Abstract
Introduction
Cancer antigen 125 (CA125) and human epididymis protein 4 (HE4) are biomarkers for ovarian cancer. Their specificity and sensitivity are often limited during pregnancy as a result of great fluctuations. The risk of ovarian malignancy algorithm (ROMA) score, which combines CA125, HE4, and menopausal status, may improve diagnostic performance. There are no reports regarding the ROMA index in pregnant women. Therefore, the aim of our study was to establish appropriate reference intervals (RIs) for the ROMA index in pregnant Chinese women and compare them with those of CA125 and HE4 during pregnancy.
Methods
Serum concentrations of CA125 and HE4 were simultaneously measured in healthy pregnant women via electrochemiluminescence immunoassay (ECLIA). The ROMA index was calculated using premenopausal algorithms.
Results
The RIs for the ROMA index calculated by premenopausal algorithms were substantially closer to the normal range in the first 2 trimesters. For pregnant women, the great misclassifications identified in CA125 may be reversed by the use of ROMA index.
Conclusions
We established the RIs for HE4 and CA125, as well as the ROMA index, in pregnant women at different gestational periods. The ROMA index is suggested to be a more promising tumor marker for pregnant women diagnosed with malignance.
Keywords: carbohydrate antigen 125, human epididymis protein 4, pregnancy, reference interval, tumor marker
1. INTRODUCTION
Tumor markers are useful parameters to help diagnose cancer and to monitor treatment.1 However, their clinical applications are often limited in pregnant women as a result of pregnancy‐induced physiological changes.2
Carbohydrate antigen 125 (CA125) is the most widely used tumor marker for ovarian cancer. In pregnant women, the specificity of CA125 is limited because of the marked increase, particularly during the first trimester of pregnancy.3 In recent years, human epididymis protein 4 (HE4) has been proposed as a novel biomarker for ovarian cancer, with higher specificity and sensitivity.4, 5, 6 It is reported that HE4 and CA125 are complimentary. The risk of ovarian malignancy algorithm (ROMA), which is a qualitative serum test that combines the assessment of HE4 and CA125 levels with menopausal status to generate numerical score, has been shown to have the best diagnostic performance in the assessment of epithelial OC risk.7, 8
Numerous studies have shown that CA125 and HE4 values in pregnant women varied according to different pregnancy stages and ethnic factor.9, 10, 11 Therefore, the currently used reference intervals (RIs) for non‐pregnant women do not appear to be available for pregnant women. For the Chinese population, it is necessary to establish accurate gestational stage‐dependent RIs. However, the establishment of RIs for CA125 during pregnancy has been limited because of wider fluctuations.12, 13, 14, 15 Previous studies have also shown that age, fertility status, menopause, and ethnicity may affect HE4 serum levels.11, 16, 17, 18 In this state, the ROMA index may be a more ideal tumor marker for pregnant women.
To date, there are no studies on the ROMA index for pregnant women, partly because it is difficult to define menopause in the condition of pregnancy. In this study, we initially established RIs for the ROMA index during pregnancy, which were calculated using the premenopausal algorithms according to the definition reported by Moore et al19 The aim of this study was to establish the RIs for HE4, CA125, and the ROMA index during pregnancy. Furthermore, we determined misclassification of having abnormal values of these tumor markers in this study and compared these findings to evaluate the clinical application of the RIs we established for pregnant women.
2. MATERIALS AND METHODS
2.1. Study population and subject recruitment
This cross‐sectional study was approved by the Sir Run Run Shaw Hospital, College of Medicine, Zhejiang University, and written informed consent was obtained from all participants following an explanation of the protocol. According to the CLSI C28‐A3 document, we selected apparently healthy pregnant individuals as the reference population for the healthy pregnancy‐related reference intervals. The exclusion criteria were as follows: (i) women with benign gynecologic disorders such as endometriosis, pelvic inflammatory disease, cysts, and benign neoplasms of the ovaries and uterus. (ii) women with a history of diabetes, cardiovascular disease, preeclampsia, or gestational hypertension, as well as women receiving drugs to treat diabetes and anemia, or anticoagulants. (iii) women with liver diseases and kidney diseases. Using these exclusion criteria, 1006 healthy female participants were enrolled from June 2013 to March 2014. The participants were all pregnant and had ages that ranged from 20 to 42 years. Among the 1006 pregnant women, 306 were in their first trimester (≤12 weeks), 350 were in their second trimester (13‐28 weeks), and another 350 were in their third trimester (≥29 weeks). The gestational age was estimated based on the measurement of the crown‐rump length via ultrasound.
2.2. Laboratory methods
Five milliliters of venous fasting blood were obtained from 1006 pregnant women for the measurement of serum tumor markers. The blood samples were collected in serum separation tubes (Becton Dickinson, Franklin Lakes, NJ, USA) and then centrifuged immediately after collection at 3000 rpm for 5 minutes. The serum levels of the tumor markers CA125 and HE4 were assayed using the ROCHE Cobas E601 system with the ECLIA method using Elecsys CA125 II kits (Roche Laboratories, Nutley, NJ, USA) and Elecsys HE4 kits (Roche Laboratories), respectively. The cutoff values for CA125 and HE4 were 35 U/mL and 140 pmol/L, respectively, according to the assay kits.
2.3. ROMA index calculation
The ROMA index was calculated according to the levels of CA125 and HE4 to classify patients as being at a low or high risk for ovarian cancer. A predictive index (PI) was calculated using the following algorithms:
Premenopausal PI: −12.0 + 2.38 × LN (HE4) + 0.0626 × LN (CA125);
Postmenopausal PI: −8.09 + 1.04 × LN (HE4) + 0.732 × LN (CA125).
The ROMA value (predictive value) was subsequently calculated using the following equation:
LN indicates the natural logarithm; e indicates the base of the natural logarithm.
Premenopausal and postmenopausal women with a ROMA value ≥ 11.4% and ≥29.9%, respectively, had a higher risk of ovarian cancer.
According to Moore et al women were considered to be premenopausal if they had a period within 1 year of the study blood draw.19 Therefore, the ROMA index in this study is calculated using the premenopausal algorithm.
2.4. Statistical analysis
All statistical analyses were performed using SPSS statistical software (version 19.0; IBM‐SPSS, IBM Inc., Armonk, NY, USA). For all measured parameters, the results are reported as median and range values. One sample from the Kolmogorov‐Smirnov test was used to define the distributions of CA125, HE4, and the ROMA index among the study individuals. The RIs were defined by nonparametric 95% confidence intervals according to the recommendations of CLSI C28‐A3. For CA125, HE4, and the ROMA index, only the upper limit is of medical importance. The reference limit was regarded as the 95th percentile of the distribution of the test results for the reference population. A Kruskal‐Wallis H test was performed to assess the differences among trimesters. The level of statistical significance was set at P < .05.
3. RESULTS
3.1. CA125, HE4, and the ROMA index values during different trimesters of pregnancy
In this study, we measured serum HE4 and CA125 concentrations in 1006 pregnant women. The results of Kolmogorov‐Smirnov test showed that HE4, CA125, and ROMA index values in our study were not in normal distribution. Therefore, we calculated the RIs with nonparametric method.
The variation trends of the CA125, HE4, and ROMA index values during pregnancy are summarized in Figure 1. In our study, elevated CA125 levels were identified in the first (median 59.5 U/mL) and third (median 31.3 U/mL) trimesters. The HE4 values increased from the first (median 36.9 pmol/L) to second (median 39.8 pmol/L) trimester (P < .0001) and from the second to third (median 54.6 pmol/L) trimester (P < .0001). The ROMA index, calculated by the premenopausal algorithm, increased from the first (median 4.1%) to second (median 4.5%) trimester (P < .05) and from the second to third (median 9.8%) trimester (P < .0001). Overviews of the CA125 and HE4 values during pregnancy are shown in Tables 1 and 2. We summarized the CA125 values in 5studies from different countries. Elevated CA125 levels in the first trimester were identified in all studies.15, 20, 21, 22, 23 One study identified elevated CA125 levels in the second trimester,23 and 2 studies identified elevated levels in the third trimester.15, 23 These levels were lower than those reported herein during each trimester. For HE4, Moore et al reported that the median concentrations of HE4 were not significantly different among trimesters; however, these concentrations were significantly lower than their premenopausal counterparts.11 The concentrations reported in our study were higher than those of Moore et al in the second and third trimesters.11
Figure 1.
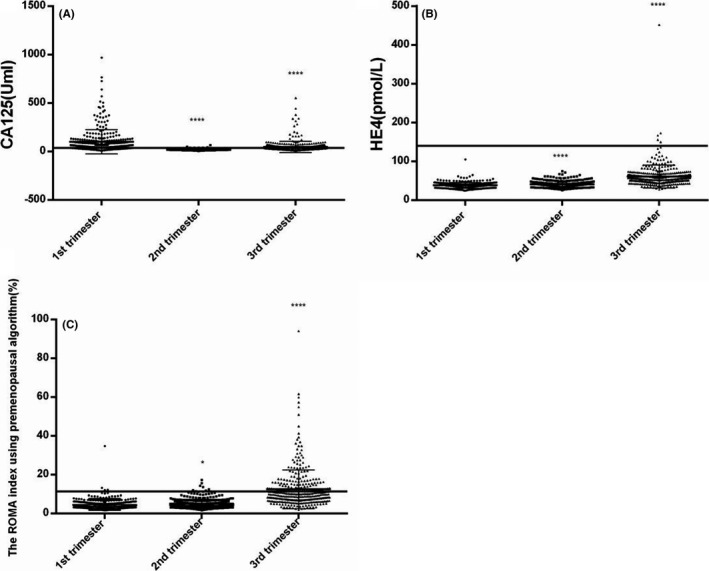
Variations of CA125 (A), HE4 (B), and ROMA (C) in healthy pregnant women during different gestational periods (*P < .05, ***P < .001, ****P < .0001)
Table 1.
Overview of selected studies on CA125 levels during pregnancy
| Region | Current study | Japan | United States | UK | Netherlands | Turkey |
|---|---|---|---|---|---|---|
| Author/method | ECLIA | Kobayashi ORIS Industry | Spitzer M., RIA | Jacobs IJ. Abbott Laboratories | Bon GG, Enzymun test | Serif Ercan, ECLIA |
| N | 1st 306; 2nd 350 | n = 122 | n = 20 | 1st 11; 2nd 7 | 1st 127; | n = 30 |
| 3rd 350 | 3rd 8 | 2nd 192; 3rd 47 | ||||
| Cutoff value | <35 | <35 | <35 | <35 | <35 | |
| 1st trimester | 59.5 (median) | 71.7 ± 71.1 | 33.1 (median) | 53.6 (median) | 23 (median) | 19.0 (median) |
| 7.7‐967.4 (range) | (mean ± SD) | 3.7‐251.2 (range) | 15.6‐268.3 (range) | 4‐108 (range) | 4.9‐61 (range) | |
| 2nd trimester | 16.6 | 19.1 ± 7.0 | <35 | 18.5 | 14 | 15.6 |
| 5.3‐64.1 | (range) | 12.0‐25.1 | 1‐73 | 4.7‐32.1 | ||
| 3rd trimester | 31.3 | 28.1 ± 14.1 | <35 | 19.2 | 21 | 19.6 |
| 7.5‐551.8 | (range) | 16.8‐43.8 | 8‐144 | 9.8‐41.2 | ||
| References | 9, 20 | 9, 21 | 9, 22 | 1, 9, 23 | 9, 15 |
Table 2.
Overview of selected studies on HE4 levels during pregnancy
| Region | Current study | United States |
|---|---|---|
| Author/method | ECLIA | Richard G., EIA |
| N | n = 1006 | n = 67 |
| Cutoff value (pmol/L) | <140 | <140 |
| 95% RI for premenopausal women | <65.8 | <89.1 |
| 1st trimester | ||
| Median (range) | 36.9 (24.2‐104.8) | 31.2 (23.1‐66.4) |
| 95% RI | 50.3 | 49.6 |
| 2nd trimester | ||
| Median (range) | 39.8 (25.4‐74.2) | 30.0 (18.6‐44.8) |
| 95% RI | 56.4 | 35.1 |
| 3rd trimester | ||
| Median (range) | 54.6 (27.6‐451.7) | 35.0 (23.0‐51.0) |
| 95% RI | 101.9 | 50.2 |
| References | 11 | |
3.2. RIs for pregnant women
In this study, the most obvious variations were identified in the RIs for CA125 during pregnancy (Table 3). The cutoff values for CA125 in the first (309.5 U/mL) and third (113.3 U/mL) trimesters were substantially higher than the cutoff value for non‐pregnant women (35 U/mL). In contrast, the cutoff values for HE4 in each of the 3 trimesters were lower than the cutoff value of 140 pmol/L. The cutoff values for the ROMA index in the first (8.7%) and second (10.0%) trimesters were close to the cutoff value of 11.4%.
Table 3.
Percentile distributions of CA125, HE4, and ROMA values in different gestational periods
| Items | Gestation weeks | n | 5th | 50th | 95th |
|---|---|---|---|---|---|
| CA125 (U/mL) | 1st trimester | 306 | 14.9 | 59.5 | 309.5 |
| 2nd trimester | 350 | 8.9 | 16.6 | 32.5 | |
| 3rd trimester | 350 | 12.4 | 31.4 | 113.3 | |
| 1 + 2 + 3 trimester | 1006 | 10.5 | 26.5 | 183.1 | |
| HE4 (pmol/L) | 1st trimester | 306 | 28.0 | 36.9 | 50.3 |
| 2nd trimester | 350 | 30.0 | 39.8 | 56.4 | |
| 3rd trimester | 350 | 38.1 | 54.7 | 101.9 | |
| 1 + 2 + 3 trimester | 1006 | 30.4 | 42.3 | 75.5 | |
| 1st trimester | 306 | 2.1 | 4.1 | 8.7 | |
| ROMA (%) | 2nd trimester | 350 | 2.3 | 4.5 | 10.0 |
| 3rd trimester | 350 | 4.1 | 9.8 | 31.0 | |
| 1 + 2 + 3 trimester | 1006 | 2.5 | 5.4 | 18.0 |
3.3. Comparisons of RIs for the ROMA index, CA125, and HE4
For the ROMA index, only 2% of participants were misclassified as out of the normal range in the first 2 trimesters. For HE4, the RI for Chinese premenopausal women (<65.8 pmol/L) was more suitable for evaluation of HE4 levels in pregnant women because of the relationship between increasing serum levels of HE4 and increasing age.17 Approximately 0.3%, 1.1%, and 24.9% of pregnant women were misclassified as being out of normal range in the first, second, and third trimesters, respectively.
Using the cutoff value for non‐pregnant women (35 U/mL), approximately 216 (70.6%), 14 (4%), and 150 (42.9%) pregnant women had CA125 values that were misclassified as out of the normal range in the first, second, and third trimesters, respectively. We analyzed the participants who were misclassified as having abnormal CA125 values. Among these participants, only 0.9%, 0%, and 40% of the pregnant women had ROMA index values that were above the cutoff value of 11.4%, and 0.5%, 0%, and 23.3% had HE4 values that were above the cutoff value of 65.8 pmol/L in the first, second, and third trimesters, respectively. In the participants who had CA125 values that were above the cutoff values for pregnant women established in this study, only 0%, 0%, and 47% of pregnant women had HE4 and ROMA index beyond the normal range (Table 4).
Table 4.
Proportions of pregnant women whose concentrations of HE4, CA125, and the ROMA index that were out of the normal range
| Parameter | n | HE4for whole>65.8 pmol/L, n (%) | ROMAfor whole>11.4%, n (%) | CA125for whole>35 U/mL, n (%) | HE4>65.8 pmol/L, n (%) | ROMA>11.4%, n (%) | CA125 | HE4 | ROMA>11.4%, n (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| >95% RI in this study | ||||||||||
| n | >65.8 pmol/L, n (%) | |||||||||
| 1st trimester | 306 | 1 (0.3) | 4 (1.3) | 216 (70.6) | 1/216 (0.5) | 2/216 (0.9) | >309.5 | 15 | 0/15 (0) | 0/15 (0) |
| 2nd trimester | 350 | 4 (1.1) | 8 (2.3) | 14 (4.0) | 0/14 (0) | 0/14 (0) | >32.5 | 17 | 0/17 (0) | 0/17 (0) |
| 3rd trimester | 350 | 87 (24.9) | 133 (38) | 150 (42.9) | 35/150 (23.3) | 60/150 (40) | >113.3 | 17 | 8/17 (47) | 8/17 (47) |
4. DISCUSSION
In this study, clear variations in HE4, CA125, and the ROMA index were identified during pregnancy. These findings suggest that gestational stage is a critical factor and plays an important role in the variations of these tumor markers. The establishment of gestational stage‐dependent RIs for these tumor markers appears to have greater clinical significance.
Carbohydrate antigen 125 is the most studied tumor marker during pregnancy. Although the different reports are inconsistent, elevated levels were identified in all studies, with the highest level in the first trimester. For the second and third trimesters, the CA125 values were generally reported below the cutoff value of 35 U/mL in the United States, UK, and Japan.20, 21, 22 Nevertheless, Bon G.G. in Netherlands reported elevated CA125 levels during the second and third trimesters.23 Ercan et al identified elevated CA125 levels in the third trimester.15 Our results showed higher CA125 levels than those reported previously, particularly in the first and third trimesters, with wider fluctuations. These differences may be caused by the different methods and study populations. Therefore, we recommend appropriate RIs for CA125 for the pregnant women in China.
According to our results, the RI for non‐pregnant women (<35 U/mL) was only suitable for pregnant women in the second trimester. Approximately 70.6% and 42.9% of pregnant women had CA125 values that were above the cutoff value of 35 U/mL in the first and third trimesters, respectively. Thus, the use of the RIs for non‐pregnant women implies a risk of false positive results in pregnant women in the absence of ovarian cancer. Moreover, the RIs we established during the first (<309.5 U/mL) and third (<113.3 U/mL) trimesters appear to have lower clinical significance because of wider fluctuations. Therefore, we suggest that CA125 is not applicable to clinical interpretation during these 2 trimesters even if the cutoff values for pregnant women are used.
Human epididymis protein 4 has been suggested as a more specific marker for ovarian cancer. There are a limited number of studies regarding the level of HE4 during pregnancy. Moore et al reported that HE4 varied based on age, and menopausal and pregnancy status, and the RIs for pregnant women were significantly lower than their premenopausal counterparts (<89 pmol/L).11 The current results are in agreement with the findings reported by Moore et al11 It implied that false negative results may occur in pregnant women if the cutoff value for premenopausal women is used.
The RIs for HE4 established in this study were approximately 2.0‐fold higher than those by Moore et al in the second and third trimesters, and lower than those for premenopausal Chinese women in the first 2 trimesters. It implied that HE4 serum levels could be affected not only by age and pregnancy, but also by ethnic background. Therefore, we suggest the use of RIs for HE4 for Chinese pregnant women. The cutoff values of 50.3 pmol/L, 56.4 pmol/L, and 101.9 pmol/L are recommended during the first, second, and third trimesters, respectively, and further clinical confirmations are required.
Both CA125 and HE4 with menopausal status are incorporated into the ROMA index, which appears to show the best diagnostic performance to differentiate epithelial ovarian cancer from benign disease.8, 19 In this study, the ROMA index is calculated using premenopausal algorithms according to the definition reported by Moore et al19 Moore considered that women were considered to be premenopausal if they had a period within 1 year of the study blood draw.19 To prove that the premenopausal algorithm is more appropriate for pregnant women, we also established the RIs for the ROMA index calculated by postmenopausal algorithm in Appendix S1. To the best of our knowledge, this study is the first time that the ROMA index has been evaluated in pregnant women. Therefore, we could not summarize the ROMA index variability during pregnancy based on factors such as race and ethnicity. Using the premenopausal algorithms, the 95th RIs for the ROMA index in the first and second trimesters were very close to the RIs for non‐pregnant women (<11.4%), which implied that the ROMA index was not heavily influenced by pregnancy during these 2 stages. The elevated values in the third trimester may be a result of the increases in the HE4 and CA125 values. However, the RIs for the ROMA index calculated by the postmenopausal algorithms were clearly different from the normal range (<29.9%). Therefore, we recommend premenopausal algorithms for the ROMA index during pregnancy. For Chinese pregnant women, the cutoff value of 31% could be used in the third trimester, and the RIs in the first and second trimesters should not be altered.
Using the cutoff values of 35 U/mL, the most misclassification (approximately 70.6%) was identified in CA125 in the first trimester. In pregnant women who were misclassified as having abnormal CA125 values, few participants had HE4 values that were above the cutoff value of 65.8 pmol/L in the first 2 trimesters. However, as a result of the risk of false negatives, it cannot be concluded that HE4 is more suitable to be detected during pregnancy except the RIs for pregnant women was used. In 216 pregnant women who were misclassified as having abnormal CA125 values in the first trimester, only 2 participants had a ROMA index that was above the cutoff value of 11.4%. The upper 5th percentile of the CA125 values in the first 2 trimesters returned to the normal range using the ROMA index. Therefore, we recommend the clinical application of the ROMA index in pregnant women, especially during the first 2 trimesters.
In conclusion, we established gestational stage‐dependent RIs for CA125, HE4, and the ROMA index in healthy Chinese pregnant women. The ROMA index calculated by the premenopausal algorithms is recommended for pregnant women, and additional clinical studies are required to verify our findings.
Supporting information
Lu J, Zheng Z, Zhang Q, et al. Measurement of HE4 and CA125 and establishment of reference intervals for the ROMA index in the sera of pregnant women. J Clin Lab Anal. 2018;32:e22368 10.1002/jcla.22368
Funding information
This study was supported by National Natural Science Foundation of China (81271914), National Science and Technology Ministry Foundation (2012BAI35B01), Zhejiang Provincial Natural Science Foundation of China (LY12H16025) and Zhejiang Provincial Innovative Medical Discipline (11‐CX18).
Jie Lu and Zhipeng Zheng authors contributed equally to this article.
Contributor Information
Xinyou Xie, Email: scottxie@mail.hz.zj.cn.
Jun Zhang, Email: jameszhang2000@163.com.
REFERENCES
- 1. Schutter EM, Davelaar EM, van Kamp GJ, Verstraeten RA, Kenemans P, Verheijen RH. The differential diagnostic potential of a panel of tumor markers (CA 125, CA 15‐3, and CA 72‐4 antigens) in patients with a pelvic mass. Am J Obstet Gynecol. 2002;187:385‐392. [DOI] [PubMed] [Google Scholar]
- 2. Sarandakou A, Protonotariou E, Rizos D. Tumor markers in biological fluids associated with pregnancy. Crit Rev Clin Lab Sci. 2007;44:151‐178. [DOI] [PubMed] [Google Scholar]
- 3. Bottoni P, Scatena R. The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol. 2015;867:229‐244. [DOI] [PubMed] [Google Scholar]
- 4. Zhao T, Hu W. CA125 and HE4: measurement tools for ovarian cancer. Gynecol Obstet Invest. 2016;81:430‐435. [DOI] [PubMed] [Google Scholar]
- 5. Shen Y, Li L. Serum HE4 superior to CA125 in predicting poorer surgical outcome of epithelial ovarian cancer. Tumour Biol. 2016;37:14765‐14772. [DOI] [PubMed] [Google Scholar]
- 6. Trudel D, Têtu B, Grégoire J, et al. Human epididymis protein 4 (HE4) and ovarian cancer prognosis. Gynecol Oncol. 2012;127:511‐515. [DOI] [PubMed] [Google Scholar]
- 7. Karlsen MA, Sandhu N, Høgdall C, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012;127:379‐383. [DOI] [PubMed] [Google Scholar]
- 8. Wei SU, Li H, Zhang B. The diagnostic value of serum HE4 and CA‐125 and ROMA index in ovarian cancer. Biomed Rep. 2016;5:41‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han SN, Lotgerink A, Gziri MM, Van Calsteren K, Hanssens M, Amant F. Physiologic variations of serum tumor markers in gynecological malignancies during pregnancy: a systematic review. BMC Med. 2012;10:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheli CD, Morris DL, Neaman IE, Dai J, Allard WJ, Yeung KK. Measurement of four tumor marker antigens in the sera of pregnant women. J Clin Lab Anal. 1999;13:35‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moore RG, Miller MC, Eklund EE, Lu KH, Bast RC, Lambert‐Messerlian G. Serum levels of the ovarian cancer biomarker HE4 are decreased in pregnancy and increase with age. Am J Obstet Gynecol. 2012;206:349. e341–e347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi K, Yamane Y, Yoshino K, Shibukawa T, Matsunaga I, Kitao M. Studies on serum CA125 levels in pregnant women. Nihon Sanka Fujinka Gakkai zasshi. 1985;37:1931‐1934. [PubMed] [Google Scholar]
- 13. Haga Y, Sakamoto K, Egami H, Yoshimura R, Akagi M. Evaluation of serum CA125 values in healthy individuals and pregnant women. Am J Med Sci. 1986;292:25‐29. [DOI] [PubMed] [Google Scholar]
- 14. Seki K, Kikuchi Y, Uesato T, Kato K. Increased serum CA 125 levels during the first trimester of pregnancy. Acta Obstet Gynecol Scand. 1986;65:583‐585. [DOI] [PubMed] [Google Scholar]
- 15. Ercan S, Kaymaz Ö, Yücel N, Orçun A. Serum concentrations of CA 125, CA 15‐3, CA 19‐9 and CEA in normal pregnancy: a longitudinal study. Arch Gynecol Obstet. 2012;285:579‐584. [DOI] [PubMed] [Google Scholar]
- 16. Scaletta G, Plotti F, Luvero D, et al. The role of novel biomarker HE4 in the diagnosis, prognosis and follow‐up of ovarian cancer: a systematic review. Expert Rev Anticancer Ther. 2017;17:827‐839. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Sa M, Huang M, et al. The reference intervals for HE4, CA125 and ROMA in healthy female with electrochemiluminescence immunoassay. Clin Biochem. 2013;46:1705‐1708. [DOI] [PubMed] [Google Scholar]
- 18. Li PL, Zhang X, Li TF, et al. Combined detection of sialic acid and hydroxyproline in diagnosis of ovarian cancer and its comparison with human epididymis protein 4 and carbohydrate antigen 125. Clin Chim Acta. 2015;439:148‐153. [DOI] [PubMed] [Google Scholar]
- 19. Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kobayashi F, Sagawa N, Nakamura K, et al. Mechanism and clinical significance of elevated CA 125 levels in the sera of pregnant women. Am J Obstet Gynecol. 1989;160:563‐566. [DOI] [PubMed] [Google Scholar]
- 21. Spitzer M, Kaushal N, Benjamin F. Maternal CA‐125 levels in pregnancy and the puerperium. J Reprod Med. 1998;43:387‐392. [PubMed] [Google Scholar]
- 22. Jacobs IJ, Fay TN, Stabile I, Bridges JE, Oram DH, Grudzinskas JG. The distribution of CA 125 in the reproductive tract of pregnant and non‐pregnant women. Br J Obstet Gynaecol. 1988;95:1190‐1194. [DOI] [PubMed] [Google Scholar]
- 23. Bon GG, Kenemans P, Verstraeten AA, et al. Maternal serum Ca125 and Ca15‐3 antigen levels in normal and pathological pregnancy. Fetal Diagn Ther. 2001;16:166‐172. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


