Abstract
Background
To investigate the role of pregnancy‐associated plasma protein A (PAPP‐A) in the outcome of ischemic cerebrovascular disease.
Methods
We analyzed the levels of PAPP‐A in the transient ischemic attack (TIA) patients, ischemic stroke (IS) patients and normal control, and followed up the outcome of the patients in the following 2 years. Blood samples were drawn at admission, prior to treatment with heparins.
Results
The levels of PAPP‐A in TIA patients, IS patients and normal control were 4.91 (2.11, 6.48) mIU/L, 6.77 (3.31, 10.23) mIU/L and 4.25 (1.76, 5.22) mIU/L, respectively. The follow‐up results of TIA patients and IS patients indicated the PAPP‐A concentration in the poor prognosis group were higher than those in the good prognosis group (5.90 vs 4.46 mIU/L, P<.05, 10.06 vs 5.12 mIU/L, P<.05, respectively). Serum PAPP‐A concentration emerged as a predictor of risk stratification with an OR of 1.41 and 1.25 (P<.05, P<.05).
Conclusions
Higher PAPP‐A concentration has a forecasting value on prognosis in ischemic cerebrovascular disease.
Keywords: biomarker, inflammation, ischemic stroke, pregnancy‐associated plasma protein‐A, transient ischemic attack
Abbreviations
- ACS
acute coronary syndrome
- CI
confidence intervals
- ELISA
enzyme‐linked immunosorbent assay
- IS
ischemic stroke
- LMWH
low molecular weight heparin
- OR
odds ratio
- PAPP‐A
pregnancy‐associated plasma protein A
- SD
standard deviation
- TIA
transient ischemic attack
1. Introduction
Stroke is a leading cause of long‐term adult disability, and is the second leading cause of death in China.1, 2 Ischemic stroke (IS) is the most common type of stroke, accounting for 43%‐79% of all strokes.2 Often, health professionals and the public consider transient ischemic attack (TIA) benign but regard stroke as serious. However, IS and TIA are on a spectrum of serious conditions involving brain ischemia. The short‐term risk of IS after TIA is estimated to be approximately 3%‐10% at 2 days, 5% at 7 days, and 9%‐17% at 90 days.3 And patients with IS or TIA are at increased risk for future cardiovascular events despite current preventive therapies.4 Therefore, risk prediction system and early treatment is critical to reduce mortality and risk of future cardiovascular events in patients who have experienced an IS or TIA.
Pregnancy‐associated plasma protein A (PAPP‐A), a zinc‐binding matrix metalloproteinase, which was studied mainly in hemodialysis, disorders of pregnancy and atherosclerosis.5, 6, 7 It was reported rising PAPP‐A levels are prognostic in patients with coronary artery disease, especially in acute coronary syndrome (ACS) patients with troponin below 10% CV of the 99th percentile for cardiovascular mortality.8 This study was aimed to assess if PAPP‐A might be a marker not only of coronary event but also a useful parameter in ischemic cerebrovascular disease. In our recent study, it has been demonstrated that heparin and low molecular weight heparin treatments may increase serum PAPP‐A,9 which was also proved by Wang G in ACS.10 Therefore, blood samples were drawn at admission, prior to treatment with heparins from the patients with IS or TIA.
2. Subjects and Methods
2.1. Study population
Fifty‐nine TIA patients (23 women, mean±SD age, 59.8±7.4 years) and 152 IS patients (69 women, mean±SD age, 64.2±8.6 years) were recruited from the Department of Neurosurgery and Neurology at the Second Hospital of Shandong University between May 2009 and June 2012. TIA patients were diagnosed with the first‐ever TIA based on both clinical and radiological information, hospitalized no more than 24 hours after TIA occurring. The diagnosis of TIA is difficult at best,11 therefore, all of them were confirmed by two or more physicians. The IS patients were diagnosed with the first‐ever acute ischemic stroke based on both clinical and radiological information. All the patients were followed up after 2 years. Five TIA patients and six IS patients were excluded because of incomplete follow‐up data. The remaining 54 TIA patients and 146 IS patients were deemed eligible for the study. All patients were treated according to routine clinical protocols. Fifty healthy volunteers, neurologically normal and had no medical history of stroke, myocardial infarction, occlusive artery disease, inflammatory disease or systemic metabolic disorder, were also enrolled in this study. The study protocol was approved by the Medical Ethics Committee of the Second Hospital of Shandong University. Written informed consent was obtained from patients or legal representatives.
2.2. Clinical assessment
All subjects enrolled in this study underwent the standardized examination. The basic clinical information including age, sex, and major risk factors (history of arterial hypertension, diabetes, ACS, atrial fibrillation, and dyslipemia) were recorded.
2.3. Blood sampling and measurement
The first admission blood samples before any heparins administration were collected from all patients after hospitalized. And blood samples of healthy controls were also collected. Sera were separated by centrifugation (1760 g) at 4°C for 10 minutes and stored at −80°C until analysis.
Serum PAPP‐A concentrations were measured using an ultra‐sensitive enzyme‐linked immunosorbent assay kit with a polyclonal anti‐PAPP‐A antibody (DRG International, Inc., Mountainside, NJ, USA). The lower limit of detection was 0.005 mIU/L. The intra‐assay CVs were 6.86% and 4.27% at 2.7 and 38.5 mIU/L, respectively, and the inter‐assay CVs were 9.40% and 5.86% at 2.2 and 36.5 mIU/L, respectively. The clinicians had no access to the investigational information of PAPP‐A.
2.4. Follow‐up and clinical end points
All data were collected from hospital records or by patient interviews. And the outcomes were assessed during the follow‐up visit after 2 years. The clinical end point of this study was a composite of TIA or stroke recurrence, myocardial infarction, vascular death or the identification of a high‐risk stroke mechanism requiring specific early intervention (defined as >70% stenosis in a vessel referable to symptoms) during follow‐up.
2.5. Statistical analysis
Data were expressed as mean±standard deviation (SD) for continuous variables or percentages for categorical variables. Variables with more skewed distributions such as PAPP‐A concentrations were given as median and quartiles. The Chi‐square test for association was applied for categorical data at baseline. The Mann‐Whitney U‐test was applied to evaluate the differences in biomarker levels between two groups. The one‐way ANOVA test was used to test for equality of means among three groups. The binary logistic regression was performed to determine whether PAPP‐A and baseline parameters including sex, arterial hypertension, diabetes, ACS and dyslipidemia affected the outcome. All hypothesis tests were two‐tailed. P<.05 was considered statistically significant. SPSS/WIN (version 13.0; SPSS Inc., Chicago, IL, USA) software and Prism 5 for Windows were used to perform all statistical analyses.
3. Results
3.1. Baseline characteristics and serum PAPP‐A levels
The baseline characteristics of enrolled patients and normal control were shown in Table 1. No significant difference was found between TIA or IS group and normal control group on age, gender, smoking (P>.05), but a significant difference was found in the distribution of hypertension, diabetes, triglyceride, total cholesterol, low‐density lipoprotein cholesterol (LDL‐c) and high‐density lipoprotein cholesterol (P<.05). PAPP‐A levels [medians (25th, 75th percentiles)] of TIA patients, IS patients and normal control were 4.91 (2.11, 6.48) mIU/L, 6.77 (3.31, 10.23) mIU/L and 4.25 (1.76, 5.22) mIU/L, respectively. The median levels of PAPP‐A in TIA and IS patients were higher than those in normal controls (P<.05, P<.05).
Table 1.
Baseline characteristics of the TIA, IS patients, and normal control
| Characteristics | TIA patients | IS patients | Normal control | P value |
|---|---|---|---|---|
| Age, mean±SD, years | 59.8±11.4 | 62.2±8.6 | 61.1±10.2 | .2567 |
| Female/Male, n/n | 36/23 | 83/69 | 25/25 | .5027 |
| Smoke, n | 19 | 55 | 20 | .6982 |
| Hypertension, n (%) | 22 | 78 | 11 | .0009 |
| Diabetes, n (%) | 10 | 37 | 5 | .0716 |
| ACS, n (%) | 8 | 23 | 1 | .0462 |
| TG (mmol/L) | 1.93±0.88 | 1.99±0.91 | 1.50±0.66 | .0023 |
| TC (mmol/L) | 5.19±0.76 | 5.22±0.82 | 4.91±0.63 | .0466 |
| LDL‐c (mmol/L) | 3.01±0.85 | 3.09±0.98 | 2.69±0.52 | .0217 |
| HDL‐c (mmol/L) | 1.16±0.17 | 1.17±0.15 | 1.25±0.26 | .0142 |
ACS, acute coronary syndrome; TG, Triglyceride; TC, total cholesterol; LDL‐c, Low‐density lipoprotein cholesterol; HDL‐c, High‐density lipoprotein cholesterol; TIA, transient ischemic attack.
3.2. Outcome and predictive value of PAPP‐A
Five TIA patients and six IS patients were lost to follow‐up. It was found that the composite outcome end point occurred in 17 (31.5%) TIA patients and 51 (34.9%) IS patients. In the TIA group the PAPP‐A concentration of the poor outcome patients was 5.90 (3.20, 10.22) mIU/L, and the PAPP‐A concentration of the good outcome patients was 4.46 (1.75, 5.50) mIU/L. Using Mann‐Whitney U test, there were significant differences between the two groups (U=183.5, P<.05). After adjustment for several additional factors (history of arterial hypertension, diabetes, coronary syndromes, and dyslipemia), PAPP‐A emerged as a predictor of risk stratification with an OR of 1.41 (P<.05). In the IS group, the PAPP‐A concentration of the poor outcome patients was 10.06 (6.73, 13.14) mIU/L, and the PAPP‐A concentration of the good outcome patients was 5.12 (2.75, 7.54) mIU/L. Using Mann‐Whitney U test, there were significant differences between the two groups (U=1083, P<.05). After adjustment for several additional factors (history of arterial hypertension, diabetes, coronary syndromes, NIHSS and dyslipemia), PAPP‐A emerged as a predictor of risk stratification with an OR of 1.25 (P<.05) (Figure 1).
Figure 1.
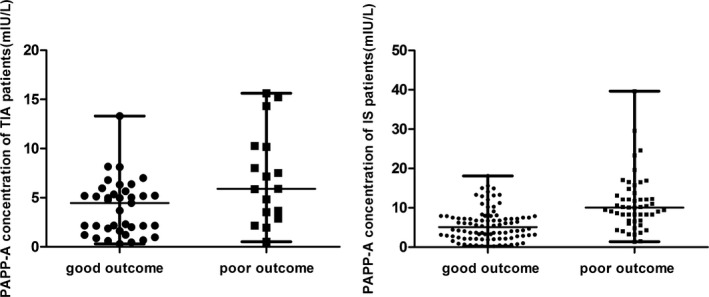
The pregnancy‐associated plasma protein A levels of poor outcome and good outcome in the TIA patients and IS patients
4. Discussion
Among patients who present with stroke, the prevalence of prior TIA has been reported to range from 7% to 40%.12, 13 The timing of a TIA before stroke was consistent, with 17% occurring on the day of the stroke, 9% on the previous day, and another 43% during the 7 days before the stroke.14, 15, 16 So the risk prediction system is very important. So we used binary logistic regression to determine whether PAPP‐A and baseline parameters including sex, arterial hypertension, diabetes, ACS and dyslipemia affected the outcome.
PAPP‐A, as a matrix metalloproteinase, plays an important role in the pathophysiology of atherosclerotic plaque formation and development.17 Studies found that PAPP‐A was abundantly expressed in eroded and ruptured plaques, but was minimally expressed in stable plaques.17 PAPP‐A may be involved in processing the plaques extracellular matrix and fibrous cap weakening, which may result in plaque more susceptible to erosion, rupture, and subsequent thrombosis. PAPP‐A as the enzyme cleaving insulin‐like growth factor (IGF)‐binding protein 4, an inhibitor of IGF, also increases the availability of IGF.18 IGF is present on many cell types found in the plaque. In macrophages, IGF promotes excess LDL‐C uptake, release of proinflammatory cytokines, and chemotaxis. The free fraction of IGF‐I induces migration of vascular smooth‐muscle cells and is important for monocyte chemotaxis and the activation and release of atherosclerotic cytokines. Then the inflammatory environment digests the fibrous cap, leading the plaque vulnerable to rupture. In short, increased PAPP‐A will increase levels of IGF, thereby causing the plaque to disruption.19 In clinical the release of PAPP‐A into blood circulation maybe reflect the increased risk of unstable atherosclerosis and the related complications, such as myocardial infarction, ACS and ischemic stroke.8, 20, 21 And it also plays an “active” role in the pathophysiology of ACS as an effector molecule able to induce a pro‐thrombotic phenotype in endothelial cells.22
Studies on the role of PAPP‐A for diagnosis and prediction of atherosclerosis and its complications are ongoing. However, recent study of plaque specimens demonstrated that elevation of PAPP‐A in patients with ACS s seems to be caused by heparin induced release of PAPP‐A from the arterial wall and not due to excretion from vulnerable plaques. In this study, we collected the first admission blood samples before any heparins administration, used an ultra‐sensitive PAPP‐A assay kit, and we discovered a significantly higher concentration of PAPP‐A in patients with TIA or IS than that in healthy controls. And we also found the prognostic value of PAPP‐A. The elevation of PAPP‐A in patients in our study was not due to the release of PAPP‐A from the arterial wall caused by heparin. The difference between our findings and previous results may be explained, at least in part, by the time of sample collection or the exclusion of patients treated with heparins, normally have more severe conditions. Although it is not clear how PAPP‐A was evaluated in those patients, and whether the elevation is related to unstable atherosclerotic plaque, our study established a potential role of PAPP‐A in the prognosis of unstable atherosclerosis and the related complications after TIA or IS.
The deficiency of our study is the lacking of the information of brain imaging and vascular imaging, the risk prediction system including those will be better at risk prediction. And further studies are needed, may be combined with other serum markers.
5. Conclusions
Our finding indicates that PAPP‐A might be as the predictive biomarker for the diagnosis and/or prognosis of ischemic cerebrovascular disease.
Acknowledgments
This work was supported by Youth Fund of the 2nd Hospital of Shandong University.
References
- 1. Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–3654. [DOI] [PubMed] [Google Scholar]
- 2. Liu M, Wu B, Wang WZ, Lee LM, Zhang SH, Kong LZ. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol. 2007;6:456–464. [DOI] [PubMed] [Google Scholar]
- 3. Wu CM, McLaughlin K, Lorenzetti DL, Hill MD, Manns BJ, Ghali WA. Early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis. Arch Intern Med. 2007;167:2417–2422. [DOI] [PubMed] [Google Scholar]
- 4. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;375:703–704. [DOI] [PubMed] [Google Scholar]
- 5. Beaudeux JL, Burc L, Imbert‐Bismut F, et al. Serum plasma pregnancy‐associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk. Arterioscler Thromb Vasc Biol. 2003;23:e7–e10. [DOI] [PubMed] [Google Scholar]
- 6. Bicik Z, Coskun A, Serteser M, Bulur A, Mese M, Unsal I. Association between serum pregnancy‐associated plasma protein‐A and bicarbonate in hemodialysis patients. J Clin Lab Anal. 2014;28:114–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim SY, Kim HJ, Park SY, Han YJ, Choi JS, Ryu HM. Early prediction of hypertensive disorders of pregnancy using cell‐free fetal DNA, cell‐free total DNA, and biochemical markers. Fetal Diagn Ther. 2016. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8. Zengin E, Sinning C, Zeller T, et al. The utility of pregnancy‐associated plasma protein A for determination of prognosis in a cohort of patients with coronary artery disease. Biomark Med. 2015;9:731–741. [DOI] [PubMed] [Google Scholar]
- 9. Wang S, Wang L, Zhang X, et al. Profile of serum pregnancy‐associated plasma protein A after sustained subcutaneous low molecular weight heparin administration in patients with cerebrovascular diseases. Clin Chem. 2011;57:526–527. [DOI] [PubMed] [Google Scholar]
- 10. Wang G, Zhang A, Han X, Zhang J, Zhang G, Sun L. Effect of routine heparins treatment in acute coronary syndrome on serum pregnancy‐associated plasma protein a concentration. Ann Clin Lab Sci. 2013;43:274–277. [PubMed] [Google Scholar]
- 11. Fred HL. TIA–treacherously inaccurate acronym. Tex Heart Inst J. 2002;29:314–315. [PMC free article] [PubMed] [Google Scholar]
- 12. Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. [DOI] [PubMed] [Google Scholar]
- 13. Dennis M, Bamford J, Sandercock P, Warlow C. Prognosis of transient ischemic attacks in the Oxfordshire Community Stroke Project. Stroke. 1990;21:848–853. [DOI] [PubMed] [Google Scholar]
- 14. Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817–820. [DOI] [PubMed] [Google Scholar]
- 15. Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK‐TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998;351:1379–1387. [PubMed] [Google Scholar]
- 17. Li X, Liu Q, Zhou T, Zhao S, Zhou S. PAPP‐A: a possible pathogenic link to the instability of atherosclerotic plaque. Med Hypotheses. 2008;70:597–599. [DOI] [PubMed] [Google Scholar]
- 18. Bayes‐Genis A, Conover CA, Schwartz RS. The insulin‐like growth factor axis: a review of atherosclerosis and restenosis. Circ Res. 2000;86:125–130. [DOI] [PubMed] [Google Scholar]
- 19. Resch ZT, Chen BK, Bale LK, Oxvig C, Overgaard MT, Conover CA. Pregnancy‐associated plasma protein a gene expression as a target of inflammatory cytokines. Endocrinology. 2004;145:1124–1129. [DOI] [PubMed] [Google Scholar]
- 20. Hajek P, Macek M Sr, Peskova M, et al. High positive predictive value of PAPP‐A for acute coronary syndrome diagnosis in heparin‐naive patients. J Thromb Thrombolysis. 2012;34:99–105. [DOI] [PubMed] [Google Scholar]
- 21. Sangiorgi G, Mauriello A, Bonanno E, et al. Pregnancy‐associated plasma protein‐a is markedly expressed by monocyte‐macrophage cells in vulnerable and ruptured carotid atherosclerotic plaques: a link between inflammation and cerebrovascular events. J Am Coll Cardiol. 2006;47:2201–2211. [DOI] [PubMed] [Google Scholar]
- 22. Cirillo P, Conte S, Pellegrino G, et al. Pregnancy‐associated plasma protein‐A promotes TF procoagulant activity in human endothelial cells by Akt‐NF‐κB axis. J Thromb Thrombolysis. 2016;42:225–232. [DOI] [PubMed] [Google Scholar]


