Abstract
Objectives: This study examines the associations among serum β2 microglobulin (B2M), malnutrition, inflammation, and atherosclerosis (MIA) in those with chronic kidney disease (CKD). Methods: CKD patients who were followed in Taoyuan General Hospital from 2009 to 2015 were enrolled. Demographic and biochemical data, including B2M and C‐reactive protein (CRP) were reviewed. The participants were stratified according to B2M tertiles. Adjusted hazard ratios (AHRs) and cumulative survival curves for death and MIA syndrome were evaluated by Cox hazard model and Kaplan–Meier method. We also calculated the area under the curve for the receiver operating characteristic curve (AUROC). Results: From a total of 312 CKD patients, mean follow‐up time was 39.7 months. Compared to those with lowest tertile of B2M, the highest tertile group had lower serum albumin, hemoglobin, and estimated glomerular filtration rate. After multivariate adjustment, the associations among tertiles of B2M, death or dialysis, cardiovascular events (CVEs), and MIA syndrome remained significant. The AHRs for the highest tertile group in death or dialysis, CVEs, and MIA syndrome were 25.91 and 65.84 and 152.50(all Ps <0.05).The AUROC for B2M in death or dialysis, CVEs, and MIA syndrome were greater than that for creatinine. The best cut‐off value of B2M for predicting death or dialysis, CVEs, and MIA syndrome were 5.39 mg/dL(sensitivity: 67.1%, specificity 62.5%), 4.21 mg/dL(sensitivity: 85.1%, specificity 52.1%), and 5.40 mg/dL(sensitivity: 79.7%, specificity 64.1%). Conclusions: In those with CKD, serum B2M was more sensitive than creatinine in predicting CVEs and MIA syndrome.
Keywords: cardiovascular event, C‐reactive protein, MIA syndrome, Mortality, β2 microglobulin
Introduction
Chronic kidney disease (CKD) is an important public health challenge 1. The high prevalence of CKD, especially end stage renal disease (ESRD), is associated with a high burden of morbidities and mortality 2. Several biomarkers, such as low estimated glomerular filtration rate (eGFR) and albuminuria, were associated with mortality in those with CKD 3, 4. There was a graded association between reduced eGFR and cardiovascular events (CVEs), which was the leading cause of mortality in patients with CKD 1. Patients with advanced CKD, especially those undergoing maintenance hemodialysis, had a high prevalence of protein‐energy malnutrition and inflammation 5. Chronic inflammation had also been known as a risk factor for CVEs 6. As these two conditions, malnutrition and inflammation, usually occurred concomitantly in those with advanced CKD, they had been referred together as malnutrition‐inflammation and atherosclerosis (MIA) syndrome 5.
There was a graded association between CKD stages and incidence of MIA syndrome. There were 7.9% and 30% of uremic patients with malnutrition and chronic inflammation, whereas 14.2%–22% of them suffered from CVEs 7. Several etiologies might lead to malnutrition, such as inflammation, gastropathy, medication, depressive mood, and decreased synthesis of glucose and amino acid. Besides, elevated serum leptin and hemodialysis related factors, such as inadequacy of dialysis and postdialysis fatigue, might result in malnutrition 5. Potential causes of inflammation included cytokine, acidosis, oxidative stress, and hemodialysis related factors, such as access infection, bio‐incompatibility of dialyzer, and dialysate contamination 5.
Beta 2 microglobulin (B2M) is a middle molecule with a molecular weight 11,800 Da 8. It is present on the surface of human nucleated cell and forms part of the major histocompatibility complex class (MHC) I family 9. Elevated B2M had been observed in malignancy, renal, immunodeficiency, and autoimmune disease 8, 9, 10. B2M was associated with cardiac performance and mortality in those with CKD and those on maintenance hemodialysis 11, 12, 13. It was also a thrombotic marker for peripheral artery occlusive disease 14. However, the association between B2M and MIA syndromes was not well investigated. The aim of the study is to evaluate whether serum B2M is superior to serum creatinine (SCr) in predicting MIA syndrome and mortality or not. We analyzed multiple risk factors and comorbid conditions at baseline, including serum B2M levels. The impacts of those factors on mortality in those undergoing dialysis were followed over 7 years.
Materials and Methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki (2000) of the World Medical Association, and the protocol was approved by the institutional review board of Taoyuan General Hospital (TYGH105009) and waived the need for informed consent, because there was no breach of privacy or interference with decision‐making processes related to patient care. This study was investigated in a single center, and all patients in the study were directly diagnosed and followed up at Taoyuan General Hospital.
Materials
This is a retrospective observational cohort study that included 707 adult patients who were followed in Taoyuan General Hospital from January of 2009 to December of 2015. We reviewed clinical characteristics and biochemical data from first time of presentation to nephrologists to the end of the cohort time. According to previous guideline, laboratory values, such as hemoglobin, white blood cell (WBC) count, blood urine nitrogen (BUN), creatinine (SCr), albumin, sodium, potassium, calcium, phosphate, uric acid, total cholesterol, triglyceride, Low density lipoprotein‐cholesterol (LDL‐c) were measured quarterly in subjects with CKD stage 3 and 4, whereas laboratory values were measured monthly when subjects progressed to CKD stage 5 15. Glycosylated hemoglobin was also measured every 3 months, especially in those with diabetes. There is no current consensus on frequency of C‐reactive protein (CRP) follow‐up. However, some authors recommended repeat CRP measures from three times in 2 years to annually; thus we reviewed those with CRP measures annually 16. Baseline and follow‐up eGFR were estimated according to the equation Modification Diet Renal Disease: Estimated GFR = 186 × serum creatinine−1.154 × age−0.203 × 1.21 (if black) × 0.742 (if female) 15. Those referred at early stage (CKD stage <3) (n = 76), whose length of follow‐up was less than 1 year (n = 115), lacked demographic and biochemical data (n = 98), and were lost to follow‐up during cohort time (n = 5) were excluded. Because malignancy and autoimmune disease might confound the serum B2M levels, those with history of cancer (n = 16) or autoimmune disease (n = 14) were also excluded 8, 9, 10. To assess the incidence of MIA syndrome, we also excluded those with any component of MIA syndrome, including history of CVEs, serum albumin <3.5 g/dL, and CRP >1.0 mg/dL, at baseline (n = 71). (Fig. 1) Consequently, a total of 312 eligible patients were included for analysis.
Figure 1.
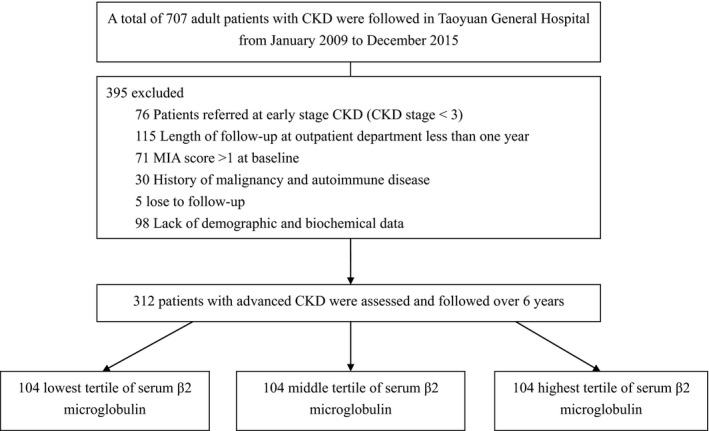
Data flow diagram.
Methods
Demographic data, comorbidity, clinical characteristics, and serial modifiable biochemical data were recorded and shown in Table 1. All cause mortality, CVEs, and start of dialysis were recorded. According to tertiles of baseline B2M, the study subjects were stratified as followed: lowest tertile: <4.08 mg/dL; middle tertile: 4.08–5.65 mg/dL; highest tertile: >5.65 mg/dL. Diagnosed comorbidities were also recorded at referral, such as diabetes and hypertension. Dialysis was started when subjects suffered from either hyperkalemia with arrhythmia, refractory metabolic acidosis, fluid overload with respiratory distress azotemia (blood urine nitrogen >100 mg/dL) or uremic symptoms, such as uremic bleeding and uremic encephalopathy. Cardiovascular events, including coronary artery disease, congestive heart failure class III or IV, and cerebrovascular accident 17, were documented or defined according to coronary angiography or history of myocardial infarction, functional classification of New York Heart Association (NYHA), and history of neurologic deficit with computerized tomography (CT) scan or magnetic resonance image (MRI) of brain. Malnutrition was defined as serum albumin <3.0 g/dL measured by bromocresol purple (BCP) assay, whereas inflammation was defined as serum CRP >1.0 mg/dL 18. CRP was measured by latex assay (AU system; Beckman Coulter Taiwan Inc, Taipei, Taiwan), whereas serum albumin was determined using bromocresol green (BCG)assay (AU640; Beckman Coulter Taiwan Inc, Taipei, Taiwan). Serum creatinine values were measured by an enzymatic IDMS traceable method (AU system; Beckman). According to previous definition of malnutrition, we converted albumin values determined by bromocresol green (BCG) assay to albumin values determined by bromocresol purple (BCP) assay based on following equation: AlbBCG (g/dL) = 0.72 + 0.98 × AlbBCP (g/dL) 19. All laboratory measures were performed by laboratory department in Taoyuan General Hospital using standardized and automated methods. Serum B2M was measured by microparticle enzyme immunoassay (Abbott AXsym, Wiesbaden, Germany; reference range: 0.97–1.84 mg/dL). The occurrence of components of MIA syndrome was evaluated annually. We also assessed the associations among B2M levels and outcomes in different subgroup, including elderly (Age ≥ 65) or nonelderly (Age < 65), those with or without diabetes, and those with or without CKD stage‐to‐stage progression. Compared to baseline CKD stage, those without stage‐to‐stage progression in the cohort time was regarded as non‐CKD progression.
Table 1.
Baseline patients' characteristics stratified by tertiles of serum beta2 microglobulin
| Parameters at referral | Lowest tertile (n = 104) | Middle tertile (n = 104) | Highest tertile (n = 104) | P value |
|---|---|---|---|---|
| Age (year) | 70 (58–82) | 72 (62.5–82) | 71 (56.5–84) | 0.310 |
| Male (%) | 75 (72.8) | 65 (62.5) | 53 (50.5) | 0.004a |
| BMI (kg/m2) | 26.5 (22.4‐29.0) | 26.1 (22.5‐29.5) | 25.6 (22.5‐28.6) | 0.226 |
| Length of follow‐up (m) | 42 (25–58) | 39.5 (24–54) | 33.2 (13.5–51) | 0.001a |
| eGFR, MDRD4 (ml/min/1.73 m2) | 43.5 ± 5.8 | 27.6 ± 5.1 | 14.0 ± 5.5 | <0.001a |
| Hemoglobin (g/dL) | 12.8 ± 2.2 | 11.2 ± 1.8 | 9.9 ± 1.8 | <0.001a |
| WBC count (×103/μL) | 6.9 ± 2.5 | 6.9 ± 2.5 | 6.8 ± 2.9 | 0.937 |
| BUN (mg/dL) | 26.4 ± 11.6 | 38.3 ± 15.1 | 57.2 ± 26.0 | <0.001a |
| Creatinine (mg/dL)+ | 1.6 (1.3–1.8) | 2.3 (1.9–2.7) | 4.7 (2.3–6.1) | <0.001a |
| Albumin (BCG) (g/dL) | 4.2 (3.9–4.5) | 4.2 (3.8–4.5) | 4.1 (3.6–4.4) | 0.018a |
| Sodium (mEq/L) | 139.3 ± 2.5 | 138.6 ± 2.6 | 136.0 ± 16.5 | 0.364 |
| Potassium (mEq/L) | 4.4 ± 0.6 | 4.5 ± 0.6 | 4.5 ± 0.8 | 0.190 |
| Calcium (mg/dL) | 9.3 ± 0.5 | 9.2 ± 0.5 | 8.9 ± 0.7 | <0.001a |
| Phosphate (mg/dL) | 3.8 ± 0.7 | 3.9 ± 0.6 | 4.7 ± 1.2 | <0.001a |
| Uric acid (mg/dL) | 8.0 (6.6–9.2) | 8.4 (7.1–9.7) | 8.4 (6.9–9.9) | 0.103 |
| Cholesterol (mg/dL) | 199.9 ± 46.0 | 195.5 ± 48.8 | 188.4 ± 46.4 | 0.206 |
| Triglyceride (mg/dL) | 157.3 ± 131.5 | 173.8 ± 110.9 | 154.4 ± 97.4 | 0.422 |
| LDL‐C (mg/dL) | 113.7 ± 17.7 | 111.6 ± 19.5 | 107.57 ± 19.8 | 0.059 |
| B2M (mg/dL) | 3.8 (2.7–3.9) | 4.7 (4.2–5.3) | 7.7 (5.2–10.3) | <0.001a |
| C‐reactive protein (mg/dL) | 0.51 (0.20–0.70) | 0.54 (0.37–0.71) | 0.50 (0.31–0.71) | 0.226 |
| HbA1c (%) | 6.5 (5.2–7.8) | 6.6 (5.0–8.2) | 6.5 (5.0–7.8) | 0.361 |
| Underlying disease | ||||
| DM | 48 | 52 | 40 | 0.204 |
| Hypertension | 53 | 67 | 51 | 0.050 |
| Outcomes during cohort time | ||||
| Coronary artery disease | 1 | 6 | 10 | 0.022a |
| Cerebrovascular accident | 0 | 1 | 3 | 0.170 |
| Congestive heart failure, NYHA III or IV | 2 | 2 | 3 | 0.873 |
| Mortality | 5 | 9 | 15 | 0.056 |
| Commencing dialysis | 1 | 2 | 4 | 0.371 |
| MIA syndrome | 3 | 5 | 14 | 0.006a |
BMI, body mass index; eGFR, estimated glomerular filtration rate; MDRD4, equation of Modification Diet Renal Disease, 4 parameters; BUN, blood urine nitrogen; WBC, white blood cell; LDL‐C, low density lipoprotein‐cholesterol; B2M, beta2 microglobulin; HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; MIA, malnutrition, inflammation, and atherosclerosis.
P < 0.05, Data were percentage, mean ± standard deviation, median (interquartile range (IQR)).
Statistical analysis
Continuous variables with a normal distribution were summarized as mean ± SD unless otherwise stated. Variables with a non‐normal distribution are expressed as a median (interquartile range (IQR)). Pearson's Chi‐square, Mann–Whitney U, one‐way analysis of variance (ANOVA) or Kruskal–Wallis test are used to determine the differences in the demographic data, the laboratory variables, and clinical characteristics among different tertiles of baseline B2M. A multivariate regression model was used to relate a number of baseline variables to baseline B2M levels. Baseline variables in the model included age, gender, and independent variables with α < 0.05, such as hemoglobin, albumin, CRP, serum calcium and phosphate. The all cause mortality, initiation of dialysis, any component of MIA syndrome, including malnutrition, inflammation, and CVEs were recorded and calculated using Kaplan–Meier method and a log‐rank test. The adjusted hazard ratios (AHRs) and 95% confidence intervals (CIs) for all cause mortality, initiation of dialysis, any component of MIA syndrome was calculated using Cox proportional hazard model after adjusted for age, gender, BMI, underlying disease (diabetes, hypertension), eGFR, serum albumin, hemoglobin, uric acid, HbA1c, C‐reactive protein, LDL‐cholesterol, serum calcium, and phosphate at baseline. We chose “Enter” method for multivariate adjustment; thus, all variables were entered together. The discriminative ability of the baseline B2M and SCr to predict mortality, initiation of dialysis, MIA syndrome was determined using the area under the curve for the receiver operating characteristic (AUROC) curve. All statistical analyses were performed using PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) and a two‐tailed P value of <0.05 was considered statistically significant.
Results
In total, 312 patients with advanced CKD were enrolled between January 2009 and December 2015. The cohort of the patients was followed up for a mean of 39.7 months since first referral to nephrologists’ outpatient department. None of these patients received organ transplantation or were diagnosed with human immunodeficiency virus (HIV) infection. The mean age of the patients was 70.7 years, with 193 (61.9%) males and 119 (38.1%) females. Most of the patients were referred with hypertension (54.8%) and diabetes (44.9%). More than 45% of the subjects were referred at CKD stage 3 (n = 142), whereas 36.2% and 18.3% were referred at stage 4 and stage 5 (n = 113 and 57). The median B2M level was 5.43 mg/dL and subjects were stratified by B2M tertiles (n = 104 in all subgroups). Distributions of B2M levels were shown in Figure 2. Hemodialysis was started when subjects suffered from hyperkalemia with arrhythmia (n = 4), refractory metabolic acidosis (n = 1), and fluid overload with respiratory distress (n = 2). The mortality rate at the end of the 6‐year study period was 9.3% (n = 29), including seven subjects (2.24%) who died within 3 months after commencing dialysis. The mean time to mortality was 43.6 months. There were 180 subjects (57.7%) without any component of MIA syndrome during cohort time (MIA score = 0), whereas 22 subjects (7.1%) developed all components of MIA syndrome (MIA score = 3). The rate for death or dialysis in those with none, one, two, and three components of MIA syndrome were 1.7%, 8.7%, 33.4%, and 77.3%, respectively.
Figure 2.
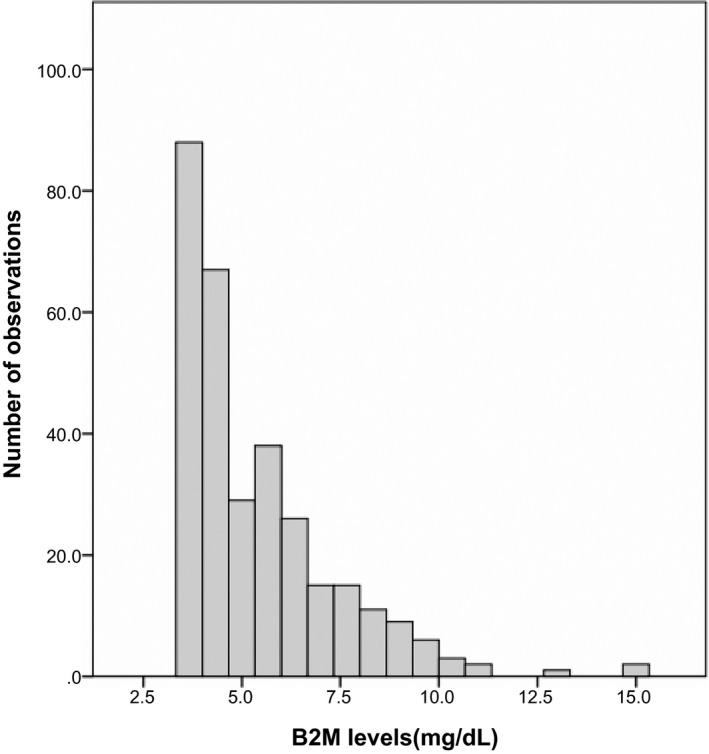
Distribution of B2M in study population. Notes: the mean ± standard deviation of B2M levels in all subjects.
Table 1 shows the comparisons among subjects with different tertiles of B2M levels. Compared to the lowest tertile group, those with highest tertile of B2M levels had higher serum phosphate, SCr, and greater incidence of MIA syndrome (3.8 vs. 4.7 mg/dL; 1.6 vs. 4.7 mg/dL, and 2.9% vs. 13.4%, all Ps <0.05). Besides, lower serum albumin, hemoglobin, and eGFR were also found in highest tertile group (4.2 vs. 4.1 g/dL; 12.8 vs. 9.9 g/dL and 43.5 mL/min/1.73 m2 vs. 14.0 mL/min/1.73 m2, all Ps <0.05). The incidence of coronary artery disease in high tertile group was also higher than that in those with lowest B2M levels (9.5% vs. 0.9%, P = 0.025). The proportion of male patients was greater in the lowest tertile group than that in the highest tertile group (72.8% vs. 50.5%, P = 0.004). There was no difference among tertiles of B2M levels in age, body mass index (BMI), serum sodium, potassium, uric acid, lipid profile, CRP, WBC count, and underlying disease (Table 1). Table 2 shows the association of various factors with baseline serum B2M in a multivariate regression model. Serum B2M was positively correlated with SCr, age, and CRP. Besides, there was a trendy inverse correlation between B2M and serum albumin, as well as hemoglobin.
Table 2.
Multivariable regressions of B2M at baseline
| Variables | Regression coefficient (mg/L) | Standard error | P value |
|---|---|---|---|
| Age (per year) | 0.016 | 0.003 | <0.001a |
| Gender (male) | ‐0.139 | 0.169 | 0.410 |
| Hemoglobin (per mg/dL) | ‐0.022 | 0.016 | 0.174 |
| Calcium (per mmol/L) | 0.039 | 0.058 | 0.670 |
| Phosphate (per mg/dL) | 0.005 | 0.035 | 0.149 |
| Albumin (per g/dL) | ‐0.100 | 0.083 | 0.129 |
| C‐reactive protein (per mg/dL) | 0.397 | 0.192 | 0.039a |
| Creatinine (per mg/dL) | 1.069 | 0.018 | <0.001a |
P < 0.05.
In multivariate analysis for continuous variables using Cox proportional hazards model, the age and serum B2M were significant predictors for death, death or dialysis, and CVEs. The risk for death, death or dialysis, and CVEs increased 5.1%, 4.1%, and 2.2% for every 1‐year increase in age (P = 0.029, 0.038, and 0.303), whereas a 0.1 unit increase in nature logarithms of B2M (Ln B2M) produced a significant increase (36.3%, 25.8%, and 35.0%) in death, death or dialysis, and CVEs (all Ps <0.05). In univariate analysis, subjects with the highest tertile of B2M had strong association with death, death or dialysis, and CVEs (Hazard ratio (HR) and 95% confidence intervals (CIs): 3.13 (1.14–8.63), 3.19 (1.27–8.00), and 3.89 (1.29–11.73), all Ps <0.05). After multivariate adjustment, the associations among tertiles of B2M, death, death or dialysis, and CVEs remained significant. Compared to the lowest tertile group, the adjusted hazard ratios (AHRs) and 95% CIs for the highest tertile group in death, death or dialysis, and CVEs were 21.52 (2.06–225.05), 25.91 (3.63–184.58), and 65.84 (6.33–684.26). (all Ps <0.05) (Table 3). The associations among tertiles of B2M, CVEs, and MIA syndrome in subgroups are also shown in Table 4. Those with highest tertile of B2M were associated with an increased risk for CVEs and MIA syndromes, especially in those older than 65 years old, those with CKD progression, and those with or without diabetes. There was a trendy association between cardiovascular outcomes and mortality in those without stage‐to‐stage progression (Table 4). Compared to the lowest tertile group, the highest tertile of B2M levels remained a significant predictor for occurrence of MIA syndrome. For occurrence of one, two, and three component of MIA (MIA score = 1,2 and 3), the AHR (95% CIs) were 6.86 (2.33–20.14), 20.30 (1.67–124.29), and 152.50 (9.96–2335.20) (all Ps<0.05).
Table 3.
Multivariate Cox regression analysis of tertiles of B2M for mortality, dialysis, and MIA syndrome
| β coefficient | SE | Hazard ratio | 95% CI | P value | |
|---|---|---|---|---|---|
| All cause mortality (n = 29) | |||||
| Lowest tertile | – | – | 1 (reference) | 1 (reference) | (reference) |
| Middle tertile | 1.64 | 0.86 | 5.18 | 0.95–28.23 | 0.057 |
| Highest tertile | 3.06 | 1.20 | 21.52 | 2.06–225.05 | 0.010a |
| Commencing dialysis (n = 7) | |||||
| Lowest tertile | – | – | 1 (reference) | 1 (reference) | (reference) |
| Middle tertile | 2.30 | 1.86 | 9.90 | 0.26–385.90 | 0.217 |
| Highest tertile | 5.00 | 2.44 | 149.60 | 1.25–1786.63 | 0.004a |
| Commencing dialysis or mortality (n = 35) | |||||
| Lowest tertile | – | – | 1 (reference) | 1 (reference) | (reference) |
| Middle tertile | 1.75 | 0.73 | 5.78 | 1.38–24.27 | 0.016a |
| Highest tertile | 3.25 | 1.00 | 25.91 | 3.63–184.58 | <0.001a |
| Cardiovascular event (n = 27) | |||||
| Lowest tertile | – | – | 1 (reference) | 1 (reference) | (reference) |
| Middle tertile | 2.19 | 0.84 | 8.93 | 1.61–49.31 | 0.012a |
| Highest tertile | 4.36 | 1.17 | 65.84 | 6.33–684.26 | <0.001a |
| MIA score = 3 (n = 22) | |||||
| Lowest tertile | – | – | 1 (reference) | 1 (reference) | (reference) |
| Middle tertile | 2.48 | 1.04 | 11.94 | 1.54–92.29 | 0.017a |
| Highest tertile | 5.03 | 1.39 | 152.50 | 9.96–2335.20 | <0.001a |
B2M, β2 microglobulin; MIA, malnutrition, inflammation, and atherosclerosis; SE, standard error.
Adjusted for age, gender, BMI, underlying disease (DM, hypertension), eGFR, serum albumin, hemoglobin, uric acid, HbA1c, C‐reactive protein, LDL‐cholesterol, calcium, and phosphate at baseline.
P < 0.05.
Table 4.
Associations among tertiles of B2M, cardiovascular events, and MIA syndrome in different subgroup
| Parameter | Lowest tertile | Middle tertile | Highest tertile | |||||
|---|---|---|---|---|---|---|---|---|
| AHR | 95%CI | AHR | 95%CI | P value | AHR | 95%CI | P value | |
| Cardiovascular events (n = 13/14) | ||||||||
| Without stage‐to‐stage progression | 1.0 | –(reference) | 2.81 | 0.23–34.00 | 0.415 | 26.23 | 0. 97–866.33 | 0.057 |
| With stage‐to‐stage progression | 1.0 | –(reference) | 282.26 | 4.34–1832.03 | 0.008a | 445.50 | 17.41–1136.80 | 0.003a |
| Cardiovascular events (n = 7/20) | ||||||||
| Age < 65 | 1.0 | –(reference) | 6.03 | 0.24–13.77 | 0.171 | 27.33 | 0.72–87.08 | 0.071 |
| Age ≥ 65 | 1.0 | –(reference) | 4.82 | 0.72–32.23 | 0.105 | 47.31 | 3.39–658.54 | 0.004a |
| Cardiovascular events (n = 10/17) | ||||||||
| Without DM | 1.0 | – (reference) | 45.63 | 1.53–1358.58 | 0.027a | 345.30 | 17.47–7706.14 | 0.003a |
| With DM | 1.0 | –(reference) | 8.64 | 0.88–84.51 | 0.064 | 41.65 | 1.756–987.76 | 0.021a |
| MIA score = 3 (n = 10/12) | ||||||||
| Without stage‐to‐stage progression | 1.0 | –(reference) | 1.65 | 0.37–5.67 | 0.967 | 27.14 | 0.37–1969.99 | 0.131 |
| With stage‐to‐stage progression | 1.0 | –(reference) | 127.48 | 2.09–776.31 | 0.021a | 315.69 | 11.776–8530.16 | 0.005a |
| MIA score = 3 (n = 4/18) | ||||||||
| Age <65 | 1.0 | –(reference) | 6.03 | 0.24–13.77 | 0.171 | 27.33 | 0.72–87.08 | 0.071 |
| Age ≥65 | 1.0 | –(reference) | 6.15 | 0.66–57.38 | 0.111 | 100.01 | 5.56–1797.34 | 0.002a |
| MIA score = 3 (n = 9/13) | ||||||||
| Without DM | 1.0 | –(reference) | 126.39 | 1.91–8354.85 | 0.024 | 807.69 | 21.17–3081.23 | 0.003a |
| With DM | 1.0 | –(reference) | 5.61 | 0.36–87.69 | 0.219 | 55.30 | 1.24–2450.05 | 0.038a |
P < 0.05
Cumulative survival curves over 6 years according to tertiles of B2M using Kaplan–Meier method for death, death or dialysis, MIA syndrome, and CVEs were evaluated. Figure 3 shows Kaplan–Meier curves for cumulative risk of MIA syndrome among different tertile of B2M levels (Ps <0.05). The discriminative power of serum B2M and SCr for death, death or dialysis, CVEs and MIA syndrome were also calculated. The discriminative power (AUROC) for MIA syndrome between B2M and SCr levels are shown in Figure 4. Serum B2M was superior to SCr in predicting occurrence of death, death or dialysis, and MIA syndrome. The discriminative power for death, death or dialysis, CVEs, and MIA syndrome in B2M and SCr were as follows: death: 0.627 (0.522–0.732) vs. 0.570 (0.463–0.677); death or dialysis: 0.614 (0.516–0.712) vs. 0.564 (0.465–0.664); CVEs: 0.684 (0.582–0.753) vs. 0.563 (0.460–0.667); MIA score = 1:0.600 (0.537–0.662) vs. 0.558 (0.495–0.662); MIA score = 2:0.593 (0.489–0.698) vs. 0.530 (0.432–0.627); and MIA score = 3: 0.703 (0.587–0.780) vs. 0.582 (0.478–0.687) (Ps <0.05 only in B2M for death, death or dialysis, CVEs and those with one or three components of MIA syndromes). (Ps <0.05 only in death, death or dialysis, CVEs and those with one or three components of MIA syndromes). Based on Youden index, the best cut‐off values of B2M for predicting death or dialysis, CVEs, and MIA syndrome were 5.39 mg/dL (sensitivity: 67.1%, specificity 62.5%), 4.21 mg/dL (sensitivity: 85.1%, specificity 52.1%), and 5.40 mg/dL (sensitivity: 79.7%, specificity 64.1%).
Figure 3.
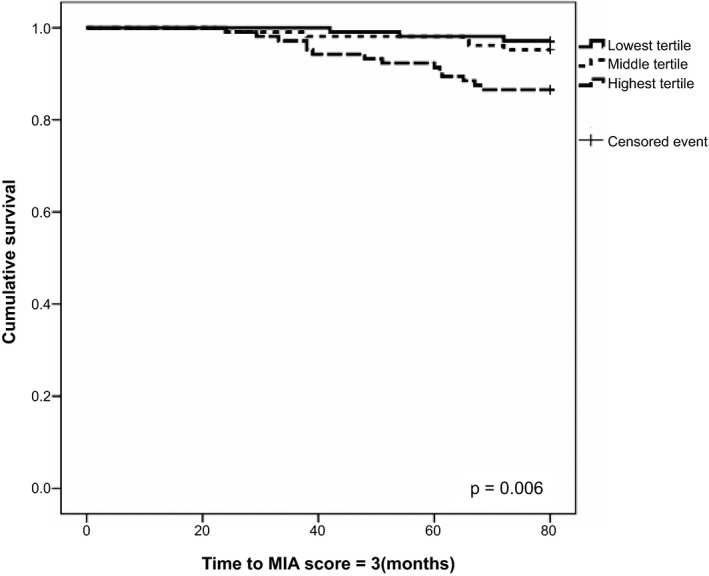
Cumulative survival curves for occurrence of MIA syndrome stratified by B2M tertiles. P < 0.05.
Figure 4.
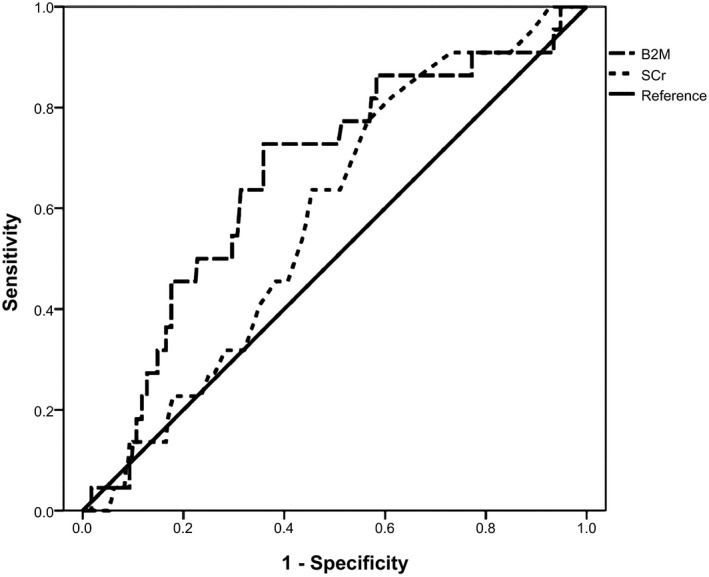
The discriminative power for occurrence of MIA syndrome in B2M and SCr. Ps < 0.05.
Discussion
This retrospective observational cohort study demonstrated not only advanced age, but also high serum B2M associated with death, death or dialysis, and CVEs in those with CKD. Serum B2M was more sensitive than SCr in predicting advanced CVEs and MIA syndrome, after adjusting for age, gender, BMI, eGFR, serum albumin, hemoglobin, uric acid, HbA1c, C‐reactive protein, LDL‐cholesterol, calcium, phosphate as well as underlying disease at baseline. There was a graded association between the number of components of MIA syndrome and death or dialysis.
Some studies had demonstrated the association between B2M and mortality as well as CVEs in those with CKD 7, 11, 13, 14, 20. Nevertheless, the association between serum B2M and MIA syndrome had not been well investigated. As previous studies, B2M was associated with stroke, cardiac performance, and CVEs 10, 12, 20. Besides, B2M was also associated with inflammation 10, which played an important role in pathophysiology of MIA syndrome 5. B2M was a part of the MHC class I and an initiator of inflammatory response that trigger inflammatory process 21. It was also reported to be a marker after reperfusion injury in peripheral arterial disease and affected endothelial progenitor cell in those with vascular injury 14, 22. Moreover, B2M was associated with heart valve calcifications in those under dialysis 23. Not surprisingly, Serum B2M can predict the occurrence of MIA syndrome. Most of the studies demonstrated the association among malnutrition, inflammation, and atherosclerosis syndromes cross‐sectionally because CRP measurements were not repeated regularly 7, 17, 24. We assessed CRP and components of MIA syndrome annually in CKD subjects as per some authors’ recommendation 16. In subjects with CKD, we demonstrated that the high baseline serum B2M was associated with death, death or dialysis, and CVEs, as well as the occurrence of MIA syndrome. Those with higher serum B2M had higher incidence of MIA syndrome, either in those with or without diabetes (Table 4).
The reported proportions of each component of MIA syndrome in those with CKD were as followed: malnutrition: 7.9%–44%; inflammation: 30%–50%, and atherosclerosis: 14.2%–72% 7, 24, 25. Moreover, the prevalence of none, one, two, and three components of MIA syndrome were 22%–32%, 29%–44%, 20.7%–27%, and 2.3%–22% 17, 24. However, the prevalence of components of MIA syndrome was lower in our study (57.7%, 29.5%, 5.8%, and 7.1%). One of the explanations is the method of CRP measurements. Latex assay for CRP is less sensitive to latex‐enhanced immunoturbidimetric or particle‐enhanced assay for high sensitive‐CRP (hs‐CRP); thus, the incidence of MIA syndrome in the study might be underestimated. There were several biomarkers to define inflammation, such as interleukin‐6 (IL‐6), and tumor necrosis factor α (TNFα) 5, 18. The utilization of more than one biomarker for inflammation might increase the sensitivity for inflammation. Besides, the definition of atherosclerosis was different in these studies 17, 24. Some authors defined atherosclerotic CVEs including asymptomatic carotid artery plaque and intimal hyperplasia; thus, the prevalence of atherosclerosis ranged from 14.2% to 72% 24. Despite the predictive value of B2M in peripheral arterial disease, some authors did not agree with it 26, 27. This may be the reason as to why the B2M was less sensitive to predict MIA syndrome and death or dialysis.
Serum B2M had been known to be an inverse association with eGFR. Nevertheless, SCr‐based eGFR was less predictive for MIA syndrome, death, death or dialysis than serum B2M in the study. One of the explanations was eGFR levels did not appear to influence the serum CRP levels, especially in those with early stage CKD 28. Serum CRP was independently associated with albumin, CVEs, and B2M 28, 29, 30. As previous study, the discriminative power of B2M for death was superior to CRP and renal function 30.
There were several limitations in this study. First, it is an observational, rather than an interventional study and is within one medical center; thus, the result might not be extrapolated into other area or population. Second, the exclusion of those lost to follow‐up and those without demographic and biochemical data may lead to selection bias. Third, we did not evaluate the strategies for anti‐inflammation, such as diet modification, exercise, smoking cessation, anti‐inflammatory medicine ,and treatment of comorbidity. For example, treatment of periodontal disease and medicine, such as statin, angiotensin‐converting enzyme inhibitors, sevelamer, pentoxifylline, and N‐acetylcysteine might be anti‐inflammatory 16. Finally, only single baseline measurement of B2M might be inadequate; therefore, repeat B2M measurements may provide more information. Nevertheless, B2M was strongly correlated with SCr and good for GFR estimation at steady state 31, 32. SCr was measured quarterly during cohort time in nonacute setting; thus, single measurement of baseline B2M might provide some prognostic information as previous study 11.
In summary, not only advanced age, but also high serum B2M levels in CKD subjects is associated with death, death or dialysis, CVEs, and occurrence of MIA syndrome after adjusting for baseline demographic and biochemical data. B2M was more sensitive than SCr in predicting CVEs and MIA syndrome. Serum B2M is positively correlated with CRP and SCr. There is a trendy inverse correlation between B2 m and serum albumin as well as hemoglobin. Optimal interventions to reduce chronic inflammation in CKD subjects, such as anti‐inflammatory medicine, diet and lifestyle modifications, might be important. This study was limited by the retrospective nature and sample size. A multicenter observational cohort study is warranted to confirm the predictive value of B2M for occurrence of MIA syndrome.
Conflict of interest
The authors report no conflicts of interest.
Special thanks to Dr. Lin‐Chien Lee in statistical analysis.
Contributor Information
Hung‐Chieh Wu, Email: torsade1@yahoo.com.tw.
Wei‐Jie Wang, Email: whitakerwang@gmail.com.
References
- 1. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004;351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 2. Wen CP, Cheng TY, Tsai MK, et al. All‐cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462,293 adults in Taiwan. Lancet 2008;371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 3. de Boer IH, Katz R, Cao JJ, et al. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care 2009;32:1833–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis 2009;54:205–226. [DOI] [PubMed] [Google Scholar]
- 5. Rao P, Reddy GC, Kanagasabapathy AS. Malnutrition‐inflammation‐atherosclerosis syndrome in Chronic Kidney disease. Indian J Clin Biochem 2008;23:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107:499–511. [DOI] [PubMed] [Google Scholar]
- 7. Beddhu S, Pappas LM, Ramkumar N, Samore MH. Malnutrition and atherosclerosis in dialysis patients. J Am Soc Nephrol 2004;15:733–742. [DOI] [PubMed] [Google Scholar]
- 8. Winchester JF, Salsberg JA, Levin NW. Beta‐2 microglobulin in ESRD: an in‐depth review. Adv Ren Replace Ther 2003;10:279–309. [DOI] [PubMed] [Google Scholar]
- 9. Chitra P, Bakthavatsalam B, Palvannan T. Beta‐2 microglobulin as an immunological marker to assess the progression of human immunodeficiency virus infected patients on highly active antiretroviral therapy. Clin Chim Acta 2011;412:1151–1154. [DOI] [PubMed] [Google Scholar]
- 10. Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta‐2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int 2012;82:1297–1303. [DOI] [PubMed] [Google Scholar]
- 11. Cheung CL, Lam KS, Cheung BM. Serum β‐2 microglobulin predicts mortality in people with diabetes. Eur J Endocrinol 2013;169:1–7. [DOI] [PubMed] [Google Scholar]
- 12. Sedighi O, Abediankenari S, Omranifar B. Association between plasma Beta‐2 microglobulin level and cardiac performance in patients with chronic kidney disease. Nephro Urol Mon 2014;7:e23563. doi: 10.5812/numonthly.23563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okuno S, Ishimura E, Kohno K, et al. Serum beta2‐microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant 2009;24:571–577. [DOI] [PubMed] [Google Scholar]
- 14. Wilson AM, Kimura E, Harada RK, et al. Beta2‐microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation 2007;116:1396–1403. [DOI] [PubMed] [Google Scholar]
- 15. Disease Kidney. Improving Global Outcomes (KDIGO): KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 2013;3:1–150. [DOI] [PubMed] [Google Scholar]
- 16. Cobo G, Qureshi AR, Lindholm B, Stenvinkel P. C‐reactive protein: repeated measurements will improve dialysis patient care. Semin Dial 2016;29:7–14. [DOI] [PubMed] [Google Scholar]
- 17. Hung SC, Kuo KL, Peng CH, et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 2014;85:703–709. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Coresh J, Eustace JA, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA 2004;291:451–459. [DOI] [PubMed] [Google Scholar]
- 19. Clase CM, St Pierre MW, Churchill DN. Conversion between bromcresol green and bromcresol purple‐measured albumin in renal disease. Nephrol Dial Transplant 2001;16:1925–1929. [DOI] [PubMed] [Google Scholar]
- 20. Amighi J, Hoke M, Mlekusch W, et al. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke 2011;42:1826–1833. [DOI] [PubMed] [Google Scholar]
- 21. Chen NX, O'Neill KD, Niwa T, Moe SM. Signal transduction of beta2 m‐induced expression of VCAM‐1 and COX‐2 in synovial fibroblasts. Kidney Int 2002;61:414–424. [DOI] [PubMed] [Google Scholar]
- 22. Jourde‐Chiche N, Dou L, Sabatier F, et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J Thromb Haemost 2009;7:1576–1584. [DOI] [PubMed] [Google Scholar]
- 23. Ikee R, Honda K, Oka M, et al. Association of heart valve calcification with malnutrition‐inflammation complex syndrome, beta‐microglobulin, and carotid intima media thickness in patients on hemodialysis. Ther Apher Dial 2008;12:464–468. [DOI] [PubMed] [Google Scholar]
- 24. Stenvinkel P, Heimbürger O, Paultre F, et al. Strong association between malnutrition, inflammation, and atherosclerosis in chronic renal failure. Kidney Int 1999;55:1899–1911. [DOI] [PubMed] [Google Scholar]
- 25. Pecoits‐Filho R, Lindholm B, Stenvinkel P. The malnutrition, inflammation, and atherosclerosis (MIA) syndrome – the heart of the matter. Nephrol Dial Transplant 2002;17(Suppl 11):28–31. [DOI] [PubMed] [Google Scholar]
- 26. Real de Asúa D, Puchades R, García‐Polo I, Suárez C. A study on the relationship between serum beta 2‐microglobulin levels, underlying chronic kidney disease, and peripheral arterial disease in high‐vascular‐risk patients. Int Cardiovasc Res J 2012;6:107–112. [PMC free article] [PubMed] [Google Scholar]
- 27. Kals J, Zagura M, Serg M, et al. β2‐Microglobulin, a novel biomarker of peripheral arterial disease, independently predicts aortic stiffness in these patients. Scand J Clin Lab Invest 2011;71:257–263. [DOI] [PubMed] [Google Scholar]
- 28. Menon V, Wang X, Greene T, et al. Relationship between C‐reactive protein, albumin, and cardiovascular disease in patients with chronic kidney disease. Am J Kidney Dis 2003;42:44–52. [DOI] [PubMed] [Google Scholar]
- 29. Shimoda M, Kaneto H, Yoshioka H, et al. Influence of atherosclerosis‐related risk factors on serum high‐sensitivity C‐reactive proteinlevels in patients with type 2 diabetes: Comparison of their influence in obese and non‐obese patients. J Diabetes Investig 2016;7:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shinkai S, Chaves PH, Fujiwara Y, et al. Beta2‐microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C‐reactive protein. Arch Intern Med 2008;168:200–206. [DOI] [PubMed] [Google Scholar]
- 31. Vilar E, Boltiador C, Wong J, et al. Plasma levels of middle molecules to estimate residual kidney function in haemodialysis without urine collection. PLoS ONE 2015;10:e0143813. doi: 10.1371/journal.pone.0143813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inker LA, Tighiouart H, Coresh J, et al. GFR estimation using β‐trace protein and β2‐microglobulin in CKD. Am J Kidney Dis 2016;67:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]


