Abstract
Background
Cancer prevention is essential after transplantation (Tx). The use of HE4 and Risk of Ovarian Malignancy Algorithm (ROMA) is recommended as a tool for selective ovarian cancer screening; however, creatinine is a known confounder. This study assessed the reliability of HE4, CA125, and ROMA after Tx.
Methods
We matched a total of 202 women without gynecological malignancies and 236 men by age and serum creatinine. Each pair consisted of a patient after Tx (kidney, liver, heart, and pancreas) and a diseased but non‐Tx consecutive patient. Serum HE4, CA125 (Roche Cobas 6000), and creatinine (enzymatic, Abbott Architect) were measured in all patients.
Results
Creatinine correlated with HE4 (women: r = .864, P < .0001; men: r = .848, P < .0001). Age correlated slightly with HE4 in women (r = .250, P < .005) and men (r = .240, P < .0005). HE4 in women after Tx (median of 84.8 pmol/L) was significantly higher than non‐Tx women (53.7 pmol/L, P < .0001) in the reference range of serum creatinine. Neither HE4 nor CA125 correlated with tacrolimus concentration, but anemia, hyperparathyroidism, kidney, liver, and lung diseases were possible confounders for HE4 after transplantation (P < .05).
Conclusion
Human epididymis protein 4 (HE4) was significantly increased in women after solid organ transplantation compared to levels without transplants matched by age and serum creatinine. HE4 results may be misleading in these patients.
Keywords: CA125, creatinine, human epididymis protein 4, Risk of Ovarian Malignancy Algorithm, transplantation
Abbreviations
- CKD
chronic kidney disease
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- HE4
human epididymis protein 4
- ROMA
Risk of Ovarian Malignancy Algorithm
- Tx
transplantation
1. INTRODUCTION
Human epididymis protein 4 (HE4), CA125, and the probability of ovarian cancer calculated using Risk of Ovarian Malignancy Algorithm (ROMA) are recommended as practical tools for the assessment of risk of ovarian malignancy in premenopausal and postmenopausal women with pelvic mass.1, 2, 3, 4, 5, 6, 7 However, benign pelvic mass is not uncommon in premenopausal women. There are confounding factors influencing the use of HE4 and interpretation of ROMA. Granata recently published a critical review of HE4 and recommended the consideration of age, menstrual status, smoking habits, and renal function while interpreting HE4.8 Glomerular filtration rate (GFR) decreases with age, and few reports described the relationship between HE4 and GFR in detail.9, 10, 11, 12 No recommendation was given for the use of ROMA in populations with chronic kidney disease (CKD). After solid organ transplantation (Tx), patients are at increased risk of malignancy due to intensive immunosuppressant treatment.13 The cumulative incidences of ovarian cancer after kidney transplantation were 0.04%, 0.09%, and 0.14% at 12th‐, 24th‐, and 36th‐month post‐Tx, respectively.14 The incidence of ovarian cancer is 0.26% in women after liver transplantation, and the incidence is much higher (15.4%, ie, 1:6.5) in women with a previous history of breast cancer before Tx.15 Impaired GFR and immunosuppressant‐related malignancy often occur simultaneously in kidney and heart Tx. Therefore, the use of preventive assessments, including gynecological cancer prevention, is recommended in young post‐transplant patients. We found positive ROMA with a higher frequency in women after Tx with negative specialist ultrasound examination and normal renal function compared to nontransplant, but polymorbid, women, which is in contrast to the clinical experience. Our study compared transplant and diseased nontransplant pairs of women and men matched by age and serum creatinine and assessed the role of different confounding factors influencing the reliability of HE4, CA125, and ROMA in solid organ transplant recipients.
2. MATERIALS AND METHODS
2.1. Patients
Our pilot study retrospectively evaluated 155 consecutive ROMA index results in 155 women with pelvic mass. Our cohort included 50 women after solid organ Tx (premenopausal status 72%) and 105 diseased women without organ transplantation (56% premenopausal). The transplanted organs were liver (16 patients), kidney (14 pts), combined kidney and pancreas (14 pts), heart (5 pts), and pancreas (1 patient). Immunosuppressant regimens consisted primarily of tacrolimus (approximately 80% of patients) and everolimus, sirolimus, and rarely cyclosporin A, which was used to a lesser extent.
Our case‐control study matched 202 women and 236 men by age and serum creatinine to eliminate the 2 main known factors that influence HE4 and ROMA. The purpose of the case‐control study was not to assess the risk of malignancy, but to evaluate the confounding factors that influence levels of HE4 and CA125. The pair consisted of patients (woman or man) after solid organ transplantation and diseased, but nontransplant, patients matched by sex, age, and serum creatinine. The reliability of matching was assessed by calculating the relative differences between paired data. Detailed analyses of relationships between HE4, CA125, creatinine, morbidity, and transplant status were performed for women only. The necessary criteria for inclusion, except for matched age and creatinine, were at least 1‐year survival after Tx without any known gynecological malignancy (breast, ovary, cervix, and uterus). The transplanted organs were kidney (41), combined kidney and pancreas (21), liver (29), liver and kidney (2), liver and Langerhans islets (1), pancreas (3), and heart (4). Tacrolimus was used in 98 women (97%), 2 women were treated with cyclosporin, and 1 woman received a combination of cyclosporin and sirolimus.
2.2. Clinical data
Clinical data of the women with transplants were retrieved from patient records. Information on diabetes mellitus, primary or secondary hyperparathyroidism, hypoparathyroidism, anemia, intestinal disease, lung disease, and ischemic heart disease was extracted from patient history. Information on the presence of hypertension or hyperlipidemia was derived from patient history or medications (antihypertensives or hypolipidemics). Infection was defined as any acute or chronic infection at the time of evaluation or when actual treatment with antibiotics was recorded. Tumor was defined as any malignant tumor (of nongynecological origin) in the patient history. Hypothyroidism and hyperthyroidism were derived from patient history or medications (actual treatment with hormonal supplementation or the use of thyreostatics). Nephropathy (kidney disease) was derived from patient history (in patients without renal Tx) or kidney transplantation. Similarly, hepatopathy (liver disease) was derived from patient history (in patients without liver Tx) or liver transplantation.
2.3. Laboratory methods
All measurements were performed in an accredited laboratory (according to the ISO 15189:2012). HE4 and CA125 were measured using automated immunoanalysis on the Roche Cobas 6000 analyzer (Roche Diagnostics, Rotkreuz, Switzerland) with the Roche Elecsys HE4 and CA125 II kits (Cat. No. 05950929 and 11776223, respectively; Roche Diagnostics, Mannheim, Germany). Creatinine was measured using an automated enzymatic method on the Abbott Architect analyzer with Architect Creatinine (Enzymatic) kits (Cat. No. 8L24‐31; Abbott Laboratories, Abbott Park, IL, USA). The cutoff values for HE4 were 70 pmol/L (84th percentile) for premenopausal women and 140 pmol/L (97th percentile) for postmenopausal women. The cutoff limit for CA125 was 35 kU/L (95th percentile) for the pre‐ and postmenopausal women. Reference values for serum creatinine were 0.55‐1.02 mg/dL (49‐90 μmol/L) for women and 0.72‐1.18 mg/dL (64‐104 μmol/L) for men. Estimated glomerular filtration rate (eGFR) was calculated using the CKD‐EPI 2012 creatinine formula according to the KDIGO 2012 Guidelines.
2.4. Statistical evaluation
We used the Mann‐Whitney test, Kruskal‐Wallis test with Conover post hoc analysis, the Spearman's nonparametric coefficient of correlation, and Fisher's exact test.
2.5. Ethics committee approval
The Ethics Committee of the Institute for Clinical and Experimental Medicine and the Thomayer Hospital with multicenter competence approved the study under No. 1537/15 G‐15‐09‐02. The study was performed in accordance with the 2000 Declaration of Helsinki and the 2008 Declaration of Istanbul.
3. RESULTS
We present the data in 2 parts. The pilot study described the problem of possible false elevations of HE4 and ROMA in women after transplantation. The case‐control study analyzed the results of HE4 and CA125 measured in women and men with and without transplantation, who were matched by age and serum creatinine. We also focused on the relationship between post‐transplant clinical status, immunosuppressant therapy, and tested biomarkers (HE4, CA125).
3.1. Pilot study
There were significant correlations between serum creatinine and HE4 (r = .545, P < .0001, N = 155) and between serum creatinine and ROMA (r = .525, P < .0001). However, only a weak correlation was found between serum creatinine and CA125 (r = .170, P < .05), while age did not correlate to ROMA, HE4, or CA125.
We further divided the group of women into tertiles (T1‐T3) based on the concentration of serum creatinine. Table 1 summarizes these results.
Table 1.
Pilot study. Women: Measured and calculated parameters in tertiles (T1‐T3) of serum creatinine
| T1 (N = 52, 0.38‐0.79 mg/dL) | T2 (N = 51, 0.79‐0.94 mg/dL) | T3 (N = 52, 0.95‐6.65 mg/dL) | |
|---|---|---|---|
| Age (y) | 42.5 (33.5‐55.5) | 50.0 (32.5‐61.0) | 45.5 (37.5‐55.5) |
| Serum creatinine (mg/dL) | 0.72 (0.66‐0.77) | 0.86 (0.82‐0.89) | 1.23 (1.02‐1.99) |
| CA125 (kU/L) | 13.2 (8.9‐17.8) | 11.6 (8.3‐17.9) | 17.6 (11.1‐28.4) |
| HE4 (pmol/L) | 52.6 (43.8‐70.0) | 53.7 (45.6‐70.9) | 142.0 (84.8‐2272.8) |
| ROMA (%) | 8.5 (6.2‐14.8) | 9.1 (5.9‐14.3) | 39.6 (18.8‐68.0) |
HE4, human epididymis protein 4; ROMA, Risk of Ovarian Malignancy Algorithm.
Divide by 0.01131 to convert creatinine from mg/dL to μmol/L. Data are given as median (interquartile range).
We compared ROMA with specific cutoff limits for increased risk of epithelial ovarian cancer to assess the risk of malignancy. The cutoffs of 11.4% and 29.9% were used for premenopausal and postmenopausal women, respectively. A significantly higher proportion of cases with increased risk of cancer were found in women after transplantation compared to that of the non‐Tx cohort (OR 18.2, 95% CI 7.7‐43.4, χ2 = 54.3, P < .0001, N = 155). This pattern was also present in first 2 tertiles of serum creatinine (up to 0.94 mg/dL (83.4 μmol/L), OR 14.8, 95% CI 4.4‐49.6, χ2 = 25.5, P < .0001, N = 103).
The highest concentration of serum creatinine in the second tertile of creatinine was 0.94 mg/dL (83.4 μmol/L), and the lowest serum concentration of creatinine in the third tertile was 0.95 mg/dL (84 μmol/L), which is close to the upper reference limit for serum creatinine in women. Therefore, we analyzed the differences between women with and without transplants in the reference interval of serum creatinine (up to 1.02 mg/dL [90 μmol/L]) to exclude impaired renal function as the source of variability for HE4 and CA125 values. HE4, CA125, and ROMA were significantly higher (P < .0001) in women with transplants in this subgroup of patients (Table 2). Increased concentrations of HE4, CA125, and ROMA were found in 90%, 81%, and 90% of Tx women, respectively; in contrast, increased values of HE4, CA125, and ROMA were present in 41%, 44%, and 43% of non‐Tx women, respectively. However, women with serum creatinine above 1.02 mg/dL (90 μmol/L) exhibited no differences between Tx and non‐Tx patients for all 3 evaluated biomarkers.
Table 2.
Pilot study. Measured and calculated parameters with respect to the upper reference limit for serum creatinine (1.02 mg/dL) in women with and without transplants
| Serum creatinine ≤1.02 mg/dL N = 115 | Serum creatinine >1.02 mg/dL N = 40 | |||||
|---|---|---|---|---|---|---|
| Non‐Tx (N = 94) | Tx (N = 21) | P (Mann‐Whitney test) | Non‐Tx (N = 11) | Tx (N = 29) | P (Mann‐Whitney test) | |
| CA125 (kU/L) | 10.7 (8.3‐16.1) | 19.8 (13.6‐26.9) | .000 075 | 21.7 (11.1‐48.8) | 19.1 (12.9‐37.8) | .5855 |
| HE4 (pmol/L) | 50.4 (42.8‐64.5) | 82.8 (63.4‐105.4) | <.000 001 | 345.1 (143.4‐1143.0) | 153.8 (112.3‐263.1) | .0440 |
| Serum creatinine (mg/dL) | 0.79 (0.72‐0.86) | 0.86 (0.79‐0.92) | .017 | 3.08 (1.09‐4.92) | 1.40 (1.21‐1.97) | .2960 |
| ROMA (%) | 7.8 (5.8‐12.6) | 22.1 (13.3‐29.5) | <.000 001 | 85.4 (29.2‐94.8) | 42.7 (30.6‐59.5) | .0814 |
| Age (y) | 47.5 (38.0‐60.0) | 34.0 (29.8‐48.5) | .01 | 47.0 (35.0‐65.8) | 45.0 (37.8‐52.0) | .5443 |
ROMA, Risk of Ovarian Malignancy Algorithm.
Divide by 0.01131 to convert creatinine from mg/dL to μmol/L. Data are given as median (interquartile range).
3.2. Case‐control study
3.2.1. Relationship between serum creatinine and measured variables
A significant correlation was found between serum creatinine and HE4 in women (Spearman r = .864, P < .0001, N = 202). Age only slightly correlated to HE4 (r = .250, P < .005) but not CA125. Similarly, the correlation between serum creatinine and CA125 was also low (r = .227, P < .01).
Similar relationships were found in men. HE4, but not CA125, and creatinine correlated significantly (r = .848, P < .0001, N = 236). Similarly, HE4, but not CA125, slightly correlated with age (r = .240, P < .0005).
Table 3 compares the values of measured variables in tertiles (T1‐T3) of serum creatinine. Values of HE4 increased significantly with increasing serum creatinine and decreasing eGFR (Kruskal‐Wallis test, P < .0001), and post hoc analysis revealed significant differences in HE4 concentrations between respective tertiles. HE4 in T2 was significantly higher than HE4 in T1 (P < .05), and HE4 in T3 was significantly higher than HE4 in T2 (P < .05). The increase in CA125 with increasing creatinine was significant (P = .0052), but CA125 concentrations in T3 were significantly higher than CA125 in T1 only (P < .05).
Table 3.
Case‐control study
| T1 (N = 67, 0.49‐0.98 mg/dL) | T2 (N = 68, 0.98‐1.73 mg/dL) | T3 (N = 67, 1.77‐7.93 mg/dL) | |
|---|---|---|---|
| Age (y) | 46.0 (32.0‐62.8) | 57.5 (44.0‐65.0) | 58.0 (50.0‐65.0) |
| Serum creatinine (mg/dL) | 0.84 (0.78‐0.90) | 1.32 (1.09‐1.53) | 3.01 (2.02‐3.96) |
| CA125 (kU/L) | 12.9 (9.9‐22.9) | 17.2 (12.2‐26.2) | 20.5 (13.8‐36.4) |
| HE4 (pmol/L) | 71.3 (53.8‐94.4) | 159.1 (98.6‐217.5) | 354.0 (294.1‐847.2) |
HE4, human epididymis protein 4.
Values of measured variables in tertiles (T1‐T3) of serum creatinine in women (N = 202). Median and interquartile ranges are given. Borders of respective quintiles of serum creatinine are given in mg/dL. Divide by 0.01131 to convert creatinine from mg/dL to μmol/L. Data are given as median (interquartile range).
Similar results (ie, increasing concentrations of HE4 with the decrease in GFR) were found in the case‐control study in men (Table S1, online only).
We combined the tertiles of age and serum creatinine to demonstrate differences in HE4 in respective subgroups to further examine the relationships between HE4, age, and creatinine. Figure 1 displays these relationships.
Figure 1.
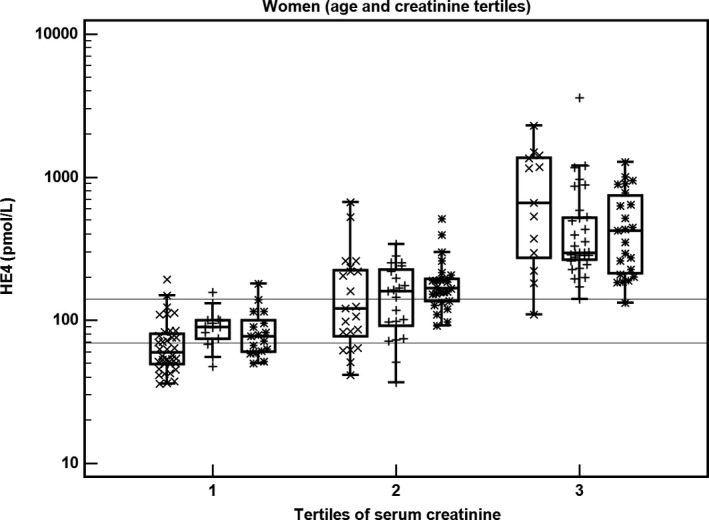
The relationship between HE4, age, and serum creatinine in women (N = 202). Tertiles of age and serum creatinine are combined, tertiles of age are marked as x (1st tertile), + (2nd tertile), and * (3rd tertile). Lines represent recommended reference values for premenopausal (70 pmol/L) and postmenopausal (140 pmol/L) women
An almost identical relationship between HE4 and tertiles of age and serum creatinine was found in men (Figure S1).
The relationship between serum creatinine, age, and HE4 for all patients is also highlighted in Figures S3 and S4. We confirmed the relationship between serum creatinine and HE4, which was statistically (r = .847, P < .0001) and clinically significant. However, the statistically significant relationship between age and HE4 (r = .309, P < .0001) likely exerted negligible clinical impact.
3.2.2. The influence of post‐transplant clinical status on HE4 and CA125
Figure 2 compares HE4 concentrations in women after transplantation (+) and without transplantation (x) in respective tertiles of serum creatinine.
Figure 2.
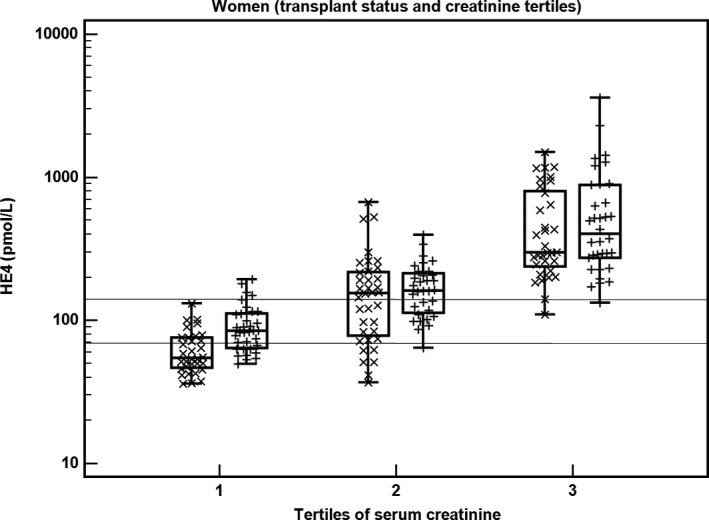
The relationship between HE4, transplant status, and serum creatinine in women (N = 202). Tertiles of serum creatinine are combined with transplant status, which is marked as x (nontransplant) and + (transplant). Lines represent recommended reference values for premenopausal (70 pmol/L) and postmenopausal (140 pmol/L) women. HE4, human epididymis protein 4
We present data of measured variables in 2 subgroups with respect to the upper reference limit of serum creatinine to eliminate the strong influence of impaired renal function (represented by heavily increased serum creatinine) on HE4 values (Table 4). HE4 (P < .0001) and CA125 (P = .0012) are significantly higher in women after Tx compared to those in non‐Tx patients in the reference range of serum creatinine. In this subgroup of women with serum creatinine in the reference range, increased concentrations of HE4 and CA125 were found in 69% and 66% of Tx women, respectively; in contrast, increased values of HE4 and CA125 were present in 32% and 35% of non‐Tx women, respectively.
Table 4.
Women: Case‐control study
| Serum creatinine ≤1.02 mg/dL N = 72 | Serum creatinine >1.02 mg/dL N = 130 | |||||
|---|---|---|---|---|---|---|
| Non‐Tx (N = 37) | Tx (N = 35) | P (Mann‐Whitney test) | Non‐Tx (N = 64) | Tx (N = 66) | P (Mann‐Whitney test) | |
| CA125 (kU/L) | 11.0 (8.9‐14.3) | 16.8 (11.6‐35.5) | .0012 | 20.7 (12.5‐30.5) | 18.2 (13.7‐28.6) | .4032 |
| HE4 (pmol/L) | 53.7 (45.7‐75.8) | 84.8 (64.0‐112.2) | .000 026 | 239.8 (155.4‐428.5) | 229.7 (160.5‐431.8) | .7321 |
| Serum creatinine (mg/dL) | 0.84 (0.79‐0.91) | 0.84 (0.78‐0.92) | .7739 | 1.80 (1.42‐3.05) | 1.83 (1.40‐3.01) | .8760 |
| Age (y) | 46.0 (34.2‐60.8) | 46.0 (32.0‐62.8) | .8658 | 58.0 (48.5‐66.0) | 58.5 (47.0‐65.0) | .6632 |
HE4, human epididymis protein 4.
Measured and calculated parameters with respect to the upper reference limit for serum creatinine (1.02 mg/dL) in women with transplants (Tx) and women without transplants (non‐Tx). Divide by 0.01131 to convert creatinine from mg/dL to μmol/L. Data are given as median (interquartile range).
There was no significant difference in evaluated variables in the reference range of serum creatinine for men (up to 1.18 mg/dL [104 μmol/L]) and above the upper reference limit. HE4 was 71.4 pmol/L in men without transplants and 83.3 pmol/L in men with transplants (N.S.) and normal serum creatinine (Figure S2).
3.2.3. Relationship between tacrolimus, HE4, and CA125
We examined the relationship between the concentration of tacrolimus (used in 98 of 101 women and in all men after Tx) and HE4 or CA125. No significant relationship (Spearman r) between tacrolimus and HE4 was found in women or men. However, there was a significant but weak relationship between tacrolimus and CA125 in women (r = .238, P = .018) but not in men.
3.2.4. Clinical data
We analyzed differences in clinical data in non‐Tx and Tx women in the case‐control study. Table 5 summarizes data for all women and women with normal concentrations of serum creatinine (up to 1.02 mg/dL [90 μmol/L]). Anemia, hyperparathyroidism, kidney, liver, and lung diseases were more frequent in the group of women after transplantation (P < .05). In a subset of women with serum creatinine in reference range, a significantly higher occurrence of anemia (P = .017), hyperparathyroidism (P = .026), kidney disease (P = .0029), and liver disease (P = .00003) was found in women after transplantation.
Table 5.
Women: Summary of clinical diagnoses
| Non‐Tx (N=101) | Tx (N=101) | P (Fisher exact test) | Non‐Tx (N=37) | Tx (N=35) | P (Fisher exact test) | |
|---|---|---|---|---|---|---|
| Anemia | 20 | 46 | .00 016 | 3 | 11 | .017 |
| Diabetes mellitus | 34 | 42 | .3093 | 10 | 12 | .611 |
| Hyperlipidemia | 42 | 37 | .5643 | 10 | 8 | .788 |
| Hyperparathyroidism | 7 | 27 | .00 026 | 1 | 7 | .026 |
| Hypertension | 61 | 71 | .1831 | 11 | 19 | .0551 |
| Hyperthyroidism | 2 | 6 | .2791 | 0 | 2 | .2328 |
| Hypothyroidism | 22 | 27 | .5117 | 5 | 6 | .7503 |
| Infection | 17 | 27 | .1244 | 5 | 10 | .1509 |
| Intestinal disease | 8 | 8 | 1 | 5 | 4 | 1 |
| Ischemic heart disease | 18 | 13 | .4353 | 3 | 2 | 1 |
| Kidney disease | 52 | 73 | .0036 | 4 | 15 | .0029 |
| Liver disease | 19 | 46 | .00 008 | 6 | 23 | .00 003 |
| Lung disease | 15 | 5 | .0318 | 3 | 4 | .7066 |
| Tumor | 10 | 9 | 1 | 3 | 5 | .4727 |
The difference between women with and without transplants was tested using Fisher's exact test. Data are given both for all women (N = 202) and for subset of women (N = 72) with serum creatinine up to 1.02 mg/dL (90 μmol/L).
4. DISCUSSION
We confirmed increased HE4 concentrations in women and men with decreased eGFR and increased serum creatinine. However, there were increased concentrations of HE4 in the post‐transplant patients with serum creatinine in reference ranges in the pilot and case‐control studies in women. None of the women with transplants exhibited gynecological malignancy, and there was no relationship between immunosuppressant concentration and HE4. The only difference was more pronounced polymorbidity in women with transplants.
Data on the frequency of ovarian cancer in women with transplants are inconsistent with different rates in other studies.16, 17, 18 HE4, CA125, and ROMA are the best predictors of malignancies in women with pelvic mass. However, ROMA is falsely increased in patients with decreased eGFR and/or increased creatinine9, 10, 19 because of the higher HE4 concentrations in renal failure. Age, smoking, BMI, and menstrual status also influence HE4.8, 19 There are likely other factors influencing the concentrations of HE4, CA125, and ROMA. Shin et al20 found falsely elevated ROMA in women with confirmed ovarian endometrioma, which was likely due to the elevated serum HE4.
4.1. Pilot study
The significant relationships between serum creatinine, HE4, and ROMA in the pilot study are consistent with data in the literature. However, there were also increased values of HE4, CA125, and ROMA in the group of women with transplants with serum creatinine within the reference range, despite the younger age of this cohort (Table 1). Therefore, serum creatinine may be the major factor contributing to the differences in HE4 and ROMA between women with and without transplants.9, 10 However, other factors may also play a role. Age likely plays only a minor role in the observed pattern. The role of eGFR in this situation seems controversial. The median value of eGFR was not different from women without transplants in the reference interval of serum creatinine due to the younger age of women with transplants. Nevertheless, the increased creatinine concentrations were the primary reason for the increased HE4 and ROMA values. However, the use of recommended cutoff limits (to reveal the increased risk of ovarian cancer) demonstrated that the frequency of positive results in women with transplants was approximately 2 times higher than in women without transplants, even within the subgroup with normal renal function (serum creatinine in reference interval) (Table 2). Therefore, the difference in the concentration of HE4 between women with and without transplants is determined by factors other than decreased renal function. Four women without transplants with the highest concentrations of HE4 were dialyzed polymorbid patients who were on a waiting list for kidney Tx. Therefore, their classification as nontransplant is correct, but the very high concentration of creatinine is the primary cause of the increased HE4.
4.2. Case‐control study
The purpose of our case‐control study was to assess possible confounders in selected pairs of women and men matched by age and serum creatinine, but not to use HE4 and ROMA for detection of malignancies. HE4 increased with higher concentrations of serum creatinine in women and men (Table 3 and Table S1). The differences in HE4 between tertiles of serum creatinine were highly significant. The relationship between HE4 and creatinine was more pronounced than the relationship between HE4 and age (Figure 1 and Figure S1). In women, CA125 was significantly higher only in the 3rd tertile of serum creatinine compared to the 1st tertile. There was no relationship between CA125 and creatinine in men.
General risk factors for malignancies after kidney Tx include age (below 18 years and above 34 years), male sex, Caucasian race, and dialysis before Tx for 3 or more years. No significant role was noted for BMI, type of initial immunosuppression or antibody induction.14 None of the listed risk factors (except for dialysis time, which was not analyzed) differed significantly between non‐Tx and post‐Tx women in our cohort. Groups were matched by age and creatinine, and all women were Caucasian. None of the women with transplants exhibited gynecological malignancy, and there was no relationship between tacrolimus concentration and HE4. In contrast, post‐Tx women exhibited anemia, secondary hyperparathyroidism, and chronic kidney, liver, or lung disorders more frequently (Table 5). There are likely other possible factors that may have influenced the studied biomarkers (eg, type of immunosuppressive agent, phase of menstrual cycle, hormonal replacement therapy, and contraceptives). However, data in the literature are controversial.12, 21, 22, 23 Specific reference ranges were published for pregnant women.24
It is advisable to measure serum creatinine or serum cystatin C consistently to detect even mild kidney disease before examinations of HE4, CA125, and ROMA are requested.11 The use of the CKD‐EPI equation played a limited role compared to that of serum creatinine, which was likely because of the incorporation of age in the equation. The clinical situation of the patient should always be analyzed. Risky, polymorbid patients with chronic diseases and patients after solid organ transplantation may display higher HE4 concentrations, even within the reference values of serum creatinine and a younger age group.
An ultrasound in expert hands is a superior tool to tumor markers for the diagnosis and differential diagnosis of pelvic tumors in patients with increased serum creatinine levels (>1.02 mg/dL) and in patients after transplant. The use of ultrasound becomes more imperative in patients with comorbidities that influence CA125 and/or HE4 concentrations in sera.25, 26, 27 The use of a universal equation for the calculation of ovarian malignancy risk is questionable. HE4 values can hardly be interpreted without the knowledge of age, menstrual status, serum creatinine, organ complications, hormonal therapy and other medication, smoking status, body mass index and possibly other, yet unidentified, factors.10, 11, 28 Several ongoing prospective randomised trials evaluate the diagnostic and prognostic roles of HE4 in gynecological malignancies and other conditions.29 However, the prognostic potential of HE4 can be diminished by many confounding factors, while the data on diagnostic efficacy in patients after solid organ transplantation are scarce.
5. CONCLUSIONS
Serum creatinine influenced HE4 much more than age in women and men. However, the concentration of HE4 was significantly higher in polymorbid women after kidney, liver, pancreas, and heart transplantation than in women without Tx, matched by age and serum creatinine, even within the reference range of serum creatinine. The concentration of tacrolimus exhibited no relationship with HE4 in women or men. The universal use of HE4 and ROMA for screening purposes in polymorbid patients and patients after solid organ transplantation is therefore questionable. Only the negative result of ROMA could possibly serve as a filter for reducing the number of unnecessary ultrasound examinations.
5.1. Study limitations
We did not analyze menstrual status in paired women matched by age and creatinine because we analyzed women and men. Some younger women were on contraceptives, and older women used hormonal replacement therapy. We were unable to analyze patient medication in details, except immunosuppressant therapy.
Supporting information
ACKNOWLEDGMENT
We thank Ms Štěpánka Strnadová for her technical assistance.
Franeková J, Cindr J, Lavríková P, et al. Falsely elevated human epididymis protein 4 results and Risk of Ovarian Malignancy Algorithm in polymorbid women after solid organ transplantation: A pilot and case‐control study. J Clin Lab Anal. 2018;32:e22432 10.1002/jcla.22432
REFERENCES
- 1. Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402‐408. [DOI] [PubMed] [Google Scholar]
- 2. Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moore RG, Miller MC, Disilvestro P, et al. Evaluation of the diagnostic accuracy of the risk of ovarian malignancy algorithm in women with a pelvic mass. Obstet Gynecol. 2011;118:280‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chudecka‐Głaz AM. ROMA, an algorithm for ovarian cancer. Clin Chim Acta. 2015;440:143‐151. [DOI] [PubMed] [Google Scholar]
- 5. Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzoli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013;66:273‐281. [DOI] [PubMed] [Google Scholar]
- 6. Lin J, Qin J, Sangvatanakul V. Human epididymis protein 4 for differential diagnosis between benign gynecologic disease and ovarian cancer: a systematic review and meta‐analysis. Eur J Obstet Gynecol Reprod Biol. 2013;167:81‐85. [DOI] [PubMed] [Google Scholar]
- 7. Zhang Q, Wang CR, Yu JP, Ma Q, Xu WW. The establishment of an HE4‐CLIA method and the combined analysis of HE4 and CA125 in ovarian cancer. J Clin Lab Anal. 2016;30:709‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Granato T, Porpora MG, Longo F, Angeloni A, Manganaro L. Anastasi E HE4 in the differential diagnosis of ovarian masses. Clin Chim Acta. 2015;446:147‐155. [DOI] [PubMed] [Google Scholar]
- 9. Nagy B Jr, Krasznai ZT, Balla H, et al. Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem. 2012;49:377‐380. [DOI] [PubMed] [Google Scholar]
- 10. Kappelmayer J, Antal‐Szalmás P, Nagy B Jr. Human epididymis protein 4 (HE4) in laboratory medicine and an algorithm in renal disorders. Clin Chim Acta. 2015;438:35‐42. [DOI] [PubMed] [Google Scholar]
- 11. Gizzo S, Ancona E, Saccardi C, D'Antona D, Nardelli GB, Plebani M. Could kidney glomerular filtration impairment represent the “Achilles heel” of HE4 serum marker? A possible further implication. Clin Chem Lab Med. 2014;52:e45‐e46. [DOI] [PubMed] [Google Scholar]
- 12. Escudero JM, Auge JM, Filella X, Torne A, Pahisa J, Molina R. Comparison of serum human epididymis protein 4 with cancer antigen 125 as a tumor marker in patients with malignant and nonmalignant diseases. Clin Chem. 2011;57:1534‐1544. [DOI] [PubMed] [Google Scholar]
- 13. Engels EA, Pfeiffer RM, Fraumeni JF Jr, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891‐1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905‐913. [DOI] [PubMed] [Google Scholar]
- 15. Molmenti EP, Molmenti H, Weinstein J, et al. Syndromic incidence of ovarian carcinoma after liver transplantation, with special reference to anteceding breast cancer. Dig Dis Sci. 2003;48:187‐189. [DOI] [PubMed] [Google Scholar]
- 16. Krynitz B, Edgren G, Lindelöf B, et al. Risk of skin cancer and other malignancies in kidney, liver, heart and lung transplant recipients 1970 to 2008–a Swedish population‐based study. Int J Cancer. 2013;132:1429‐1438. [DOI] [PubMed] [Google Scholar]
- 17. Zhou J, Hu Z, Zhang Q, et al. Spectrum of de novo cancers and predictors in liver transplantation: analysis of the scientific registry of transplant recipients database. PLoS One. 2016;11:e0155179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Buell JF, Woodle ES. Syndromic incidence of ovarian cancer after liver transplantation: is breast cancer an antecedent risk? Liver Transpl. 2004;10:156‐157. [DOI] [PubMed] [Google Scholar]
- 19. Bolstad N, Øijordsbakken M, Nustad K, Bjerner J. Human epididymis protein 4 reference limits and natural variation in a Nordic reference population. Tumour Biol. 2012;33:141‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin JJ, Lee YJ, Kim R, da Lee Y, Won KH, Jee BC. Analysis of falsely elevated risk of ovarian malignancy algorithm in women with ovarian endometrioma. Obstet Gynecol Sci. 2016;59:295‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anastasi E, Granato T, Marchei GG, et al. Ovarian tumor marker HE4 is differently expressed during the phases of the menstrual cycle in healthy young women. Tumour Biol. 2010;31:411‐415. [DOI] [PubMed] [Google Scholar]
- 22. Hallamaa M, Suvitie P, Huhtinen K, Matomäki J, Poutanen M, Perheentupa A. Serum HE4 concentration is not dependent on menstrual cycle or hormonal treatment among endometriosis patients and healthy premenopausal women. Gynecol Oncol. 2012;125:667‐672. [DOI] [PubMed] [Google Scholar]
- 23. Karlsen NS, Karlsen MA, Høgdall CK, Høgdall EV. HE4 tissue expression and serum HE4 levels in healthy individuals and patients with benign or malignant tumors: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:2285‐2295. [DOI] [PubMed] [Google Scholar]
- 24. Lu J, Zheng Z, Zhang Q, et al. Measurement of HE4 and CA125 and establishment of reference intervals for the ROMA index in the sera of pregnant women. J Clin Lab Anal. 2017;e22368 10.1002/jcla.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Valentin L, Jurkovic D, Van Calster B, et al. Adding a single CA 125 measurement to ultrasound imaging performed by an experienced examiner does not improve preoperative discrimination between benign and malignant adnexal masses. Ultrasound Obstet Gynecol. 2009;34:345‐354. [DOI] [PubMed] [Google Scholar]
- 26. Van Calster B, Timmerman D, Bourne T, et al. Discrimination between benign and malignant adnexal masses by specialist ultrasound examination versus serum CA‐125. J Natl Cancer Inst. 2007;99:1706‐1714. [DOI] [PubMed] [Google Scholar]
- 27. Van Gorp T, Veldman J, Van Calster B, et al. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. Eur J Cancer. 2012;48:1649‐1656. [DOI] [PubMed] [Google Scholar]
- 28. Qu W, Li J, Duan P, et al. Physiopathological factors affecting the diagnostic value of serum HE4‐test for gynecologic malignancies. Expert Rev Mol Diagn. 2016;16:1271‐1282. [DOI] [PubMed] [Google Scholar]
- 29. Capriglione S, Plotti F, Miranda A, et al. Further insight into prognostic factors in endometrial cancer: the new serum biomarker HE4. Expert Rev Anticancer Ther. 2017;17:9‐18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


