 genotype, ABCB1 C/C was detected in 59 patients with 35.12% mutation frequency. ABCB1 T/C was found in 83 patients with 49.40% mutation frequency. ABCB1 T/T was observed in 26 patients with 15.48% mutation frequency.
Table 4.
Distribution of genotypes
| Gene | SNP ID | Genotype | Number | Incidence (%) |
|---|---|---|---|---|
| CYP2C19*2 | rs4244285 | G/G | 74 | 44.05 |
| G/A | 70 | 41.67 | ||
| A/A | 24 | 14.28 | ||
| CYP2C19*3 | rs4986893 | G/G | 135 | 80.36 |
| G/A | 33 | 19.64 | ||
| A/A | 0 | 0.00 | ||
| ABCB1 3435CT | rs1045642 | C/C | 59 | 35.12 |
| T/C | 83 | 49.40 | ||
| T/T | 26 | 15.48 |
The gene mutation rate of patients with CYP2C19*3 in HRP group (81.82%) was significantly higher than that in normal group (18.18%). Average PIR of patients with CYP2C19* was also significantly higher than that of other patients (Table 5, Figure 2). All the above results demonstrated that CYP2C19*3 mutation could be considered as an independent predictive factor of post‐PCI HPR and cardiovascular adverse events, whereas CYP2C19*2 and ABCB1 genetic mutation was not significantly correlated with HPR.
Table 5.
Gene mutation distribution among the patients
| Gene | Overall (n = 168) | Normal (n = 118) | HPR (n = 50) | P value |
|---|---|---|---|---|
| CYP2C19*2 | 94 | 70 (74.47%) | 24 (24.74%) | .234 |
| CYP2C19*3 | 33 | 6 (18.18%) | 27 (81.82%) | <.001 |
| ABCB1 3435CT | 109 | 81 (74.31%) | 28 (25.69%) | .157 |
CYP2C19: cytochrome P450 family 2 subfamily C member 19; CYP2C19*2: rs4244285; CYP2C19*3: rs4986893; ABCB1: ATP binding cassette subfamily B member 1; ABCB1 3435CT: rs1045642. Bold value <.001 indicates that the gene mutation rate of patients with CYP2C19*3 was significantly higher than that in normal group.
Figure 2.
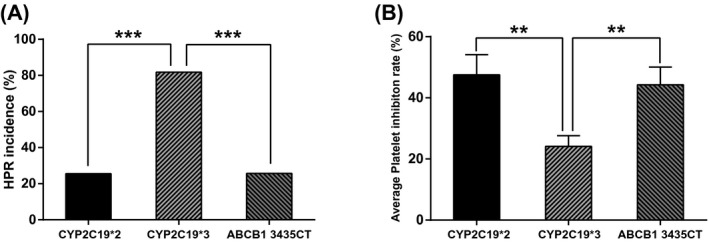
Correlation between genotype and platelet inhibition ratio (PIR) and high on‐treatment platelet reactivity (HPR). (A) HPR rate of patients with CYP2C19*3 was significantly higher than that of patients with CYP2C19*2 and ABCB1 3435CT. (B) Average PIR of patients with CYP2C19*3 was significantly lower than that of patients with CYP2C19*2 and ABCB1 3435CT. PIR: platelet inhibition ratio; HPR: high on‐treatment platelet reactivity. **P < .01, ***P < .001, compared with CYP2C19*3 group. CYP2C19: cytochrome P450 family 2 subfamily C member 19; CYP2C19*2: rs4244285; CYP2C19*3: rs4986893; ABCB1: ATP binding cassette subfamily B member 1; ABCB1 3435CT: rs1045642
3.4. Multivariate logistic regression analysis
Multivariable logistic regression analysis showed that CYP2C19*3 might serve as an independent predictive factor of post‐PCI HPR (OR: 22.97, 95% CI: 8.309‐63.502, P < .001, Table 6), and CYP2C19*3 as well as post‐PCI HPR could be independent predictive factors of ischemic cardiovascular adverse events (OR: 8.562, 95% CI: 1.035‐70.823, P = .046; OR: 7.220, 95% CI: 1.983‐26.293, P = .003, respectively, Table 7).
Table 6.
Logistic regression analysis for HPR
| Variables | B (SE) | Sig. | OR (Exp[B]) | 95% Cl for OR (Exp[B]) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| CYP2C19*2 | −0.658 (0.419) | 0.116 | 0.518 | 0.228 | 1.178 |
| CYP2C19*3 | 3.314 (0.519) | <0.001 | 22.970 | 8.309 | 63.502 |
| ABCB1 3435CT | −0.559 (0.425) | 0.188 | 0.572 | 0.249 | 1.314 |
CYP2C19: cytochrome P450 family 2 subfamily C member 19; CYP2C19*2: rs4244285; CYP2C19*3: rs4986893; ABCB1: ATP binding cassette subfamily B member 1; ABCB1 3435CT: rs1045642. Bold value Sig. <0.001 stands for a P value smaller than 0.001, indicating CYP2C19*3 might serve as an independent predictive factor of post‐PCI HPR.
Table 7.
Logistic regression analysis for ischemic cardiovascular adverse events
| Variables | B (SE) | Sig. | OR (Exp[B]) | 95% CI for OR (Exp[B]) | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| CYP2C19*2 | 0.094 (0.368) | 0.798 | 1.099 | 0.534 | 2.262 |
| CYP2C19*3 | 2.147 (1.078) | 0.046 | 8.562 | 1.035 | 70.823 |
| ABCB1 3435CT | −0.677 (0.396) | 0.087 | 0.508 | 0.234 | 1.103 |
| HPR | 1.977 (0.659) | 0.003 | 7.220 | 1.983 | 26.293 |
CYP2C19: cytochrome P450 family 2 subfamily C member 19; CYP2C19*2: rs4244285; CYP2C19*3: rs4986893; ABCB1: ATP binding cassette subfamily B member 1; ABCB1 3435CT: rs1045642. Bold values Sig. 0.046 and 0.003 mean that CYP2C19*3 as well as post‐PCI HPR could function as independent predictive factors of ischemic cardiovascular adverse events.
4. DISCUSSION
Patients with HPR had a higher recurrence rate of cardiovascular adverse events after PCI operation and clopidogrel treatment. Besides of that, patients with CYP2C19*3 mutation had a significantly higher incidence of HPR, whereas CYP2C19*2 and ABCB1 mutation was not significantly correlated with HPR incidence. CYP2C19*3 might act as an independent predictive factor of post‐PCI HPR. In addition, CYP2C19*3 as well as post‐PCI HPR could function as independent predictive factors of cardiovascular adverse events.
Genetic factors contribute to an increase in CAD occurrence and incur major complications such as HPR‐related stent thrombosis, which is commonly presented as myocardial infarction.20 Although the combined therapeutic strategy of DAPT with aspirin and clopidogrel has been widely applied to the treatment of CAD, the efficacy of clopidogrel can be influenced by CYP2C19*3 LOF allele.21 In our study, higher incidence of cardiovascular ischemic adverse events was found in HPR group. Therefore, we speculated that HPR might function as an independent predictive factor of cardiovascular ischemic adverse events. Among the mutated genotypes of CYP2C19 and ABCB1, CYP2C19*3 LOF was highly correlated with HPR occurrence and PIR, whereas CYP2C19*2 and ABCB1 3435CT had no significant relationship with HPR and PIR. Taken together, CYP2C19*3 LOF could be an independent predictive factor of HPR and ischemic cardiovascular adverse events.
In the current study, 168 CAD patients were follow‐up visited within 24 months after PCI operation and clopidogrel treatment, with all clinical data recorded. Collected data indicated that patients in HPR group had significantly higher recurrence rate of cardiovascular ischemic events. Several previous study results were also consistent with the current findings. For instance, Bagai et al22 reported that a high residual platelet reactivity on clopidogrel treatment was found in their study, in which 40% of patients had HPR. Zhang et al23 revealed that the occurrence of HPR could be regarded as an independent predictor of 2‐year stent thrombosis following DES implantation in patients treated with both aspirin and clopidogrel.
The correlation of mutated CYP2C19 and ABCB1 with HPR was explored in our study. The results demonstrated that CYP2C19*3 genetic polymorphism might function as an independent predictive factor of post‐PCI HPR and ischemic cardiovascular adverse events, while CYP2C19*2 and ABCB1 polymorphisms were found less correlated with the occurrence of HPR. Similarly, Kim et al and Zhang et al reported that ABCB1 polymorphism was not significantly associated with the increased risk of HPR, whereas CYP2C19 polymorphisms influenced antiplatelet response after clopidogrel treatment.24, 25 In addition, patients with CYP2C19 LOF polymorphisms might have increased the risk of adverse cardiovascular events after PCI.26 CYP2C19*2 mutation was considered to increase the risk of major adverse cardiovascular events in patients 27 and induce stent thrombosis.28 We found specifically that CYP2C19*3 polymorphism was closely correlated with HPR. Although CYP2C19*2 was indicated to have higher gene mutation rate in patients with HPR, while CYP2C19*3 had lower mutation rate in patients with HPR.29 CYP2C19*3 genetic polymorphism has higher predictive value for HPR. In addition, in our study, there was a remarkably higher gene mutation rate of CYP2C19*3 in CAD patients with HRP, and these patients with HPR presented a significantly higher incidence of ischemic cardiovascular adverse events. Therefore, we concluded that CYP2C19*3 genetic polymorphism could function as an independent predictive factor of ischemic cardiovascular events. These study results contributed to not only the understanding of correlation of CYP2C19 and ABCB1 genetic polymorphisms with ischemic cardiovascular adverse events, but also the development of effective therapeutic approach of adverse cardiovascular events after clopidogrel treatment of CAD patients.
However, there were still some limitations in the present study. For one thing, a limited number of study objects might not be sufficient to completely substantiate the conclusion. The results would be more convincing when a larger cohort was employed in the future. In addition, stronger evidence for the risk factor analysis of incidence of cardiovascular adverse effects should be further investigated.
In conclusion, CYP2C19*3 might function as an independent predictive factor for HPR and ischemic cardiovascular adverse events, whereas CYP2C19*2 and ABCB1 mutant genes could not predict HPR and ischemic cardiovascular adverse events. Therefore, it should be tested before clopidogrel treatment for CAD patients, which could help make individualized treatment plans for patients.
CONFLICTS OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Research conception and design: Xumin Hou and Wenzheng Han; data analysis and interpretation: Qian Gan; statistical analysis: Yuan Liu; drafting of the study: Xumin Hou; critical revision of the study: Weiyi Fang; and approval of final study: all authors.
Hou X, Han W, Gan Q, Liu Y, Fang W. CYP2C19 and ABCB1 genetic polymorphisms correlate with the recurrence of ischemic cardiovascular adverse events after clopidogrel treatment. J Clin Lab Anal. 2018;32:e22369 10.1002/jcla.22369
REFERENCES
- 1. Nelson CP, Goel A, Butterworth AS, et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat Genet. 2017;49:1385‐1391. [DOI] [PubMed] [Google Scholar]
- 2. Klarin D, Zhu QM, Emdin CA, et al. Genetic analysis in UK Biobank links insulin resistance and transendothelial migration pathways to coronary artery disease. Nat Genet. 2017;49:1395‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xiao FY, Liu M, Chen BL, et al. Effects of four novel genetic polymorphisms on clopidogrel efficacy in Chinese acute coronary syndromes patients. Gene. 2017;623:63‐71. [DOI] [PubMed] [Google Scholar]
- 4. Stefanini GG, Holmes DR Jr. Drug‐eluting coronary‐artery stents. N Engl J Med. 2013;368:254‐265. [DOI] [PubMed] [Google Scholar]
- 5. Task Force on Myocardial Revascularization of the European Society of C and the European Association for Cardio‐Thoracic S , European Association for Percutaneous Cardiovascular I , Wijns W, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501‐2555.20802248 [Google Scholar]
- 6. Lee S, Vargova K, Hizoh I, et al. High on clopidogrel treatment platelet reactivity is frequent in acute and rare in elective stenting and can be functionally overcome by switch of therapy. Thromb Res. 2014;133:257‐264. [DOI] [PubMed] [Google Scholar]
- 7. Jiang M, You JHS. Cost‐effectiveness analysis of 30‐month vs 12‐month dual antiplatelet therapy with clopidogrel and aspirin after drug‐eluting stents in patients with acute coronary syndrome. Clin Cardiol. 2017;40:789‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chatterjee A, Hillegass WB. Individualizing dual antiplatelet therapy duration: prediction tools, genomics, and clinical judgment. Catheter Cardiovasc Interv. 2017;90:38. [DOI] [PubMed] [Google Scholar]
- 9. Park Y, Franchi F, Rollini F, Angiolillo DJ. Dual antiplatelet therapy after coronary stenting. Expert Opin Pharmacother. 2016;17:1775‐1787. [DOI] [PubMed] [Google Scholar]
- 10. Ebisawa S, Ueki Y, Mochidome T, et al. Comparison of platelet aggregation response in switching regimen from prasugrel to clopidogrel between CYP2C19 extensive versus non‐extensive metabolizers. Cardiovasc Interv Ther. 2017. [DOI] [PubMed] [Google Scholar]
- 11. Levine M. Aspirin plus clopidogrel was not linked to risk for cancer compared with aspirin alone or no antiplatelets. Ann Intern Med. 2017;167:JC10. [DOI] [PubMed] [Google Scholar]
- 12. Rudolph TK, Fuchs A, Klinke A, et al. Prasugrel as opposed to clopidogrel improves endothelial nitric oxide bioavailability and reduces platelet‐leukocyte interaction in patients with unstable angina pectoris: a randomized controlled trial. Int J Cardiol. 2017;248:7‐13. [DOI] [PubMed] [Google Scholar]
- 13. Podda GM, Grossi E, Palmerini T, et al. Prediction of high on‐treatment platelet reactivity in clopidogrel‐treated patients with acute coronary syndromes. Int J Cardiol. 2017;240:60‐65. [DOI] [PubMed] [Google Scholar]
- 14. Xu K, Liu X, Li Y, et al. Safety and efficacy of policosanol in patients with high on‐treatment platelet reactivity after drug‐eluting stent implantation: two‐year follow‐up results. Cardiovasc Ther. 2016;34:337‐342. [DOI] [PubMed] [Google Scholar]
- 15. Zhang L, Chen Y, Jin Y, et al. Genetic determinants of high on‐treatment platelet reactivity in clopidogrel treated Chinese patients. Thromb Res. 2013;132:81‐87. [DOI] [PubMed] [Google Scholar]
- 16. Lin Y, Wang A, Li J, et al. Impact of glycemic control on efficacy of clopidogrel in transient ischemic attack or minor stroke patients with CYP2C19 genetic variants. Stroke. 2017;48:998‐1004. [DOI] [PubMed] [Google Scholar]
- 17. So DY, Wells GA, McPherson R, et al. A prospective randomized evaluation of a pharmacogenomic approach to antiplatelet therapy among patients with ST‐elevation myocardial infarction: the RAPID STEMI study. Pharmacogenomics J. 2016;16:71‐78. [DOI] [PubMed] [Google Scholar]
- 18. Simon T, Verstuyft C, Mary‐Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363‐375. [DOI] [PubMed] [Google Scholar]
- 19. Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON‐TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312‐1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo Y, Wang F, Li L, et al. Genome‐wide linkage analysis of large multiple multigenerational families identifies novel genetic loci for coronary artery disease. Sci Rep. 2017;7:5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tantray JA, Reddy KP, Jamil K, Kumar YS. Pharmacodynamic and cytogenetic evaluation in CYP2C19*2 and CYP2C19*3 allelomorphism in South Indian population with clopidogrel therapy. Int J Cardiol. 2017;229:113‐118. [DOI] [PubMed] [Google Scholar]
- 22. Bagai A, Peterson ED, McCoy LA, et al. Association of measured platelet reactivity with changes in P2Y12 receptor inhibitor therapy and outcomes after myocardial infarction: insights into routine clinical practice from the TReatment with ADP receptor iNhibitorS: longitudinal Assessment of Treatment Patterns and Events after Acute Coronary Syndrome (TRANSLATE‐ACS) study. Am Heart J. 2017;187:19‐28. [DOI] [PubMed] [Google Scholar]
- 23. Zhang JJ, Gao XF, Ge Z, et al. High platelet reactivity affects the clinical outcomes of patients undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2016;16:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim IS, Jeong YH, Park Y, et al. Interaction analysis between genetic polymorphisms and pharmacodynamic effect in patients treated with adjunctive cilostazol to dual antiplatelet therapy: results of the ACCEL‐TRIPLE (Accelerated Platelet Inhibition by Triple Antiplatelet Therapy According to Gene Polymorphism) study. Br J Clin Pharmacol. 2012;73:629‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang HZ, Kim MH, Guo LZ, Serebruany V. CYP2C19 but not CYP2B6, CYP3A4, CYP3A5, ABCB1, PON1 or P2Y12 genetic polymorphism impacts antiplatelet response after clopidogrel in Koreans. Blood Coagul Fibrinolysis. 2017;28:56‐61. [DOI] [PubMed] [Google Scholar]
- 26. Trenk D, Hochholzer W. Genetics of platelet inhibitor treatment. Br J Clin Pharmacol. 2014;77:642‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen Y, Huang X, Tang Y, Xie Y, Zhang Y. Both PON1 Q192R and CYP2C19*2 influence platelet response to clopidogrel and ischemic events in Chinese patients undergoing percutaneous coronary intervention. Int J Clin Exp Med. 2015;8:9266‐9274. [PMC free article] [PubMed] [Google Scholar]
- 28. Cayla G, Hulot JS, O'Connor SA, et al. Clinical, angiographic, and genetic factors associated with early coronary stent thrombosis. JAMA. 2011;306:1765‐1774. [DOI] [PubMed] [Google Scholar]
- 29. Liu X, Luo Y, Lai Y, et al. Effect of genetic and coexisting polymorphisms on platelet response to clopidogrel in Chinese Han patients with acute coronary syndrome. J Genet. 2016;95:231‐237. [DOI] [PubMed] [Google Scholar]


