Abstract
Background
The Moreau score is essential for the diagnosis of B‐cell lymphoproliferative disorders (B‐LPD).
Methods
We assessed the consistency of the Moreau score in a series of 138 patients with at least two samples involved by a B‐LPD (316 samples) other than germinal center‐derived malignancies, hairy cell leukemia, and mantle cell lymphomas. Patients with evidence of two distinct B‐LPDs were also excluded.
Results
We found 53 inconsistencies in 44 of 138 (32%) patients. FMC7 was the most inconsistent (18 cases) and CD5 the least (5 cases). CD200 was inconsistent in 6 of 67 (9%) cases. The most important predictive factor for the finding of antigenic inconsistencies was sampling of a different anatomic site. Other factors, including number of samples, time between samples, or cytogenetic group, were not predictive. For the most part, these inconsistencies did not appear to be clinically relevant.
Conclusion
Inconsistencies in the Moreau score are common, supporting the importance of integrated laboratory diagnosis. However, the practical implications of these antigenic inconsistencies are probably limited.
Keywords: B‐cell lymphoproliferative disorder, CD200, CD5, consistency, Moreau chronic lymphocytic leukemia score
1. INTRODUCTION
Flow cytometry (FC) is essential for the characterization of B‐cell lymphoproliferative disorders (B‐LPD). In particular, the expression of the 5 markers included in the Moreau score (CD5, CD23, CD79b, FMC7, and intensity of surface Immunoglobulin [sIg]) is used for the classification of many B‐LPD.1 Patients fulfilling ≥4 Moreau criteria are overwhelmingly more likely to have chronic lymphocytic leukemia (CLL) as opposed to any other B‐LPD.1 Conversely, fulfillment of ≤3 criteria by a B‐LPD, in the absence of a tissue biopsy and its histological examination, is not diagnostic of any specific entity because the predictive value for CLL is low.1, 2, 3
However, antigenic expression is a continuum rather than a dichotomic process, and B‐LPD may evolve or show differences based on the involved anatomic site. Therefore, we set out to analyze the changes in the Moreau score in a series of patients with B‐LPDs from whom more than one sample was analyzed.
2. MATERIALS AND METHODS
All patients with more than one sample involved by a B‐LPD between January 2010 and December 2016 were considered for this study. Patients with disorders characterized by the expression of highly specific markers (ie, CD10 or CD123) were excluded, as were those diagnosed with mantle cell lymphoma with translocation t(11;14), given that the Moreau score is irrelevant in these cases. The remaining cases were all included, regardless of lymphocyte count. This study was approved by the internal review board (REF. CEI: PI‐17‐178).
The main purpose of the study was (i) to determine the percentage of these patients who showed inconsistencies in the expression of the antigens that make up the Moreau score and (ii) the characterization of these differences.
One hundred and thirty‐eight patients and 316 samples fulfilled the criteria for study entry, including 105 patients with 2 samples, 27 with 3 samples, 5 with 4 samples, and 1 with 5. Seventy‐eight patients had a diagnosis of CLL/small lymphocytic lymphoma/CLL‐phenotype monoclonal B‐cell lymphocytosis. The rest had other diagnoses, largely non‐CLL non‐nodal B‐LPD with exclusive Peripheral blood (PB) and/or bone marrow (BM) involvement, without a histological diagnosis. The samples were as follows: PB (n = 209), BM (n = 42), lymph node (LN) excision (n = 43) and LN fine needle aspiration (FNA, n = 14) and malignant effusion (n = 8).
Samples were processed as previously described.4 Briefly, EDTA‐collected samples were incubated with 5 μL of monoclonal antibodies (MoAb) for 10 minutes, in the dark and at room temperature. After incubation, red cells were lysed with Optilyse (Beckman Coulter, Hialeah, FL, USA), centrifuged, decanted and washed and resuspended with PBS. A FC500 flow cytometer with CXP software (Beckman Coulter, Hialeah, FL, USA) was used for all analyses. The antibodies and clones used are shown in Table 1. The target population was selected with the lymphocyte gate, and normal lymphocyte populations were used as controls. More than 20 000 events were generally analyzed but at least 2000 were required for inclusion.
Table 1.
Panel of monoclonal antibodies (MoAb), fluorochromes, and clones employed during the study period. (a) From January to November 2010 and (b) From December 2010 onwards
| FITC (Fluorescein isothiocyanate) | PE (phycoerythrin) | ECD (PE‐Texas red) | PCy5 (Phycoerythrin Cyanin 5.1) | PCy7 (Phycoerythrin Cyanin 7) |
|---|---|---|---|---|
| (a) | ||||
| Basic panel | ||||
|
CD4 13B8.2 |
CD8 B9.11 |
CD19 HD37 |
CD3 UCHT1 |
|
| B‐cell panel | ||||
|
FMC7 FMC7 CD10 ALB1 |
CD23 9P25 CD38 LS198‐4‐3 |
CD19 J4.119 CD19 J4.119 |
CD5 BL1a CD20 B9E9 |
|
|
CD103 2G5 |
CD11C BU15 |
CD19 J4.119 |
CD25 B1 49.9 |
|
|
CD43 DFT1 |
CD79B CB3‐1 |
CD19 J4.119 |
CD22 SJ10.1H11 |
|
|
κ polyclonal |
λ polyclonal |
‐ |
CD19 HD37 |
|
| (b) | ||||
| Basic panel | ||||
|
CD4 13B8.2 |
CD8 B9.11 |
CD3 UCHT1 |
CD19 HD37 |
CD56 N901 |
| B‐cell panel | ||||
|
FMC7 FMC7 |
CD23 9P25 |
CD19 J4.119 |
CD5 BL1a |
CD38 LS198‐4‐3 |
|
CD103 2G5 |
CD11c/CD200a
BU15/MRC OX‐104 |
CD19 J4.119 |
CD22 SJ10.1H11 |
CD25/CD11cb
B1 49.9/BU15 |
|
CD43 DFT1 |
CD79B CB3‐1 |
CD19 J4.119 |
CD10 ALB1 |
CD20 B9E9 |
|
κ polyclonal |
λ polyclonal |
‐ |
CD19 HD37 |
‐ |
Other MoAb are used in special cases (ie, CD123 in hairy cell leukemia). Manufacturers: all MoAb purchased from Beckman Coulter, except CD200 (Becton Dickinson) and anti kappa/lambda/CD19 (Dako).
CD11c from December 2010 to October 2012 and CD200 from November 2012 onwards.
CD25 from December 2010 to October 2012 and CD11c from November 2012 onwards.
Inconsistency was defined as a different Moreau score or the same score with positivity for different markers. The expression of any antigen was considered positive when expressed in ≥20% of CD19‐positive events. However, a 10% difference was required for two samples to be considered inconsistent in regard to any one antigen (to avoid inconsistencies based on insignificant differences, such as 19% in one sample and 21% in another). sIg was considered negative/dim when its mean fluorescence intensity (MFI, and ranging from 0 to 103 in a logarithmic scale) was <10 and medium/bright when ≥10. Given our own experience and what has been reported,5, 6 sIg in malignant effusions was disregarded, as there is evidence of artifactual loss.
Clonality testing by IGH gene rearrangement was not systematically determined in this retrospective study. However, patients with two B‐LPDs, either by IGH V(D)J gene rearrangement or by discordant kappa/lambda light chain restriction, were excluded. We also excluded cases with two clearly distinct (clonal) populations, that is, with obviously different expression of at least two antigens. We did not exclude patients with a double population with the same kappa or lambda light chain restriction (or with negative sIg) or with a sIg smear pattern. In a subanalysis of this study, patients with these patterns (Figure 1) were compared with patients with a standard sIg image.
Figure 1.
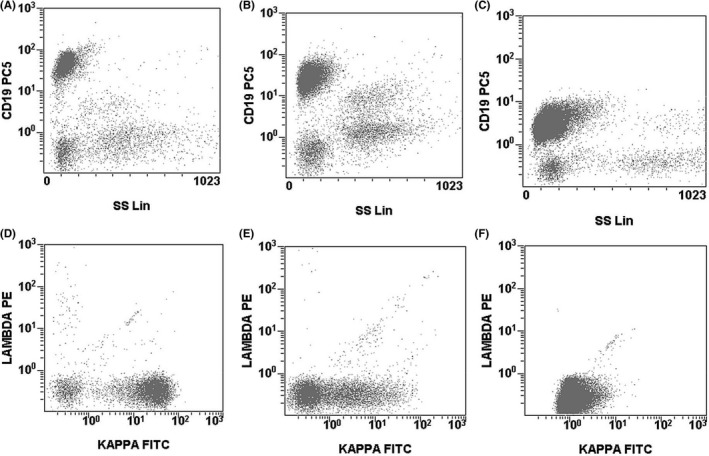
Examples of 1) a double population in the Kappa/lambda histogram (plots A and D), 2) a smear sIg pattern (plots B and E) and 3) a single (standard) sIg image (plots C and F). Patients with any of those patterns were included, unless there was phenotypic evidence of two different lymphoproliferative disorders or IGH rearrangement testing showed evidence of biclonal disease
Frequencies and percentages are given for categorical variables while, for continuous variables, median and interquartile range (IQR) are provided. For comparisons involving categorical variables, the Fisher exact test was used. After adjustment for multiple comparison testing, statistical significance was set at P < .005.
3. RESULTS
As mentioned, 138 patients and 316 samples were included. In 94 (68%) patients, all samples showed an identical Moreau score with positivity for the same markers, while in 44 (32%) patients, there was at least one antigenic inconsistency among their samples. There was one single inconsistency in 35 patients and two inconsistencies in 9. In all, there were 53 antigenic differences affecting FMC7 (n = 18), CD79b (n = 12), CD23 (n = 12), sIg (n = 6), and CD5 (n = 5). We also determined the expression of CD43, which we have previously described as of potential aid in the differential diagnosis of complex cases.4 It was inconsistent in only 3 cases. CD200 was inconsistent in 6 of 67 (9%) patients in whom it was assessed in at least two samples.
We then looked for factors that predicted for inconsistent antigenic expression.
Samples from different sites were more likely to have inconsistencies than those from the same site (considering patients with more than 2 samples as different cases for each pairing, there were 49 of 88 consistent pairings from different sites vs. 70 of 87 consistent pairings from the same site, P = .0006). No specific pairing (ie, LN/FNA vs PB, LN/FNA vs BM or PB vs BM) had a significantly higher percentage of inconsistencies.
Patients with >2 samples tended to have more antigenic differences (78 of 105 consistent for patients with 2 samples vs 16 of 33 for patients with >2 samples, P = .0094). However, patients with >2 samples were predictably more likely to have different anatomic sites tested. After correcting for this confounding factor, there were no differences based on the number of samples (Table 2a).
Table 2.
Antigenic consistency based on the (a) number of samples corrected by the anatomic site of those samples and (b) surface immunoglobulin histogram pattern
| 2 samples | >2 samples | P value | |||
|---|---|---|---|---|---|
| Consistent phenotype | Inconsistent phenotype | Consistent phenotype | Inconsistent phenotype | ||
| (a) | |||||
| Same anatomic site | 48 | 11 | 5 | 3 | .34 |
| Different anatomic site | 30 | 16 | 12 | 13 | .20 |
| Consistent phenotype | Number of cases | P valuea | P value b | ||
|---|---|---|---|---|---|
| (b) | |||||
| Double population | Maintained in all samples | 0 | 4 | .04 | .01 |
| No maintained | 2 | 4 | |||
| Smear pattern | Maintained in all samples | 11 | 18 | ||
| No maintained | 11 | 21 | |||
| Single population | 67 | 91 | |||
Resulting from the comparison between a single population and a sustained double/smear pattern in all samples.
Resulting from the comparison between a single population and a double/smear pattern not sustained in all samples.
The time between samples was not predictive of antigenic differences (median time in consistent cases 176 days [IQR 31.5‐630] vs 114.5 [IQR 22.75‐441.5] days in inconsistent cases, median test P = .51).
As the existence of biclonal (and biphenotypic) cases has been well documented,7 we analyzed whether the presence of two clearly defined populations or a smear pattern in the sIg histogram (Figure 1) correlated with the finding of inconsistencies in the expression of the five markers. The presence of two defined populations or a smear pattern showed a trend toward more antigenic inconsistencies, both when considering patients with similar patterns maintained across all samples and in patients where these patterns were seen in only one of their samples (Table 2b).
In December 2010, we changed from a 4‐ to a 5‐color antibody panel. With this, there were changes in antibody‐fluorochrome combinations (Table 1). Patients with one sample obtained before and one after December 2010 were not more likely to show antigenic inconsistencies than those with samples all obtained either before or after that date (7 of 12 vs 87 of 126, P = .52).
As the finding of trisomy 12 (tri12) in CLL (or CLL‐phenotype monoclonal B‐cell lymphocytosis) has been associated with a lower Moreau score,8, 9 we analyzed antigenic changes based on cytogenetic group among patients with CLL. The only groups large enough to consider separately were tri12 (18 cases, of which 8 were consistent) and 13q deletion (del13q) (31 cases, of which 21 were consistent) (P = .13). Three cases had both tri12 and del13q and are counted in both groups.
Given that CLL is generally diagnosed with a Moreau score ≥4, we checked the potential clinical relevance of the antigenic changes. Seventeen patients (17 of 138, 12%) had a score ≥4 in one sample and ≤3 in another. Of those, in 3 cases, there was a histological sample diagnostic of small lymphocytic lymphoma. In 8 of 9 further patients with available clinical information, CLL was the diagnosis made by the clinician, even though a majority of patients had an indolent disorder with nonspecific cytogenetic findings. In only one patient, the Moreau score changed from 2 to 4 (Figure 2) and the clinical diagnosis was CLL.
Figure 2.
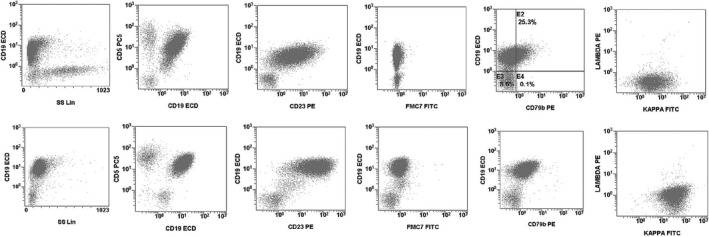
In only one patient among 138, the Moreau score changed from 2 to 4 in two samples (peripheral blood, upper row, with a Moreau score of 4 and lymph node, lower row, with a Moreau score of 2). Cytogenetic analysis showed a homozygous chromosome 13 deletions in both samples, but IGH rearrangement testing was not performed
Nine patients (9 of 138, 6.5%) had a score of ≤2 in one and 3 in another. Of those, one had a histological diagnosis of marginal zone lymphoma and one was diagnosed by their physician with “atypical CLL.” In the remainder (5 of 7 with available clinical data), the working diagnosis was that of an unspecified B‐LPD with PB involvement.
4. DISCUSSION
In this study, we found inconsistencies in the expression of the antigens in the Moreau score in an unexpectedly high proportion of cases. The clinical implication of these inconsistencies, however, appears to be limited.
Although the Moreau score is an invaluable tool in the analysis of B‐LPD, it has limitations, partly resulting from a dichotomous interpretation (CLL vs. not CLL) of a seemingly more continuous process. In their landmark study, Moreau et al1 already showed that samples with a score of 3 only had a 63% chance of being CLL (vs 37% other B‐LPD) using PB cytology as the gold standard. At present, when FC has taken this role, this study supports the idea that a molecular gold standard would be required to establish the final diagnosis of the more complex cases, including most cases with a Moreau score of 3. However, the practical value of the diagnosis is probably limited by the indolent nature of some of these cases, as well as the fact that CLL treatments are likely to be very effective for other B‐LPD of predominantly leukemic presentation.
Immunophenotypic inconsistencies are not rare in hematological malignancies, but they are often related to targeted therapies (such as anti‐CD20 therapy in non‐Hodgkin lymphoma patients leading to CD20‐negative relapses) or to leukemic relapses with a more immature phenotype than at diagnosis. Neither of these can explain the large degree of inconsistencies in our cohort.
While the search for factors predictive of antigenic inconsistencies yielded limited results, some relevant information was obtained. The most important factor associated with antigen inconsistencies was the site from where the sample was obtained. Samples obtained from different sites were more likely to show antigenic differences. This could reflect cellular adaptation to different microenvironments, such as LN or BM, where they are in close contact with other neoplastic and non‐neoplastic cells, unlike in PB or malignant effusions. Indeed, the lack of differences between patients with samples obtained before/after December 2010 and those with samples all obtained either before or after also supports the idea that inconsistencies are due to true antigenic changes rather than technical factors.
We found only a trend toward statistical significance between a double/smear image in sIg and other antigenic inconsistencies. This is probably due to the fact that patients with a double or smear pattern in the sIg histogram are not a homogenous group. On the one hand, technical factors probably play a role, as they do in sIg expression in malignant effusions.5, 6 Indeed, it seems that sIg is more easily lost from malignant cells than normal B cells,10 which would explain complete or partial loss of sIg (resulting in a double or smear pattern). On the other hand, although anecdotic, none of the 4 patients with a persistent double image in sIg had a consistent immunophenotype, suggesting that a few patients could have a biphenotypic or biclonal disease, even though we excluded all cases with clear evidence of that. Unfortunately, this being a retrospective study, IGH V(D)J gene rearrangement could not be performed.
Interestingly, there were no differences between cases with tri12 and del13q, underscoring that the immunophenotypic particularities of tri12 8, 9 remain as constant as the more antigenically standard CLL with del13q.
Previous studies have focused on the value of specific antigens for the diagnosis of CLL and the classification of B‐LPD,11, 12, 13 but we are unaware of any that have assessed the reproducibility of the Moreau score. In this case, given that every patient acts as its own control, all immunophenotypic changes should be due to a change in the neoplastic cells rather than secondary to the interindividual heterogeneity of B‐LPD, underscoring the complexity of classifying B‐LPD based on immunophenotype. Nowadays, markers other than those assessed by Moreau et al1 are used to aid in the diagnosis of CLL. CD200 is foremost among them (although its role in the differential diagnosis of CLL and B‐LPD other than mantle cell lymphoma is limited 14) and a modified CLL score including CD200 was recently published.15 Other markers, such as CD160 or CD148, have also been explored.16, 17 In our case, we have found CD43 of some value 4 and this study shows that its expression is particularly robust.
This study has limitations that must be acknowledged. Importantly, there are no data concerning the treatments patients received. Although there is no evidence that the expression of any of the antigens analyzed would be modified by any treatment in currently use, the study would have benefited from this data in being able to assess this possibility. Secondly, we could not assess whether any specific disorder is more likely to show antigenic changes, but this is an inherent limitation in a study that largely includes B‐LPD without a histological diagnosis (either due to lack of histological exam of potentially involved tissue or, more likely, due to lack of involvement of any organ other than PB). B‐LPD without a specific histological diagnosis are frequent findings in the FC laboratory but have gone largely unexamined, precisely because the lack of a definitive diagnosis makes their rigorous study particularly complex. Finally, genetic data 18, 19 would have helped in establishing the biological diagnosis, which could have then provided a solid gold standard for the difficult cases.
In conclusion, this study found antigenic inconsistencies in the Moreau score in 32% of patients, particularly those with samples from different sites, warning against establishing a specific diagnosis based on a single FC study alone, especially in borderline cases, and underscoring the need for more precise diagnostic tools for this minority of patients even if the practical relevance of these diagnostic changes is questionable.
Sorigue M, Sarrate E, Franch‐Sarto M, Feliu E, Junca J. Consistency of the Moreau CLL score. J Clin Lab Anal. 2018;32:e22376 10.1002/jcla.22376
REFERENCES
- 1. Moreau EJ, Matutes E, A'Hern RP, et al. Improvement of the chronic lymphocytic leukemia scoring system with the monoclonal antibody SN8 (CD79b). Am J Clin Pathol. 1997;108:378‐382. [DOI] [PubMed] [Google Scholar]
- 2. Eichhorst B, Robak T, Montserrat E, et al. Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2015;26(Suppl 5):v78‐v84. [DOI] [PubMed] [Google Scholar]
- 3. Oscier D, Dearden C, Eren E, et al. Guidelines on the diagnosis, investigation and management of chronic lymphocytic leukaemia. Br J Haematol. 2012;159:541‐564. [DOI] [PubMed] [Google Scholar]
- 4. Sorigue M, Juncà J, Sarrate E, Grau J. Expression of CD43 in chronic lymphoproliferative leukemias. Cytometry B Clin Cytom. 2017. (in press), 10.1002/cyto.b.21509. [DOI] [PubMed] [Google Scholar]
- 5. Sorigue M, Triguero A, Feliu E, Junca J. Difference in CD5 and CD10 expression according to anatomic site. Int J Lab Hematol. 2017. (in press), 10.1111/ijlh.12748. [DOI] [PubMed] [Google Scholar]
- 6. Horna P, Olteanu H, Kroft SH, Harrington AM. Flow cytometric analysis of surface light chain expression patterns in B‐cell lymphomas using monoclonal and polyclonal antibodies. Am J Clin Pathol. 2011;136:954‐959. [DOI] [PubMed] [Google Scholar]
- 7. Kern W, Bacher U, Schnittger S, et al. Flow cytometric identification of 76 patients with biclonal disease among 5523 patients with chronic lymphocytic leukaemia (B‐CLL) and its genetic characterization. Br J Haematol. 2014;164:565‐569. [DOI] [PubMed] [Google Scholar]
- 8. Criel A, Verhoef G, Vlietinck R, et al. Further characterization of morphologically defined typical and atypical CLL: a clinical, immunophenotypic, cytogenetic and prognostic study on 390 cases. Br J Haematol. 1997;97:383‐391. [DOI] [PubMed] [Google Scholar]
- 9. Quijano S, López A, Rasillo A, et al. Impact of trisomy 12, del(13q), del(17p), and del(11q) on the immunophenotype, DNA ploidy status, and proliferative rate of leukemic B‐cells in chronic lymphocytic leukemia. Cytometry B Clin Cytom. 2008;74:139‐149. [DOI] [PubMed] [Google Scholar]
- 10. Kaleem Z, Zehnbauer BA, White G, Zutter MM. Lack of expression of surface immunoglobulin light chains in B‐cell non‐Hodgkin lymphomas. Am J Clin Pathol. 2000;113:399‐405. [DOI] [PubMed] [Google Scholar]
- 11. McCarron KF, Hammel JP, Hsi ED. Usefulness of CD79b expression in the diagnosis of B‐cell chronic lymphoproliferative disorders. Am J Clin Pathol. 2000;113:805‐813. [DOI] [PubMed] [Google Scholar]
- 12. Monaghan SA, Peterson LC, James C, et al. Pan B‐cell markers are not redundant in analysis of chronic lymphocytic leukemia (CLL). Cytometry B Clin Cytom. 2003;56:30‐42. [DOI] [PubMed] [Google Scholar]
- 13. Sandes AF, de Lourdes Chauffaille M, Oliveira CR, et al. CD200 has an important role in the differential diagnosis of mature B‐cell neoplasms by multiparameter flow cytometry. Cytometry B Clin Cytom. 2014;86:98‐105. [DOI] [PubMed] [Google Scholar]
- 14. Challagundla P, Medeiros LJ, Kanagal‐Shamanna R, Miranda RN, Jorgensen JL. Differential expression of CD200 in B‐cell neoplasms by flow cytometry can assist in diagnosis, subclassification, and bone marrow staging. Am J Clin Pathol. 2014;142:837‐844. [DOI] [PubMed] [Google Scholar]
- 15. Köhnke T, Wittmann VK, Bücklein VL, et al. Diagnosis of CLL revisited: increased specificity by a modified five‐marker scoring system including CD200. Br J Haematol. 2017;179:480‐487. [DOI] [PubMed] [Google Scholar]
- 16. Fan L, Miao Y, Wu YJ, et al. Expression patterns of CD200 and CD148 in leukemic B‐cell chronic lymphoproliferative disorders and their potential value in differential diagnosis. Leuk Lymphoma. 2015;56:3329‐3335. [DOI] [PubMed] [Google Scholar]
- 17. Farren TW, Giustiniani J, Liu FT, et al. Differential and tumor‐specific expression of CD160 in B‐cell malignancies. Blood. 2011;118:2174‐2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Puente XS, Pinyol M, Quesada V, et al. Whole‐genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475:101‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Navarro A, Clot G, Martínez‐Trillos A, et al. Improved classification of leukemic B‐cell lymphoproliferative disorders using a transcriptional and genetic classifier. Haematologica. 2017;102:e360‐e363. [DOI] [PMC free article] [PubMed] [Google Scholar]


