Abstract
Background
Novel multiplex assays allow the simultaneous identification of a large number of plasma proteins. While these new technologies have been shown to be highly sensitive and accurate for the identification of plasma proteins, the use of this technology to quantify those proteins has not been properly investigated. In this pilot study, we tested the accuracy of the proximity extension assay (PEA) for the quantification of the cardiac biomarker brain natriuretic peptide (BNP) compared to a standard clinically approved method.
Methods
Concentrations of BNP were assessed in 120 plasma samples from 30 patients with PEA and compared to chemiluminescent microparticle immunoassay (CMIA). Venous blood samples were collected from in tubes containing ethylenediaminetetraacetic acid, centrifuged within 6 hours at 3,500 rpm for 15 minutes at 4°C, frozen and stored at −80°C until analyzed. Correlation between the CMIA and PEA techniques was tested using the Spearman’s rank correlation coefficient (rho) and the agreement was described with a Bland‐Altman plot.
Results
Brain natriuretic peptide values obtained by CMIA and PEA were highly correlated (Spearman’s rho = 0.865, P < .0001). In two patients, PEA consistently overestimated resp. underestimated BNP values compared to CMIA. After removal of those two patients, a very high correlation between the two techniques was shown (rho = 0.966, P < .0001). A high agreement between the two techniques over the whole range of tested concentrations was shown.
Conclusion
This pilot study showed for the first time an excellent correlation between a clinically approved method and the PEA‐based approach for quantification of circulating plasma BNP.
Keywords: BNP, multiplex assay, natriuretic peptide, proteomics, proximity extension assay
1. INTRODUCTION
For decades, identification and quantification of plasma proteins of potential interest as biomarkers were based on single‐protein assays. Such approach also required large quantities of plasma when testing multiple proteins. Novel multiplex assays allow the simultaneous identification of a large number of plasma proteins opening the door to a new dimension in human biomarker research and clinical application.1
The proximity extension assay (PEA), based on proximity‐dependent DNA polymerization, is one of these technologies and, according to the manufacturer’s instructions, allows the simultaneous measurement of >90 proteins using only one microliter of plasma.2 The implementation of this technology might be particularly compelling for clinical or research purposes in populations with relevant comorbidities (eg, heart failure, end‐stage renal disease), to test the concomitant activation of multiple pathways. While these new technologies have been shown to be highly sensitive and accurate for the identification of plasma proteins, the use of this technology to quantify those proteins has not been properly investigated.
In this pilot study, we tested the accuracy of PEA for the quantification of the brain natriuretic peptide (BNP) in a cohort of chronic hemodialysis patients compared to a standard clinically approved method.
2. MATERIALS AND METHODS
The study was performed from October 1st to December 31st, 2016 at the University Hospital of Zurich, Switzerland according to the standards of the Declaration of Helsinki and approved by the local ethics committee of Zurich. All patients provided written informed consent (ClinicalTrials.gov NCT02962635). The study population consisted of 30 stable chronic hemodialysis patients undergoing blood sample collection during four visits (2 visits before and 2 after 2 independent dialysis sessions).
Venous blood samples were collected in tubes containing ethylenediaminetetraacetic acid (EDTA), centrifuged within 6 hours at 3,500 rpm for 15 minutes at 4°C, frozen and stored at −80°C until analyzed at Lariboisière University Hospital, Paris, France, using two methods:
Chemiluminescent microparticle immunoassay (CMIA) using the Architect i2000 platform (Abbott Diagnostics, Abbott Park, IL, USA)
Proximity extension assay (PEA) using the Proseek Multiplex CVD II panel (Olink Proteomics AB, Uppsala, Sweden).2 This panel includes 92 cardiovascular biomarkers. The final assay readout was expressed in normalized protein expression (NPX) values, which is an arbitrary unit on a log2 scale in which a high value corresponds to high protein expression.
Correlation between the CMIA and PEA techniques was tested using the Spearman’s rank correlation coefficient (rho), and the agreement between the two techniques was described with a Bland‐Altman plot. Concentrations measured with the CMIA are depicted on a log2 scale, for consistency with the PEA values.
The null hypothesis was rejected with an adjusted two‐sided P‐value < .05. All analyses were performed with the use of IBM SPSS Statistics, Version 25.0. (IBM Corp, Armonk NY, USA).
3. RESULTS
Baseline characteristics of the study population were published elsewhere.3 Briefly, the study population consisted of chronic stable hemodialysis patients, predominantly middle‐aged men with a high prevalence of cardiovascular risk factors and coronary and peripheral artery disease. Median circulating BNP was 169 ng/L (interquartile range 71‐409 ng/L, minimum 10 ng/L, maximum 1914 ng/L), as assessed by CMIA. Median BNP assessed by PEA was 3.92 NPX (interquartile range 2.08‐6.16 NPX, minimum 0.76 NPX, maximum 9.02 NPX).
As shown in the Figure 1, upper panel (n = 120), BNP values obtained by CMIA and PEA were highly correlated (rho = 0.865, P < .0001). Notably, for two patients (Patient 1, Figure 1, white dots, and Patient 19, Figure 1, black dots), PEA consistently overestimated resp. underestimated BNP values compared to CMIA. After removal of all samples of Patient 1 and Patient 19, a very high correlation between the two techniques was shown (rho = 0.966, P < .0001, n = 112).
Figure 1.
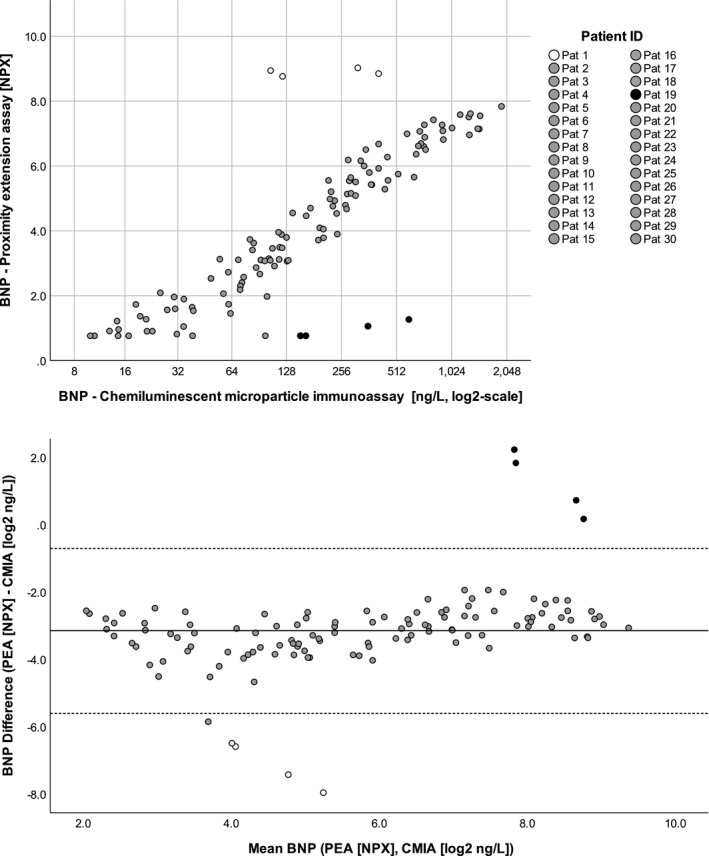
Correlation of BNP values measured with the two tested methods. Upper panel: correlation of BNP concentrations measured with chemiluminescent microparticle immunoassay (BNPCMIA) and proximity extension assay (BNPPEA) assay. BNPPEA values are expressed in NPX (normalized protein expression), an arbitrary unit on log2 scale, BNPCMIA values in ng/L on a log2 scale. After removal of the values of patient 1 and patient 19 (white and black dots), the conversion equation was determined. Using this formula, the calculated BNPCMIA has a median difference from the measured BNPCMIA of 32 [12;77] ng/L. Lower panel: Bland‐Altman plot showing the agreement between the two methods. BNPPEA values are expressed in NPX, BNPCMIA have been log2‐transformed, and expressed in log2 ng/L. The continuous line shows the mean difference between the two assays (BNPPEA‐BNPCMIA) the dotted lines the 95% confidence interval
Accordingly, the Bland‐Altman plot (Figure 1, lower panel) illustrate the high agreement between the two techniques over the whole range of tested concentrations, except for the two aforementioned patients.
4. DISCUSSION
This pilot study showed for the first time an excellent correlation between a clinically approved method and the PEA‐based approach for quantification of circulating plasma BNP. This observation is important as it shows that the gold‐standard cardiac biomarker BNP is accurately quantified by this new methodology. From an analytical standpoint, this study also showed that PEA is linear over at least a range of BNP between the limit of detection and 2000 pg/mL. This linearity contrasts with what has been observed with some point‐of‐care assays for BNP, which showed a progressive loss of correlation with CMIA at around 500 pg/mL.4 Furthermore, the fact that eight samples belonging to two patients did not fit with the correlation suggests pre‐analytical anomalies in these samples that either interfere with CMIA or PEA analysis. To check for plausibility of measured BNPs, we retrospectively reviewed patients’ charts of those two patients. Patient 1, for whom we measured normal/slightly elevated BNPs by CMIA but very high BNPs by PEA, had no dyspnea, no peripheral edema, and preserved cardiac systolic function. Patient 19, for whom we measured elevated BNPs by CMIA but very low BNPs by PEA, had dyspnea, peripheral edema, had heart failure with preserved ejection fraction, and relevant mitral and tricuspid regurgitation. Based on these observations, we believe that BNP values measured by CMIA more accurately reflected cardiac function in those two patients than PEA values. However, as we did not test PEA correlation with other conventional assays, this needs to be tested in other studies.
Therefore, we propose the comparison of BNP by CMIA and PEA as a quality‐control test of the samples before analysis; samples that do not meet the correlation criteria are likely to be inaccurate for the other biomarkers tested and therefore should be overall disregarded for further analysis. In summary, this pilot study showed that multiplex assays based on PEA technology allow linear and accurate quantification of plasma BNP over a large range of concentrations, while only requiring one microliter of plasma. Further larger scale studies are warranted to confirm this finding and to evaluate the correlation between PEA and standard methods for the other biomarkers tested alongside BNP by PEA.
Arrigo M, Vodovar N, Von Moos S, et al. High accuracy of proximity extension assay technology for the quantification of plasma brain natriuretic peptide. J Clin Lab Anal. 2018;32:e22574 10.1002/jcla.22574
Funding information
This study was supported by the Swiss Kidney Foundation, the Alfred and Erika Bär‐Spycher Foundation, the European Commission’s Seventh Framework program under grant agreement N8 305507 (HOMAGE), and departmental funds.
Arrigo and Vodovar equally contributed to this project.
REFERENCES
- 1. Solier C, Langen H. Antibody‐based proteomics and biomarker research ‐ current status and limitations. Proteomics. 2014;14:774‐783. [DOI] [PubMed] [Google Scholar]
- 2. Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96‐Plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. Hoheisel JD, ed. PLoS ONE. 2014;9:e95192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arrigo M, Moos Von S, Gerritsen K, et al. Soluble CD146 and BNP dissect overhydration in functional components of prognostic relevance in hemodialysis patients. Nephrol Dial Transplant. 2018; 10.1093/ndt/gfy113. [DOI] [PubMed] [Google Scholar]
- 4. Monfort A, Da Silva K, Vodovar N, Gayat E, Cohen‐Solal A, Manivet P. Clinical evaluation of the Heart Check system, a new quantitative measurement of fresh capillary BNP. Biomark Med. 2015;9:1323‐1330. [DOI] [PubMed] [Google Scholar]


