Abstract
Background
Vitamin C may interfere with the results of urine dipstick tests. We investigated the incidence of urinary vitamin C and its interference with urine dipstick reagents using a vitamin C dipstick.
Methods
The incidence of urinary vitamin C was determined in patients and healthy individuals undergoing routine medical check‐ups. Interference tests were performed using samples with various amounts of added vitamin C. For clinical samples, we identified false‐negative dipstick glucose, hemoglobin, and leukocyte esterase results based on the urine sediment and serum glucose results.
Results
Vitamin C was found in the urine of 18.1% of the subjects overall, and 23.1% of those undergoing medical check‐ups. Dipstick results for glucose, leukocyte esterase, and hemoglobin differed between samples without vitamin C and with added vitamin C. When vitamin C was detected in clinical urine samples, 42.3%, 10.6%, and 8.2% of the glucose, hemoglobin, and leukocyte esterase dipstick tests were rated as false negative, respectively.
Conclusions
Vitamin C was frequently found in clinical urine samples, and its concentration was higher in individuals undergoing medical check‐ups. Urinary vitamin C can interfere with the urine dipstick results. This study gives useful information for predicting false‐negative rates of urine dipstick tests caused by vitamin C.
Keywords: interference, urine dipstick test, vitamin C
1. Introduction
Urinalysis is one of the simplest and most important screening tests in clinical practice and provides essential information.1 The urine dipstick test, one of the two major components of routine urinalysis, provides information about several physiochemical properties of urine: pH, specific gravity, hemoglobin, protein, glucose, ketones, bilirubin, urobilinogen, nitrite, and leukocyte esterase in urine. However, several substances in urine can cause false‐negative or ‐positive results for some parameters.2 For example, in urine, the widely used vitamin C supplement may cause false‐negative results for glucose, hemoglobin, leukocyte esterase, and nitrite.2, 3, 4 Although the influence of vitamin C on a urine dipstick results is well known, few studies have examined its use in clinical urine samples5, 6 and only one study has reported the incidence and concentration of urinary vitamin C in a large sample.7 Recently, a new urine dipstick reagent (URiSCAN 11 strip; YD Diagnostics, Yongin, Korea) that can measure the vitamin C concentration in urine was launched in Korea.
This study investigated the incidence of urinary vitamin C in people undergoing routine urinalysis and the effects of vitamin C on the glucose, hemoglobin, leukocyte esterase, and nitrite dipstick tests using the URiSCAN 11 strip.
2. Materials and Methods
2.1. Urine specimens
This study was conducted using residual samples of random spot urines that had been submitted to laboratories at four tertiary medical centers in Korea for diagnostic urinalysis from July 2015 to November 2015. This study was conducted in accordance with the Declaration of Helsinki and approved by each hospital ethics committee.
2.2. Urine chemical screening test
Eleven urine chemistry parameters were examined, including vitamin C, with the URiSCAN 11 strip using the URiSCAN Pro III analyzer (YD Diagnostics). The results for five items analyzed in this study were classified according to the grading systems described in Table 1. The URiSCAN Pro III was calibrated according to the manufacturer's recommendations. Quality control was performed using the Liquichek™ Urinalysis Control (Bio‐Rad, Hercules, CA, USA). All urine dipstick reagents used in this study were the same lot number.
Table 1.
Grading systems used for the URiSCAN 11 strip
| Parameters | Grade | |||||
|---|---|---|---|---|---|---|
| Titrated concentration | ||||||
| Glucose (mg/dL) | Negative <100 | Trace 100‐249 | 1+ 250‐499 | 2+ 500‐999 | 3+ 1000‐1999 | 4+ ≥2000 |
| Hemoglobin (RBC/μL) | Negative | Trace <10 | 1+ 10‐49 | 2+50‐249 | 3+≥250 | |
| Leukocyte esterase (WBC/μL) | Negative | Trace <24 | 1+ 25‐74 | 2+ 75‐499 | 3+≥500 | |
| Vitamin C (mg/dL) | Negative <10 | 1+10‐24 | 2+25‐49 | 3+ ≥50 | ||
| Nitrite | Negative | Positive a | ||||
RBC, red blood cells; WBC, white blood cells.
a≥105 bacteria/mL in urine.
2.3. Urine sediment examination
Manual microscopic analysis was performed to analyze urine sediments at two medical centers. At the other two centers, UF‐1000i urine sediment analyzers (Sysmex Corporation, Kobe, Japan) were used.
The manual microscopic sediment examination was performed according to the CLSI guideline GP16‐A3.8 Each urine sample (10 mL) was centrifuged at 400 g for 5 min, after which 9.5 mL of the supernatant was removed. The pellet was resuspended and one drop was placed on a glass slide and covered with a 22×22 mm cover slip, and examined by light microscopy. For the sediment analysis, 10 high‐power fields (HPF) were evaluated and the counts were given as the average per HPF.
The urine sediment analysis on the Sysmex UF‐1000i was performed as described previously.9 The sediment results obtained as counts per microliter with the UF‐1000i were converted into counts per HPF using the equation in CLSI GP16‐A3. Each particle result was reported based on the grading systems described in Table 2.
Table 2.
Grading systems used in the analysis of urine particles
| Parameters | Grade | ||||
|---|---|---|---|---|---|
| RBC, WBC (count/HPF) | <1 | 1‐4 | 5‐9 | 10‐29 | ≥ 30 |
| Bacteria | Negative | Positive | |||
RBC, red blood cells; WBC, white blood cells; HPF, high‐power field.
2.4. Serum glucose
Blood samples collected at the same time as the spot urine were used to measure the serum glucose, using four different automatic chemistry analyzers with their exclusive reagents at each medical center: ADVIA 2400 (Siemens Healthcare Diagnostics, Erlangen, Germany), AU5800 (Beckman Coulter, Brea, CA, USA), COBAS c702 (Roche Diagnostics, Basel, Switzerland), and Modular DP (Roche Diagnostics, Mannheim, Germany).
2.5. Incidence of urinary vitamin C
The incidence of urinary vitamin C was investigated in 5006 urine samples from inpatients, outpatients, and people undergoing routine medical check‐ups in November 2015 at a tertiary medical center.
2.6. Interference test by vitamin C
2.6.1. Pooled samples
We selected clinical urine samples that were negative for vitamin C, but positive for protein (20 samples), glucose (13 samples), leukocyte esterase (9 samples), hemoglobin (15 samples), and nitrite (3 samples). To each sample, different amounts of vitamin C were added, adjusted to give final concentrations of 12.5, 25, 50, 100, and 200 mg/dL. All prepared samples were retested with reagent strips, and the results were compared with those of the original samples without vitamin C.
2.6.2. Clinical samples
From all subjects undergoing routine urinalysis at the four centers, 1509 with urinary vitamin C were analyzed. A Spearman's rank correlation was used to evaluate correlations (r, correlation coefficient) for hemoglobin, leukocyte esterase, and nitrite between the dipstick and sediment analyses. A statistical analysis was performed using Microsoft Excel 2010 (Microsoft, Redmond, WA, USA) with Analyze‐it v.4.60.1 (Analyze‐it Software, Leeds, UK).
We rated the urine dipstick results as false negative based on the urine sediment and serum glucose results: (i) glucose, the urine glucose result was negative when the serum glucose level was above 180 mg/dL; (ii) hemoglobin and leukocyte esterase, the urine dipstick result was two or more grades lower than the urine sediment result; and (iii) nitrite, the urine nitrite result was negative when the bacteria result was positive in the urine sediment analysis.
3. Results
3.1. Incidence of urinary vitamin C
Urinary vitamin C was detected in 1110 (18.1%) of 5006 samples (Table 3). The rate was higher in the group undergoing medical check‐ups (23.1%) compared with inpatients (16.3%) and outpatients (17.6%). A high vitamin C concentration (3+) was observed more frequently in the medical check‐up group than in the others.
Table 3.
Incidence of urinary vitamin C using the URiSCAN 11 strip
| Classification | No. | Vitamin C (%) | |||
|---|---|---|---|---|---|
| 1+ | 2+ | 3+ | Subtotal | ||
| Inpatients | 1030 | 80 (6.5) | 86 (7.0) | 35 (2.8) | 201 (16.3) |
| Outpatients | 3348 | 296 (7.3) | 298 (7.3) | 121 (3.0) | 715 (17.6) |
| Medical check‐ups | 628 | 26 (3.2) | 48 (5.9) | 115 (14.1) | 189 (23.1) |
| Total | 5006 | 402 (6.6) | 432 (7.1) | 271 (4.4) | 1,110 (18.1) |
3.2. Interference test with pooled samples
For protein and nitrite, no obvious interference was observed according to the vitamin C concentration (Figure 1). The results for glucose, leukocyte esterase, and hemoglobin tended to be lower based on the urinary vitamin C concentration.
Figure 1.
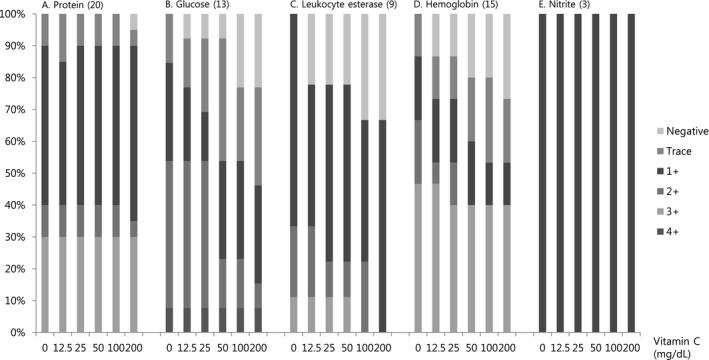
Interference of vitamin C with the results for protein, glucose, leukocyte esterase, hemoglobin, and nitrite according to the vitamin C concentration in the pooled samples
3.3. Interference test using clinical samples
In total, 175 cases were positive for urinary vitamin C and had serum glucose results >180 mg/dL. Of these, 74 (42.3%) cases gave false‐negative results on the urine dipstick glucose test (Table 4).
Table 4.
Interference of vitamin C with the urine dipstick glucose result
| Urine glucose (%) | |||||||
|---|---|---|---|---|---|---|---|
| Negative | Trace | 1+ | 2+ | 3+ | 4+ | Total | |
| Vit C | |||||||
| 1+ | 34 (44.7) | 5 (6.6) | 3 (3.9) | 8 (10.5) | 22 (28.9) | 4 (5.3) | 76 |
| 2+ | 36 (42.9) | 10 (11.9) | 7 (8.3) | 6 (7.1) | 18 (21.4) | 7 (8.3) | 84 |
| 3+ | 4 (26.7) | 3 (20.0) | 0 | 3 (20.0) | 5 (33.3) | 0 | 15 |
| Total | 74 (42.3) | 18 (10.3) | 10 (5.7) | 17 (9.7) | 45 (25.7) | 11 (6.3) | 175a |
aThe serum glucose levels were >180 mg/dL in all cases.
When vitamin C was detected in urine, the correlation coefficients between the dipstick and sediment tests for hemoglobin, leukocyte esterase, and nitrites were 0.684, 0.661, and 0.435, respectively (Tables 5, 6, 7).
Table 5.
Pairwise comparison of the RBC results between the urine dipstick and sediment tests
| Hemoglobin‐dipstick | RBC‐sediment (counts/HPF) | r | ||||||
|---|---|---|---|---|---|---|---|---|
| <1 | 1‐4 | 5‐9 | 10‐29 | ≥30 | Sum | |||
| Vit C + (total) | Negative | 691 | 255 | 91 | 20 | 7 | 1064 | .684a |
| Trace | 24 | 51 | 53 | 15 | 9 | 152 | ||
| 1+ | 3 | 21 | 42 | 23 | 18 | 107 | ||
| 2+ | 2 | 14 | 18 | 23 | 16 | 73 | ||
| 3+ | 0 | 3 | 4 | 14 | 92 | 113 | ||
| Vit C 1+ | Negative | 180 | 65 | 29 | 4 | 2 | 280 | .704a |
| Trace | 11 | 18 | 18 | 2 | 3 | 52 | ||
| 1+ | 2 | 11 | 14 | 11 | 11 | 49 | ||
| 2+ | 1 | 7 | 8 | 8 | 5 | 29 | ||
| 3+ | 0 | 0 | 1 | 8 | 31 | 40 | ||
| Vit C 2+ | Negative | 420 | 153 | 49 | 11 | 4 | 637 | .684a |
| Trace | 9 | 25 | 31 | 9 | 5 | 79 | ||
| 1+ | 0 | 7 | 22 | 10 | 6 | 45 | ||
| 2+ | 1 | 6 | 8 | 12 | 9 | 36 | ||
| 3+ | 0 | 3 | 3 | 4 | 50 | 60 | ||
| Vit C 3+ | Negative | 91 | 37 | 13 | 5 | 1 | 147 | .621a |
| Trace | 4 | 8 | 4 | 4 | 1 | 21 | ||
| 1+ | 1 | 3 | 6 | 2 | 1 | 13 | ||
| 2+ | 0 | 1 | 2 | 3 | 2 | 8 | ||
| 3+ | 0 | 0 | 0 | 2 | 11 | 13 | ||
RBC, red blood cells; HPF, high‐power field.
Number of cases [ ] within one‐grade difference and [
] within one‐grade difference and [ ] with the same grade.
] with the same grade.
r, Spearman's correlation coefficient.
a P<.05.
Table 6.
Pairwise comparison of the WBC results between the urine dipstick reagent and sediment analysis
| Leukocyte esterase‐dipstick | WBC‐sediment (counts/HPF) | r | ||||||
|---|---|---|---|---|---|---|---|---|
| <1 | 1‐4 | 5‐9 | 10‐29 | ≥30 | Sum | |||
| Vit C + (total) | Negative | 696 | 264 | 55 | 26 | 10 | 1051 | .661a |
| Trace | 41 | 70 | 39 | 14 | 3 | 167 | ||
| 1+ | 4 | 33 | 41 | 35 | 16 | 129 | ||
| 2+ | 1 | 7 | 10 | 30 | 41 | 89 | ||
| 3+ | 0 | 3 | 0 | 16 | 52 | 71 | ||
| Vit C 1+ | Negative | 179 | 84 | 21 | 12 | 7 | 303 | .595a |
| Trace | 17 | 25 | 15 | 7 | 0 | 64 | ||
| 1+ | 0 | 8 | 11 | 9 | 4 | 32 | ||
| 2+ | 1 | 2 | 4 | 8 | 11 | 26 | ||
| 3+ | 0 | 2 | 0 | 5 | 19 | 26 | ||
| Vit C 2+ | Negative | 426 | 138 | 31 | 9 | 2 | 606 | .713a |
| Trace | 16 | 31 | 19 | 7 | 3 | 76 | ||
| 1+ | 3 | 20 | 23 | 23 | 11 | 80 | ||
| 2+ | 0 | 4 | 6 | 17 | 29 | 56 | ||
| 3+ | 0 | 0 | 0 | 9 | 27 | 36 | ||
| Vit C 3+ | Negative | 91 | 42 | 3 | 5 | 1 | 142 | .578a |
| Trace | 8 | 14 | 5 | 0 | 0 | 27 | ||
| 1+ | 1 | 5 | 7 | 3 | 1 | 17 | ||
| 2+ | 0 | 1 | 0 | 5 | 1 | 7 | ||
| 3+ | 0 | 1 | 0 | 2 | 6 | 9 | ||
WBC, white blood cell; HPF, high‐power field.
Number of cases [ ] within one‐grade difference and [
] within one‐grade difference and [ ] with the same grade.
] with the same grade.
r, Spearman's correlation coefficient.
a P<.01.
Table 7.
Interference of vitamin C with the nitrite results
| Bacteria | Nitrite (%) | r | ||
|---|---|---|---|---|
| Negative | Positive | |||
| Vit C + (total) | Negative | 1332 (99.0) | 13 (1.0) | .435a |
| Positive | 118 (72.0) | 46 (28.0) | ||
| Vit C 1+ | Negative | 399 (99.3) | 3 (0.7) | .494a |
| Positive | 33 (67.3) | 16 (32.7) | ||
| Vit C 2+ | Negative | 745 (98.8) | 9 (1.2) | .438a |
| Positive | 72 (70.6) | 30 (29.4) | ||
| Vit C 3+ | Negative | 188 (99.5) | 1 (0.5) | −.018b |
| Positive | 13 (100) | 0 | ||
r, Spearman's correlation coefficient.
a P<.05, b P≥.05.
Of the 1509 cases with urinary vitamin C, 1323 (87.7%) were within a one‐grade difference for hemoglobin when the dipstick and urine sediment tests were compared, leaving 186 cases with substantial disagreement (Table 5). Of the latter cases, 160 cases (10.6%) gave false‐negative results.
For leukocyte esterase, 1368 cases (90.8%) were within a one‐grade difference for leukocyte esterase when the dipstick and urine sediment tests were compared (Table 6). Of the others, 124 cases (8.2%) were identified as false‐negative results. The interference with the nitrite results is summarized in Table 7.
4. Discussion
Vitamin C is one of the most widely used dietary supplements worldwide. However, vitamin C in urine can interfere with several urine dipstick tests. Brigden et al.7 reported that 22.8% of 4379 routine urinalysis specimens showed positive results at the vitamin dipstick test, which had the same detection limit (10 mg/dL) as the URiSCAN 11 strip. In this study, the incidence of the urinary vitamin C was slightly lower, at 18.1% of 5006 routine urinalysis specimens. We thought that this difference was based on the difference between enrolled groups. However, our data revealed an interesting result: the rate of high urinary vitamin C concentrations was higher in the medical check‐up group than in the other groups. Vitamin C is used as an antioxidant in many common chronic diseases, such as rheumatoid arthritis, cardiovascular disease, diabetes, and terminal cancer.3 However, it is taken with increasing frequency by people who are interested in their health. Consequently, about 20% of all urinary dipstick tests have a risk of false‐negative results for several parameters and this might be observed more often in individuals undergoing medical check‐ups. Brigden et al.7 suggested that screening urinalysis protocols using a dipstick should also check the urinary vitamin C to predict potential false‐negative results. The URiSCAN 11 strip also gives the urinary vitamin C result in a single test.
Our dipstick results for glucose, leukocyte esterase, and hemoglobin for samples with added vitamin C changed compared with the results for the original samples without vitamin C. The degree of interference increased with the urinary vitamin C concentration, as reported previously.6 As is well known, vitamin C did not interfere with the protein test.1 However, we also did not observe interference in the nitrite test. However, we tested only three samples that were positive for nitrite, so it is difficult to say that vitamin C does not interfere with the nitrite test.
Glucosuria is not always concomitant with hyperglycemia, but glucose usually appears in urine when the serum glucose level exceeds 180 mg/dL.1 Urine glucose is not routinely quantified in clinical laboratories. Therefore, we defined the dipstick glucose result as false negative when a patient with a blood glucose level >180 mg/dL was negative for urinary glucose. Although we did not check the degree of interference of vitamin C in the urine dipstick glucose test according to the serum glucose level, we observed false‐negative results in 42.3% of 175 cases.
There are many automated urinary sediment analyzers that give quantitative results. However, semiquantitative grading is still widely used to report urinary sediment results.9, 10 We classified the urine sediment results for red and white blood cells according to the guidelines of the Korean Society for Laboratory Medicine.11 Although the range of the grading system for urine sediments was not completely consistent with that of the urine dipstick, the dipstick results were compared with them to predict false‐negative results.
For clinical samples, when urinary vitamin C was detected, Spearman's correlation coefficients for hemoglobin and leukocyte esterase between the two methods were 0.684 and 0.661, respectively. A pattern of change with decreasing correlation coefficients according to the urinary vitamin C concentration was only observed for hemoglobin. However, our data for urine samples positive for vitamin C gave slightly lower correlations for hemoglobin and leukocyte esterase than those of random urine samples (hemoglobin, r=.865; leukocyte esterase, r=.764) in a previous study.10
The overall concordance rates between the two methods for hemoglobin and leukocyte esterase were good (87.7% and 90.8%, respectively). However, 10.6% and 8.2% were defined as false negative for hemoglobin and leukocyte esterase, respectively. Especially, among the dipstick results of the groups with urine sediment above 5/HPF, 26.5% (118/445) and 23.5% (91/388) showed ‘negative’ results for hemoglobin and leukocyte esterase. Consequently, if only a urine dipstick test is performed for people who have ingested vitamin C, about one‐quarter of the people with abnormal hemoglobin or leukocyte esterase levels in urine might be missed during screening. Therefore, it is necessary to combine the urinalysis with the urine sediment analysis or urine vitamin C test to increase the performance of the urine dipstick test as a screening tool.
Based on our definition of false negative for nitrite, vitamin C appeared to interfere with 72.0% of the cases with bacteria in urine. However, this does not truly reflect the effect of vitamin C because not all bacteria are able to reduce nitrate to nitrite, and large numbers of bacteria (≥105‐106/mL) are necessary to show a positive urine nitrite test.1
In summary, we observed that vitamin C is frequently found in samples sent for routine urinalysis. Of note, urinary vitamin C was detected more frequently in people undergoing routine medical check‐ups. Although this study has some limitations, such as using the serum glucose level instead of the urine level and using different grading systems for hemoglobin and leukocyte esterase, it should help to predict the rate of false‐negative hemoglobin and leukocyte esterase urine tests due to vitamin C.
Acknowledgment
This research was supported by the grant of the Korean Association of External Quality Assessment Service (2015).
References
- 1. McPherson RA, Ben‐Ezra J. Basic examination of urine In: McPherson RA, Pincus MR, eds. Henry's Clinical Diagnosis and Management by Laboratory Methods, 22nd edn Philadelphia, PA: Saunders;2011:445–479. [Google Scholar]
- 2. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: a comprehensive review. Am Fam Physician. 2005;71:1153–1162. [PubMed] [Google Scholar]
- 3. McGregor GP, Biesalski HK. Rationale and impact of vitamin C in clinical nutrition. Curr Opin Clin Nutr Metab Care. 2006;9:697–703. [DOI] [PubMed] [Google Scholar]
- 4. Berg B. Ascorbate interference in the estimation of urinary glucose by test strips. Clin Chem Clin Biochem. 1986;24:89–96. [DOI] [PubMed] [Google Scholar]
- 5. Daae LNW, Juell A. Ascorbic acid and test strip reactions for haematuria. Scand J Clin Lab Invest. 1983;43:267–269. [PubMed] [Google Scholar]
- 6. Ko DH, Jeong TD, Kim S, et al. Influence of vitamin C on urine dipstick test results. Ann Clin Lab Sci. 2015;45:391–395. [PubMed] [Google Scholar]
- 7. Brigden ML, Edgell D, McPherson M, Leadbeater A, Hoag G. High incidence of significant urinary ascorbic acid concentrations in a west coast population–implications for routine urinalysis. Clin Chem. 1992;38:426–431. [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute . Urinalysis; Approved Guideline – 3rd edn. CLSI Document GP16‐A3. Wayne, PA: CLSI, USA; 2009. [Google Scholar]
- 9. Lee W, Ha JS, Ryoo NH. Comparison of the automated COBAS u 701 urine microscopy and UF‐1000i flow cytometry systems and manual microscopy in the examination of urine sediments. J Clin Lab Anal. 2016;30:663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yuksel H, Kilic E, Ekinci A, Evliyaoglu O. Comparison of fully automated urine sediment analyzers H800‐FUS100 and Labumat‐Urised with manual microscopy. J Clin Lab Anal. 2013;27:312–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim KD. Urinalysis In: The Korean Society of Laboratory Medicine , ed. Laboratory Medicine, 5th edn Seoul: Panmun Education; 2014:497–508. [Google Scholar]


