Abstract
Background
Previous studies have demonstrated that dysfunctional regulatory T cells (Tregs) may be associated with Graves’ disease (GD). In this study, we evaluated four serum Treg‐associated miRNAs (miR‐210, miR‐182, miR‐155, and miR‐146a) expressions and assessed the potential of serum miRNAs as biomarkers of GD.
Methods
Foxp3 and serum miRNAs expressions both in GD patients and healthy controls were measured by RT‐PCR.
Results
Serum miR‐210 in GD patients was significantly higher than that of healthy controls (2.64‐fold, P<.001); in contrast, miR‐155 and miR‐146a were lower (P<.001 and P=.008). No significant difference was found in miR‐182. ROC curve analysis indicated that miR‐210, miR‐155, and miR‐146a with the area under ROC (AUC) of 0.803 (70.0% sensitivity and 83.1% specificity), 0.796 (76.3% sensitivity and 76.9% specificity), and 0.736 (68.8% sensitivity and 73.8% specificity), respectively, could differentiate GD patients from healthy controls. Combination of three miRNAs yielded an AUC of 0.976 (91.3% sensitivity and 93.8% specificity) with 92.41% diagnostic efficiency. In addition, serum miR‐210 and miR‐155 in GD were associated with the extent of goiter. Three miRNAs levels were different by gender. Besides, serum miR‐210 was positively correlated with free thyroxine (FT4) and thyrotrophin receptor antibody (TRAb) level.
Conclusion
The serum levels of miR‐210, miR‐155, and miR‐146a may be potential new markers for the diagnosis of GD and play important roles in GD pathogenesis.
Keywords: Graves’ disease, miR‐146a, miR‐155, miR‐210, regulatory T cells
1. INTRODUCTION
As a common organ‐specific autoimmune disease, Graves’ disease (GD) is characterized by diffuse goiter and hyperthyroidism. The diagnosis of GD is clinically based on the increased free triiodothyronine (FT3), free thyroxine (FT4), and the positive thyroid specific autoantibody, such as anti‐thyroglobulin antibody (TGAb), anti‐thyroperoxidase antibody (TPOAb), and anti‐thyrotropin receptor antibody (TRAb). Regulatory T cells (Tregs), which play important roles in the development of GD, are critical to regulating the immune response.1, 2 As a transcription factor, forkhead box protein 3 (Foxp3) is the major regulatory factor for the development and function of Tregs.3, 4 Several studies have reported that Foxp3 expression and the number of CD4+CD25+ Tregs were decreased in GD patients.5, 6, 7, 8 However, the mechanisms of Treg dysfunction were still unknown.
MicroRNAs (miRNAs), as a class of small noncoding RNA 19‐25 nucleotide in length, are identified as negative regulators of gene expression. miRNAs could affect cell apoptosis, differentiation, proliferation, and immune functions. In addition, miRNAs have been reported to play an important role in the development of autoimmunity or autoimmune disease.9 Zhao et al.10 found that miR‐210 inhibited mRNA and protein expression of Foxp3 via binding with the 3′‐UTR region of Foxp3 and modulated the immunosuppressive functions of Tregs. Besides, miR‐182 could target the 3′‐UTR of forkhead‐box O1 (FoxO1), which controls the development and function of Tregs, resulting in a decrease of Tregs.11, 12 As a direct target of Foxp3, miR‐155 is highly expressed in Tregs and maintains competitive fitness of Tregs by targeting suppressors of cytokine signaling (SOCS)‐1.13, 14 In addition, miR‐146a, which is prevalently expressed in Tregs, is critical for the suppressor function of Tregs.15 Interestingly, different serum levels of miRNAs are associated with GD, which may play a role in the pathogenesis of GD.16
In this study, we evaluated the serum levels of these four Treg‐associated miRNA (miR‐210, miR‐182, miR‐155, and miR‐146a) in patients with GD using quantitative real‐time PCR analysis. In addition, we investigated the correlations of these serum miRNAs and clinical variables of GD.
2. SUBJECTS AND METHODS
2.1. Study subjects
In this case‐control study, we recruited 80 patients who met the criteria for GD and 65 healthy controls from the Department of Endocrinology, Wuhan Union Hospital. The GD patients were diagnosed based on the American Association of Clinical Endocrinologists (AACE) guidelines. Serum samples were collected from the patients without any treatments at the first diagnosis. All participants provided written informed consent. The controls had no evidence of GD and other autoimmune diseases, infection diseases, and other diseases.
The serum levels of free triiodothyronine (FT3), free thyroxine (FT4), thyroid stimulating hormone (TSH), antithyroglobulin antibody (TGAb), thyroperoxidase antibody (TPOAb), and thyrotrophin receptor antibody (TRAb) were measured by electrochemiluminescence. The demographic data were collected from our hospital records. The study was approved by the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology.
2.2. RNA extraction
Total RNA was isolated from 200 μL of serum using the Trizol LS reagents (Invitrogen, Carlsbad, CA, USA) following manufacturer's instructions.17 Some other studies have reported that using Trizol LS reagent was effective.18, 19 Briefly, all serum samples were centrifugated at 12 000 g for 10 minutes to separate cellular debris and other solid pollutants; then 200 μL of serum was mixed with l ml of Trizol LS solution; 10 μL of synthetic cel‐miR‐39 miScript miRNA Mimic was added to all samples to be used as an exogenous reference, vortexed, then incubated at room temperature for 5 minutes and the Trizol protocol continued. Finally, total RNA was dissolved in 20 μL of RNase‐free water.
The reverse transcription reaction was in a 20 μL volume contained 1 μL reverse transcriptase, 4 μL 5× RT buffer, 2 μL antisense looped primer mix, 2 μL RNA solution, and 11 μL of RNase‐free water. The mix was incubated at 37°C for 60 minutes and 85°C for 5 minutes. The products were stored at −20°C before running the real‐time PCR.
2.3. Quantitative real‐time PCR of serum miRNAs
The reaction mixture for PCR contained 10 μL of SYBR Green PCR Mix (Takara), 1 μL of universal reverse primer, 1 μL of specific sense primer, 2 μL of cDNA, and 6 μL of RNase‐free water. The reaction conditions were as follows: 95°C for 2 minutes, followed by 45 cycles at 94°C for 5 seconds and 60°C for 40 seconds. The amplification was performed by a Stratagene Mx3000P Sequence Detection System. The specific primers of miR‐146a, miR‐155, miR‐182, and miR‐210 were bought from the company (RiboBio, Guangzhou, China). The primers of U6b and cel‐miR‐39 were ACGCAAATTCGTGAAGCGTT and CACCGGGTGTAAATCAGCTTG, respectively. Both U6b and cel‐miR‐39 were used as reference controls.17 Relative expression of each miRNA was calculated using the comparative cycle threshold (CT) method (2−ΔΔCT).20 The expression levels of miRNAs were calibrated relative to healthy controls.
2.4. Foxp3 mRNA expression
Foxp3 mRNA expression in peripheral blood mononuclear cells was measured by reverse transcription PCR (RT‐PCR). Specific PCR primers of Foxp3 were 5′‐GAGAAGGAGAAGCTGAGTGCCAT‐3′ (forward) and 5′‐AGCAGGAGCCCTTGTCGGAT‐3′ (reverse). The amplification was performed using a Stratagene Mx3000P Sequence Detection System.
2.5. Statistical analysis
The relative levels of miRNAs were expressed as mean±SEM. Differences in serum levels of miRNAs among groups were evaluated by a nonparametric test (Mann‐Whitney test or Wilcoxon paired test). Receiver operating characteristics (ROC) curves were performed to measure the diagnostic efficiency. The associations between serum miRNAs and clinical parameters were investigated by Spearman's rank correlation test. All statistical analyses were performed by SPSS 17.0. In addition, P values of less than 0.05 were considered statistically significant.
3. RESULTS
3.1. The clinical characteristics of participants
Eighty newly diagnosed GD patients and 65 healthy controls were recruited, and the clinical characteristics of the participants were shown in Table 1. The mean age was 36.43±12.82 years for GD patients and 37.60±7.21 years for healthy controls. There was no significant difference between GD patients and healthy controls in age or gender (P=.513; P=.621).
Table 1.
Clinical characteristic of the participants
| Characteristics | GD patients | Healthy controls |
|---|---|---|
| M/F | 24/56 | 22/43 |
| Age | 36.43±12.82 | 37.60±7.21 |
| M/F | M/F | |
| Alcohol abuse | 4/1 | 3/1 |
| Smoking | 7/6 | 6/5 |
| Skin lesion | 3/6 | 0/0 |
| Ophthalmopathy | 9/20 | 0/0 |
| Thyroid size | ||
| Normal/I | 7/17 | 22/43 |
| II/III | 17/39 | 0/0 |
| FT3 (p mol/L) | 26.82±14.12 | 4.52±1.36 |
| FT4 (p mol/L) | 69.82±29.33 | 16.21±3.58 |
| TSH (m IU/L) | 0.020±0.082 | 1.21±0.73 |
| TgAb (IU/mL) | 673.81±870.48 | Negative |
| TPOAb (IU/mL) | 210.47±211.92 | Negative |
| TRAb (IU/L) | 16.37±14.20 | Negative |
The quantity data are expressed as mean ± standard deviation. M/F: male/female; Normal: thyroid, impalpable and invisible; I: thyroid, palpable and invisible; II/III: thyroid, palpable and visible.
3.2. The serum levels of miRNAs in GD patients and healthy controls
As shown in Figure 1, the serum level of miR‐210 in GD patients was significantly higher than that of healthy controls (2.64‐fold, P<.001, Figure 1A). Figure 1C and D shows that the serum levels of miR155 and miR146a in GD were lower compared with healthy controls (P<.001 and P=.008, respectively). In addition, no significant difference was found in the levels of serum miR‐182 between GD patients and healthy controls (P=.290, Figure 1B).
Figure 1.
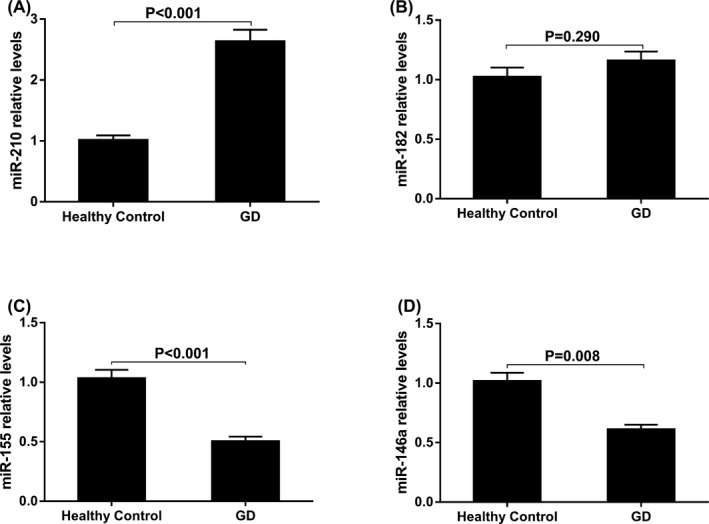
The serum levels of miR‐210 (A), miR‐182 (B), miR‐155 (C), and miR146a (D) in GD patients and healthy controls. Data given were the mean ± standard deviation. Healthy controls were normalized to 1
To evaluate the diagnostic value of these serum miRNAs, receiver operating characteristic (ROC) curve analysis was performed. The area under ROC (AUC) of miR‐210 was 0.803±0.036 (95% CI: 0.731‐0.874, P<.001; Figure 2A). At the cutoff value of 1.424 for miR‐210, the sensitivity was 70.0% and the specificity was 83.1%. miR‐155 had an AUC of 0.796±0.039 (95% CI: 0.719‐0.872, P<.001; Figure 2B) and miR‐146a had an AUC of 0.736±0.043 (95% CI: 0.653‐0.820, P<.001; Figure 2C). When the cutoff values for miR‐155 and miR‐146a reach 0.720 and 0.761, the sensitivity and specificity were 76.3% and 76.9%, and 68.8% and 73.8%, respectively. Combined analysis of three miRNAs yielded an improved AUC value of 0.976±0.010 (95% CI: 0.956‐0.997) with 91.3% sensitivity and 93.8% specificity. To determine whether the serum levels of three miRNAs have clinical values for GD diagnosis, the positive predictive value (PPV), negative predictive value (NPV), and diagnostic efficiency were calculated. As shown in Table 2, the PPVs for three miRNAs were all higher than 76%, NPVs were higher than 65%. At the same time, the diagnosis efficiencies of three miRNAs were all higher than 71%. In addition, combination of three miRNAs showed the further improved PPV (94.81%) and NPV (84.71%) with 92.41% diagnostic efficiency. These results indicated that miR‐210, miR‐155, and miR‐146a have great value on the diagnosis of GD and could be as potential biomarkers for GD.
Figure 2.
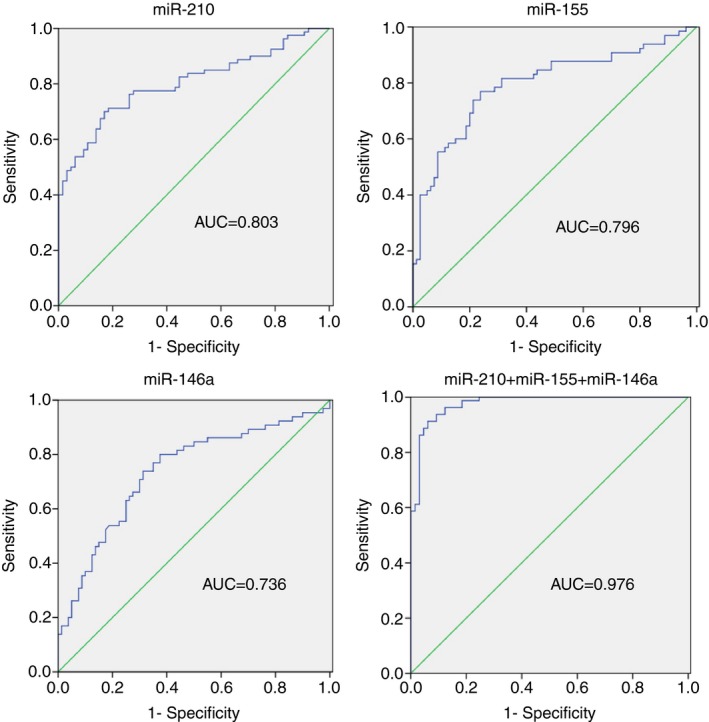
ROC curve analysis of miR‐210 (A), miR‐155 (B), and miR‐146a (C) in the discrimination of GD patients from healthy controls
Table 2.
The diagnostic value between GD and healthy controls
| Variables | miR‐210 (%) | miR‐155 (%) | miR‐146a (%) | Combination (%) |
|---|---|---|---|---|
| Sensitivity | 70.00 | 76.30 | 68.80 | 91.30 |
| Specificity | 83.10 | 76.90 | 73.80 | 93.80 |
| PPV | 83.58 | 80.26 | 76.39 | 94.81 |
| NPV | 69.23 | 72.46 | 65.75 | 89.71 |
| Diagnostic efficiency | 75.86 | 76.55 | 71.03 | 92.41 |
PPV, positive predictive value; NPV, negative predictive value.
3.3. The correlation of serum miRNAs and clinical features of GD
As the serum miRNAs (miR‐210, miR‐155, and miR‐146a) were significantly different in GD patients, we assessed the association between serum miRNAs and clinical features. Serum miRNAs were not associated with potential confounding factors such as smoking and alcohol abuse (P>.05). As shown in Figure 3A, the serum level of miR‐210 was higher in GD with II/III goiter than that of GD patients with Normal/I goiter (P=.014) and the levels of miR‐155 was lower (P=.011). However, no significant difference was found in the serum levels of miR‐146a between GD patients with Normal/I goiter and II/III goiter. Our results also indicated that serum levels of miRNAs were associated with the gender of GD patients. The level of miR‐210 was much higher in female GD patients (P=.014, Figure 3B) and the levels of miR‐155 and miR‐146a were lower (P=.019 and P=.045, respectively). No association was found between the males and females of controls (P>.05).
Figure 3.
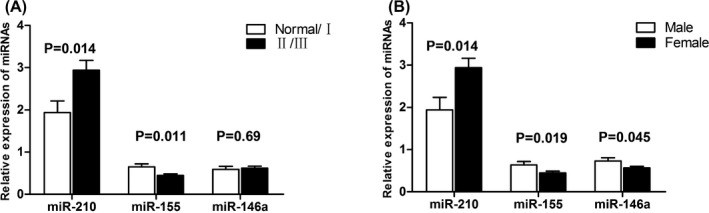
Correlation of three serum miRNAs (miR‐210, miR‐155, and miR‐146a) and the extent of goiter and the gender of GD patients. (A) Differential serum levels of three miRNAs from GD patients with normal/I goiter or II/III goiter. (B) Differential serum levels of three miRNAs from female GD patients and male GD patients. All data were normalized by healthy controls
3.4. The relationship between serum miRNAs and biochemical indexes
In order to determine whether thyroid dysfunction could affect miRNA expression, we performed analysis about the relationship between serum miRNAs and biochemical indexes. Figure 4 shows that the serum level of miR‐210 was positively correlated with FT4 (r=.3065, P=.0057) and TRAb (r=.4145, P=.0002). However, miR‐210 was not associated with FT3 (P=.5801), TSH (P=.9940), TGAb (P=.666), and TPOAb (P=.171, data not shown). In addition, the serum levels of miR‐155 and miR‐146a were not associated with FT3, FT4, TSH, TGAb, TPOAb, and TRAb (data not shown). Moreover, there was no association between the serum levels of miRNAs and the age or onset age of GD patients.
Figure 4.
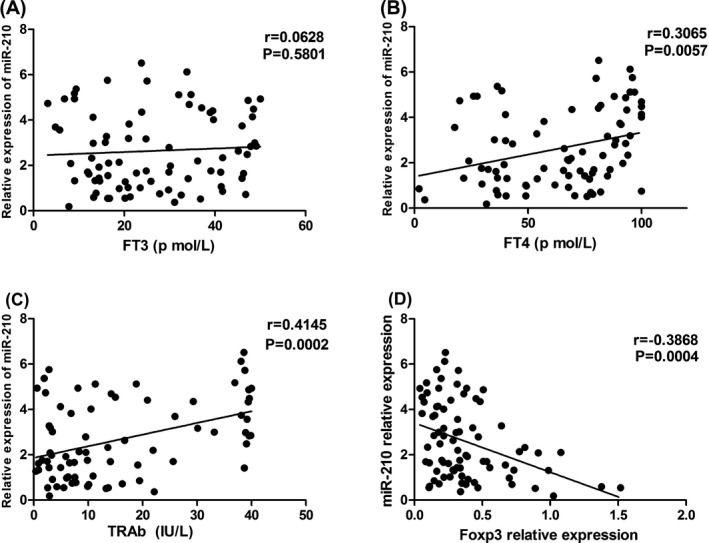
Linear relationships of serum miR‐210 with FT3 (A), FT4 (B), TRAb levels (C), and Foxp3 relative expression (D)
Our previous study showed that Foxp3 mRNA expression in GD was lower than that of healthy controls. To support the inference of the importance of Foxp3 expression and these miRNAs, the linear correlation analysis was performed. Figure 4D shows that miR‐210 was negatively correlated with Foxp3 relative expression in GD. No correlation was found in other miRNAs (P>.05, data not shown).
4. DISCUSSION
In this study, we investigated the association of four serum Treg‐associated miRNAs between GD patients and healthy controls. We found that the serum level of miR‐210 was higher in GD patients, and the levels of miR‐155 and miR‐146a were decreased compared with healthy controls.
The roles of miRNAs in autoimmune disease have attracted more and more attentions of researchers. Recently, several studies confirmed that serum levels of miRNAs showed differential profiles in autoimmune disease, such as systemic lupus erythematosus and multiple sclerosis.21, 22 Consistent with these results, our study showed that different serum miRNAs were associated with GD. Several studies suggested that miR‐210 could be used as a biomarker in congestive heart failure, breast cancer, renal cell cancer, and so on.23, 24, 25 In addition, there was a study reported that increased miR‐210 induced immune dysfunction via by Foxp3 in CD4+T cells of psoriasis vulgaris and miR‐210 targeted Foxp3 to modulate the immunosuppressive functions of Treg cells.10 Hiratsuka et al.26 indicated that GD patients had about three‐fold increase in miR‐210 level compared with healthy controls. On the other hand, we found that the serum level of miR‐210 was higher in GD patients and a positive correlation between the thyroid size of GD and serum miR‐210. In addition, miR‐210 was negatively correlated with Foxp3 relative expression. In one of our previous study, the expression of Foxp3 was decreased in GD patients compared with healthy controls.8 Besides, Mao et al.5 reported that the number of CD4+CD25+ Tregs was decreased too. These results indicated that the increased serum miR‐210, by targeting Foxp3, may be associated with the inhibited Foxp3 expression and Tregs dysfunction of GD.
Several studies have demonstrated that the Foxp3 target miR‐155 contributed to the development of Tregs.14, 27 Besides, Lu et al. have showed that Foxp3‐dependent regulation of miR155 maintained competitive fitness of Tregs subset by targeting SOCS1.13 From above, we supposed that miR‐155 may play an important role in the development of Treg‐mediated GD. In our data, the serum level of miR‐155 was lower in GD patients, especially in female GD patients. In addition, the serum level of miR‐155 was lower in GD patients with II/III goiter than Normal/I goiter. Therefore, the decreased serum miR‐155 may be associated with decreased Foxp3 expression and Tregs dysfunction in GD patients. MiR‐146a was indispensable for suppression mediated by Tregs in vivo. Besides, excessive activation of Stat1 in Tregs was kept in check by miR‐146a to ensure efficient control of spontaneous IFN‐γ‐dependent Th1‐mediated immunopathology and prevented deviation of activated Treg cells into IFN‐γ‐producing Th1‐like cells.15 We found that the serum level of miR‐146a was lower in GD in our study. A previous study has showed that miR‐146a was significantly decreased in thyroid tissue of GD than the control.28 In contrast, Nakasa et al. demonstrated that miR‐146a was highly expressed in rheumatoid arthritis (RA) synovial tissue.29 As we know, GD is characterized by the production of Th2‐cytokines, while RA is characterized by Th1‐cytokines, leading to the difference. We speculated that the decreased serum miR‐146a may be associated with low‐Th1 response and dominant Th2 response in GD.
We also found that the serum levels of miRNAs were different between female GD patients and male GD. The difference may be caused by the location of Foxp3, which is located on Xp11.23 and as a key factor controls the development and function of Tregs. Although we suppose that the underlying autoimmune condition will be responsible for the different expression of serum miRNAs, it remains possible that thyroid function could disturb the expressions of those serum miRNAs. In our study, we only found that serum miR‐210 expression was positively correlated with the TRAb and FT4 level. Serum miR‐155 and miR‐146a in GD, unaffected by thyroid dysfunction itself, may be associated with the autoimmune condition. Although our present study lacks the data regarding disease activity, prognostic and pathogenesis, the ROC analysis showed that these serum miRNAs had a moderate sensitivity and specificity to distinguish GD patients from healthy controls. Notably, combination of miR‐210, miR‐155, and miR‐146a yielded significantly improved PPV (94.81%) and NPV (84.71%) with 92.41% diagnostic efficiency.
In conclusion, serum miR‐210 level in GD patients was significantly higher in comparison to healthy controls. On the contrary, the serum levels of miR‐155 and miR‐146a were lower in GD patients. Besides, serum miR‐210 and miR‐155 in GD patients were associated with the extent of goiter. In the clinical molecular diagnosis of personalized medicine, the serum miR‐210, miR‐155, and miR‐146a may serve as potential markers for GD and play important roles in GD pathogenesis. However, larger sample‐based studies are needed to verify the usefulness of these miRNAs in the GD diagnosis and pathogenesis.
ACKNOWLEDGMENTS
This study was funded by The Science‐Technology Project of Henan Province (grant numbers: 152102410067 and 162102310142). All of the authors gave a great assistance in recruiting the subjects and the revision of manuscript. We also thank the anonymous reviewers for assistance in editing the article.
Lei Z, Zhuang C, Wang X, Ming L. Serum miR‐146a, miR‐155, and miR‐210 as potential markers of Graves’ disease. J Clin Lab Anal. 2018;32:e22266 10.1002/jcla.22266
Contributor Information
Xiaobei Wang, Email: windflower6174@gmail.com.
Liang Ming, Email: mingliang0372@163.com.
REFERENCES
- 1. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self‐tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531‐562. [DOI] [PubMed] [Google Scholar]
- 2. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self‐tolerance maintained by activated T cells expressing IL‐2 receptor alpha‐chains (CD25). Breakdown of a single mechanism of self‐tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151‐1164. [PubMed] [Google Scholar]
- 3. Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity. 2013;38:414‐423. [DOI] [PubMed] [Google Scholar]
- 5. Mao C, Wang S, Xiao Y, et al. Impairment of regulatory capacity of CD4+CD25+ regulatory T cells mediated by dendritic cell polarization and hyperthyroidism in Graves’ disease. J Immunol. 2011;186:4734‐4743. [DOI] [PubMed] [Google Scholar]
- 6. Nada AM, Hammouda M. Immunoregulatory T cells, LFA‐3 and HLA‐DR in autoimmune thyroid diseases. Indian J Endocrinol Metab. 2014;18:574‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang H, Zhao S, Tang X, Li J, Zou P. Changes of regulatory T cells in Graves’ disease. J Huazhong Univ Sci Technolog Med Sci. 2006;26:545‐547. [DOI] [PubMed] [Google Scholar]
- 8. Zheng L, Wang X, Xu L, et al. Foxp3 gene polymorphisms and haplotypes associate with susceptibility of Graves’ disease in Chinese Han population. Int Immunopharmacol. 2015;25:425‐431. [DOI] [PubMed] [Google Scholar]
- 9. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol. 2010;10:111‐122. [DOI] [PubMed] [Google Scholar]
- 10. Zhao M, Wang LT, Liang GP, et al. Up‐regulation of microRNA‐210 induces immune dysfunction via targeting FOXP3 in CD4(+) T cells of psoriasis vulgaris. Clin Immunol. 2014;150:22‐30. [DOI] [PubMed] [Google Scholar]
- 11. O'Neill LA. Outfoxing Foxo1 with miR‐182. Nat Immunol. 2010;11:983‐984. [DOI] [PubMed] [Google Scholar]
- 12. Ouyang W, Liao W, Luo CT, et al. Novel Foxo1‐dependent transcriptional programs control T(reg) cell function. Nature. 2012;491:554‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu LF, Thai TH, Calado DP, et al. Foxp3‐dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR‐155 contributes to the development of regulatory T cells. J Immunol. 2009;182:2578‐2582. [DOI] [PubMed] [Google Scholar]
- 15. Lu LF, Boldin MP, Chaudhry A, et al. Function of miR‐146a in controlling Treg cell‐mediated regulation of Th1 responses. Cell. 2010;142:914‐929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamada H, Itoh M, Hiratsuka I, Hashimoto S. Circulating microRNAs in autoimmune thyroid diseases. Clin Endocrinol. 2014;81:276‐281. [DOI] [PubMed] [Google Scholar]
- 17. Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription‐PCR (qRT‐PCR). Methods. 2010;50:298‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997‐1006. [DOI] [PubMed] [Google Scholar]
- 19. Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem. 2008;54:482‐490. [DOI] [PubMed] [Google Scholar]
- 20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods. 2001;25:402‐408. [DOI] [PubMed] [Google Scholar]
- 21. Siegel SR, Mackenzie J, Chaplin G, Jablonski NG, Griffiths L. Circulating microRNAs involved in multiple sclerosis. Mol Biol Rep. 2012;39:6219‐6225. [DOI] [PubMed] [Google Scholar]
- 22. Wang H, Peng W, Ouyang X, Li W, Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160:198‐206. [DOI] [PubMed] [Google Scholar]
- 23. Endo K, Naito Y, Ji X, et al. MicroRNA 210 as a biomarker for congestive heart failure. Biol Pharm Bull. 2013;36:48‐54. [DOI] [PubMed] [Google Scholar]
- 24. Jung EJ, Santarpia L, Kim J, et al. Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer. 2012;118:2603‐2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhao A, Li G, Peoc'h M, Genin C, Gigante M. Serum miR‐210 as a novel biomarker for molecular diagnosis of clear cell renal cell carcinoma. Exp Mol Pathol. 2013; 94:115‐120. [DOI] [PubMed] [Google Scholar]
- 26. Hiratsuka I, Yamada H, Munetsuna E, Hashimoto S, Itoh M. Circulating microRNAs in Graves’ disease in relation to clinical activity. Thyroid. 2016;26:1431‐1440. [DOI] [PubMed] [Google Scholar]
- 27. Yao R, Ma YL, Liang W, et al. MicroRNA‐155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS ONE. 2012;7:e46082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bernecker C, Lenz L, Ostapczuk MS, et al. MicroRNAs miR‐146a1, miR‐155_2, and miR‐200a1 are regulated in autoimmune thyroid diseases. Thyroid. 2012; 22:1294‐1295. [DOI] [PubMed] [Google Scholar]
- 29. Nakasa T, Miyaki S, Okubo A, et al. Expression of microRNA‐146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008;58:1284‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]


