Abstract
Background
The prevalence of coronary artery disease (CAD) is increasing globally, supporting the need for the identification of novel biomarkers. Therefore in the present study, we have explored the association of SIL2A, SIL6R, STNFRI, STNFRII, and MMP9 in CAD patients.
Methods
Twenty one patients with angiographically defined CAD with more than 50% occlusion, at least, in one coronary artery and twenty healthy subjects (n=20) without the history of cardiovascular symptoms were enrolled. Demographic and biochemical analysis (e.g. Total Cholesterol (TC), Triglyceride (TG), and HDL‐C) were measured in all the subjects. The level of cytokines receptor (SIL2A, SIL6R, SIL6R, STNFRI, STNFRII, and matrix metallopeptidase 9 (MMP9) were evaluated.
Results
Our results showed the higher level of MMP9 in patients group compared to the control subjects, while no significant differences were detected for other cytokines. In particular the level of MMP9 was significantly (P=.015) increased from 181.16 ng/mL (95%CI: 112.1‐199.2) to 192.0 ng/mL (95%CI: 181.5‐265.2). Moreover, the sensitivity and specificity of MMP9 were 95.45% and 45%, respectively, as detected by receiver operating characteristic (ROC) curves.
Conclusion
We demonstrate the significant correlation of MMP‐9 with CAD with sensitivity of 95.45%, suggesting its role as a biomarker in CAD patients. Further studies in larger population – preferably multicenter setting – are warranted to explore the functional role of this marker in coronary artery disease.
Keywords: coronary artery disease, inflammation, soluble cytokines
1. Introduction
Coronary artery disease (CAD) is a leading cause of death worldwide.1 The burden of CAD remains high and is still the main cause of death and morbidity.2 Inflammation plays an important role in CAD.3 It has been shown that cytokines stimulate the production of mediators such as endothelial adhesion molecules, proteases, which may be released in the blood stream in soluble form. Previous studies have been shown that adverse outcomes can be by elevated the circulating levels of cytokines and/or cytokine receptors in patients with heart failure.4 In particular, SIL2A, SIL6R, SIL6R, STNFRI, STNFRII, and MMP9 have been reported to be associated with CAD risk. Soluble interleukin receptors 2 and 6 (SIL2R, SIL6R) are released into the blood stream in response to the inflammation.5, 6
Matrix metalloproteinases are a large family of enzymes that are able to degrade components of extra cellular Matrix. MMPs also play an important role in proliferation, migration, differentiation, angiogenesis, and apoptosis.7 There is growing body of evidence showing the impact of MMP‐9 in inflammation and illustrating its role in the process of atherosclerosis and plaque rupture.8 It has been suggested that MMP‐9 is involved in plaque destabilization and rupture. It is also identified as a biomarker in ACS (acute coronary syndrome) and CAD patients.9 Accumulating evidence demonstrate a relationship between high level of cytokine receptors with CAD patients.3, 6, 10 However, several other studies have reported controversies.6, 10, 11 Therefore in the present study, we investigated the relationship between the serum concentrations of SIL2R, SIL6R, STNFRI, STNFRII, and MMP9 in patients with CAD.
2. Materials and Methods
2.1. Population
A total of 42 subjects were enrolled from Ghaem Educational Hospital, Mashhad, Iran. CAD patients were defined as angiographically defined coronary artery disease (CAD+) (≥50% occlusion in at least one coronary artery). Healthy subjects (n=20) referred for routine checkup or pre‐employment examination, and did not have a history of cardiovascular symptoms. This project approved by the Ethical Committee of Mashhad University of Medical Sciences.
2.2. Anthropometric measurements and biochemical analysis
Anthropometric parameters were determined as previously described.12 Total cholesterol, low density lipoprotein cholesterol, high density lipoprotein, cholesterol, and glucose were measured as described recently. The results of the fasting blood sugar (FBS) <110 mg/dL were interpreted using the American Diabetic Association criteria: normal values between 110 and 126 mg/dL, and those >126 mg/dL were considered as impaired fasting glucose (IFG) and DM, respectively.
2.3. Measurement of Cytokines
SIL2R, SIL6R, SIL6R, STNFRI, STNFRII, and MMP9 were evaluated using an Evidence Investigator analyzer (Randox Laboratories Ltd., Crumlin, Antrim, UK), according to the manufactures protocols.
2.4. Statistical analysis
Data analysis was carried out using SPSS software (SPSS Inc., Chicago, IL, USA). The Kolmogorov‐Smirnov test was used to assess normality data. Descriptive statistics (frequency, mean, and standard deviation) were determined for all variables. Data are reported as mean±SD for normally distributed variables or Median and IQR for nonparametric distribute variable. Baseline demographics and clinical characteristics were compared using student t‐test or Mann‐Whitney U‐test for normal and non‐normal continuous variables, respectively; followed by chi‐square test, and/or Fisher exact test for categorical variables. Regression modeling analyses was used. A ROC curve was generated to assess the utility of soluble cytokines concentrations. A level of P<.05 was considered as statistically significant (Figure 1).
Figure 1.
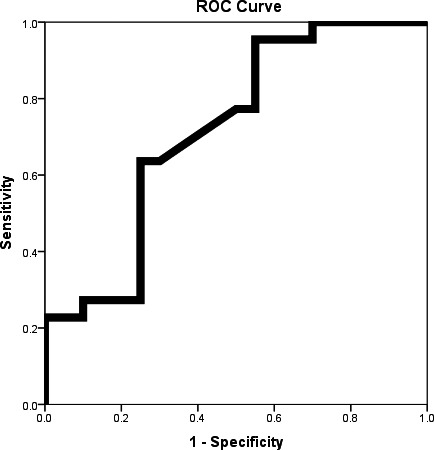
ROC Curve for MMP9 for diagnosis angiography positive subjects from normal subjects
3. Results
3.1. Clinical characteristics of population
The demographic and clinical characteristics of population are summarized in Table 1. Body mass index (BMI), waist circumference (WC), hip circumference (HC), total cholesterol (TC), triglyceride (TG), high‐density lipoprotein cholesterol (HDL‐C), Low‐density lipoprotein cholesterol (LDL‐C), blood pressure (BP) were not markedly different between patients and healthy group (P>.05; Table 1). FBS, hs‐CRP and height values were significantly (P<.05) different in CAD patient, compared to the control group (Table 1).
Table 1.
Characteristics characteristic of population
| Normal (n= 20) | CAD (n=22) | P‐value | |
|---|---|---|---|
| Age (year) (Mean±SD) | 47.4±9.1 | 56.9±11.1 | .005 |
| Sex (No. (%)) | |||
| Male | 4 (20) | 17 (77.3) | <.001 |
| Female | 16 (80) | 5 (22.7) | |
| Smoking (No. (%)) | |||
| No | 16 (80) | 8 (36.4) | .004 |
| Yes | 4 (20) | 14 (63.6) | |
| Height (cm) (Mean±SD) | 158.0±10.1 | 164.2±9.2 | .045 |
| Weight (kg) (Mean±SD) | 67.4±12.3 | 70.9±11.1 | .348 |
| BMI (kg/m2) (Mean±SD) | 27.1±5.1 | 26.3±4.2 | .616 |
| WC (cm) (Mean±SD) | 85.8±17.1 | 88.9±18.4 | .597 |
| HC (cm) (Mean±SD) | 90.0±18.9 | 90.8±18.1 | .902 |
| FBG (mg/dL) (Mean±SD) | 95.0±29.2 | 137.9±52.9 | .003 |
| TC (mg/dL) (Mean±SD) | 173.6±45.1 | 190.9±48.3 | .240 |
| TG (mg/dL) (Median (IQR)) | 124.0 (86.5‐161.2) | 131.5 (101.5‐164.2) | .562 |
| HSCRP (mg/dL) (Median (IQR)) | 4.8 (1.7‐9.6) | 12.9 (4.1‐56.3) | .032 |
| HDL‐C (mg/dL) (Mean±SD) | 51.0±10.9 | 48.1±9.0 | .351 |
| LDL‐C (mg/dL) (Mean±SD) | 102.4±32.0 | 110.3±37.4 | .467 |
| SBP (mmHg) (Mean±SD) | 133.5±22.9 | 134.6±21.7 | .865 |
| DBP (mmHg) (Mean±SD) | 78.2±11.6 | 82.6±12.5 | .247 |
BMI, Body mass index; WC, waist circumference; HC, hip circumference; FBS, fasting blood glucose; TC, total cholesterol; TG, triglyceride; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; IQR, interquartile range.
Student t‐test and Mann‐whitney U‐test were performed for normal and non‐normal continuous variables, respectively. Chi‐square test was performed for categorical variables.
3.2. Levels of soluble cytokines and MMP9
SIL2R, SIL6R, SIL6R, STNFRI, STNFRII, and MMP9 were measured in all the subjects. We observed that the level of serum MMP9 was higher in patient's group, compared to the control group (P<.05), (Table 2) while no statistically significant differences between groups for other cytokines were detected. Furthermore, the sensitivity and specificity of MMP9 were 95.45% and 45%, respectively (Table 3).
Table 2.
Comparison of serum cytokine receptors and MMP9
| Normal (n=20) | CAD (n=22) | P‐value | |
|---|---|---|---|
| SIL2R | 0.07 (0.06‐0.08) | 0.06 (0.0‐0.1) | .584 |
| SIL6R | 0.70 (0.4‐1.0) | 0.63 (0.4‐0.9) | .562 |
| STNFRI | 0.42 (0.2‐0.5) | 0.30 (0.2‐0.4) | .364 |
| STNFRII | 0.45 (0.2‐0.7) | 0.43 (0.2‐0.6) | .980 |
| MMP9 | 181.16 (112.07‐199.2) | 192.0 (181.5‐265.2) | .015 |
Mann‐whitney U‐test were performed for comparison of serum cytokine receptors in two groups.
Data are presented as median (interquartile range).
SIL2R: soluble IL‐2 receptor, SIL6R: soluble IL‐6 receptor, STNFRI: soluble TNF receptor I, STNFRII: soluble TNF receptor II.
Table 3.
Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of MMP9 as a biomarker in angiography positive patients
| Cytokine | Cut of point (pg/mL) | Sensitivity (CI 95%) | Specificity (CI 95%) | PPV (CI 95%) | NPV (CI 95%) | Area under curve | P‐value |
|---|---|---|---|---|---|---|---|
| MMP‐9 | 162.2 | 95.45% (75.1‐99.7) | 45% (0.2‐67.9) | 65% (46‐80) | 90% (54‐99) | 0.718 | .016 |
4. Discussion
To the best of our knowledge, this is the first study evaluating the association of the serum levels of sTNF‐R I, sTNF‐R II, sIL‐6‐R, sIL‐2‐R, and MMP‐9 in Iranian patients with CAD. Our results demonstrated that the level of MMP9 was significantly higher in CAD patient with respect to the control, which is in agreement with previous observations.10, 11, 13
Emerging evidence is showing the association of high serum sIL2r concentration and soluble interleukin receptors 2 with coronary artery disease.7, 10, 11 However, our finding demonstrated no significant differences in sIL2r level between the groups. Several other studies have shown that the level of IL‐6 was correlated with cardiovascular mortality and suggested its role as a potential prognostic marker for atherosclerotic disease,7, 10 although this marker was not associated with CAD in our population, supporting further studies in a larger population. Against this information, it has been suggested that TNF‐alpha plays an important role in the pathogenesis of atherosclerosis. TNF‐alpha binds to two cell surface receptors, sTNF‐R I and sTNF‐R II. Our results indicated that the serum level of TNF‐α, sTNFR1, and sTNFR2 reflect the impairment of cardiac and renal function in CAD patients.7, 10, 11, 12 However, we found no significant differences in the levels of sTNF‐R I and sTNF‐R II between angiogram positive and healthy subjects (Table 4).
Table 4.
Associations of MMP9 with CAD in present of covariates
| Dependent | Variables | Coefficient (β) | P‐value |
|---|---|---|---|
| MMP9 | Age | 1.00 | .495 |
| Sex | 46.4 | .238 | |
| Smoking | 7.7 | .807 | |
| Druga | 44.6 | .146 | |
| BMI | 2.5 | .428 | |
| Angio Positive | 98.4 | .021 | |
| Normalb | ‐ | ‐ |
Statin family drugs.
Reference category.
The matrix metalloproteinases (MMP), a large family of extracellular, act as endopeptidases.7 Konstantino and colleagues showed the role of MMP9 in plaque formation and rupture, and postulated that MMP9 levels may serve as a biomarker for acute coronary syndrome.10 Another study by Welsh and colleagues revealed an association between serum concentration of MMP9 and the incidence of coronary heart disease in the middle‐aged men.11 In addition, Ferroni et al.13 suggested that measuring the serum level of MMP9 might be used as a novel biomarker in subjects with CAD and might bring an index of plaque activity in clinic. Another study illustrated the importance of single‐nucleotide polymorphism of rs3918242 in the MMP‐9 gene with respect to CAD in the Chinese Han population.8 Mirsh and colleagues observed that the MMP9 R668Q genetic variant was significantly higher in reduced left ventricular ejection fraction in CAD patients.14 Since MMP‐9 is suggested to be involved with inflammation in atherosclerotic plaques, it may help in the evaluation of the severity of cardiovascular disease.15 Consistant with this investigation, we observed a significant association of MMP9 level with angiographically CAD positive group, compared to the healthy group. This study revealed MMP9 level in addition to serum cytokines levels16, 17 can be as the biomarker of CAD.
In conclusion, we demonstrate the significant association of MMP‐9 with CAD with sensitivity and specificity of 95.45% and 45%, respectively; supporting further investigations on evaluating the functional role of emerging markers in CAD patients.
Funding
This study was support by grant from Mashhad University of Medical Sciences.
References
- 1. Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148:7–15. [DOI] [PubMed] [Google Scholar]
- 2. Tardif JC. Coronary artery disease in 2010. Euro Heart J Suppl. 2010;12:C2–C10. [Google Scholar]
- 3. El‐Menyar AA. Cytokines and coronary artery disease: the state of the art. Crit Pathw Cardiol. 2008;7:139–151. [DOI] [PubMed] [Google Scholar]
- 4. Deswal A, Petersen NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure an analysis of the cytokine database from the Vesnarinone Trial (VEST). Circulation. 2001;103:2055–2059. [DOI] [PubMed] [Google Scholar]
- 5. Wang Q, Chen X, Feng J, Cao Y, Song Y, Wang H. Soluble interleukin‐6 receptor‐mediated innate immune response to DNA and RNA viruses. J Virol. 2013;87:11244–11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Testa M, Yeh M, Lee P, Berman JW, Lejemth TH. Fanelli, Circulating levels of cytokines and their endogenous modulators in patients with mild to severe congestive heart failure due to coronary artery disease or hypertension. J Am Coll Cardiol. 1996;28:964–971. [DOI] [PubMed] [Google Scholar]
- 7. Munger MA, Johnson B, Amber IJ, Callahan KS, Gilbert EM. Circulating concentrations of proinflammatory cytolcines in mild or moderate heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1996;77:723–727. [DOI] [PubMed] [Google Scholar]
- 8. Wu HD, Bai X, Chen DM, Cao HY, Qin L. Association of genetic polymorphisms in matrix metalloproteinase‐9 and coronary artery disease in the Chinese Han population: a case‐control study. Genet Test Mol Biomarkers. 2013;17:707–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Konstantino Y, Nguyen TT, Wolk R, Aiello RJ, Terra SG, Fryburg DA. Potential implications of matrix metalloproteinase‐9 in assessment and treatment of coronary artery disease. Biomarkers. 2009;14:118–129. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari R, Bachetti T, Confortini R, Opasich C, Febo O, Corti A. Tumor necrosis factor soluble receptors in patients with various degrees of congestive heart failure. Circulation. 1995;92:1479–1486. [DOI] [PubMed] [Google Scholar]
- 11. Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D. Interleukin‐6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin‐6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol. 1998;31:391–398. [DOI] [PubMed] [Google Scholar]
- 12. Oladi M, Nohtani M, Avan A, Mirhafez SR, Tajbakhsh A, Ghasemi F. Impact of the C1431T polymorphism of the peroxisome proliferator activated receptor‐gamma (PPAR‐gamma) gene on fasted serum lipid levels in patients with coronary artery disease. Ann Nutr Metab. 2015;66:149–154. [DOI] [PubMed] [Google Scholar]
- 13. Ferroni P, Basili S, Martini F, Cardarello CM, Ceci FDi, Franco M. Serum metalloproteinase 9 levels in patients with coronary artery disease: a novel marker of inflammation. J Invest Med. 2003;51:295–300. [DOI] [PubMed] [Google Scholar]
- 14. Mishra A, Srivastava A, Mittal T, Garg N, Mittal B. Association of matrix metalloproteinases (MMP2, MMP7 and MMP9) genetic variants with left ventricular dysfunction in coronary artery disease patients. Clin Chim Acta. 2012;413:1668–1674. [DOI] [PubMed] [Google Scholar]
- 15. Kalela A, Koivu TA, Sisto T, Kanervisto J, Hoyhtya M, Sillanaukee P. Serum matrix metalloproteinase‐9 concentration in angiographically assessed coronary artery disease. Scand J Clin Lab Invest. 2002;62:337–342. [DOI] [PubMed] [Google Scholar]
- 16. Mirhafez SR, Zarifian A, Ebrahimi M, Ali RF, Avan A, Tajfard M. Relationship between serum cytokine and growth factor concentrations and coronary artery disease. Clin Biochem. 2015;48:575–580. [DOI] [PubMed] [Google Scholar]
- 17. Mirhafez SR, Pasdar A, Avan A, Esmaily H, Moezzi A, Mohebati M. Cytokine and growth factor profiling in patients with the metabolic syndrome. Br J Nutr. 2015;113:1911–1919. [DOI] [PubMed] [Google Scholar]


