Abstract
Background
Platelets have emerged as key players in tumorigenesis and tumor progression. Tumor‐educated platelet (TEP) RNA profile has the potential to diagnose non‐small‐cell lung cancer (NSCLC). The objective of this study was to identify potential TEP RNA biomarkers for the diagnosis of NSCLC and to explore the mechanisms in alternations of TEP RNA profile.
Methods
The RNA‐seq datasets GSE68086 and GSE89843 were downloaded from Gene Expression Omnibus DataSets (GEO DataSets). Then, the functional enrichment of the differentially expressed mRNAs was analyzed by the Database for Annotation Visualization and Integrated Discovery (DAVID). The miRNAs which regulated the differential mRNAs and the target mRNAs of miRNAs were identified by miRanda and miRDB. Then, the miRNA‐mRNA regulatory network was visualized via Cytoscape software.
Results
Twenty consistently altered mRNAs (2 up‐regulated and 18 down‐regulated) were identified from the two GSE datasets, and they were significantly enriched in several biological processes, including transport and establishment of localization. Twenty identical miRNAs were found between exosomal miRNA‐seq dataset and 229 miRNAs that regulated 20 consistently differential mRNAs in platelets. We also analyzed 13 spliceosomal mRNAs and their miRNA predictions; there were 27 common miRNAs between 206 differential exosomal miRNAs and 338 miRNAs that regulated 13 distinct spliceosomal mRNAs.
Conclusion
This study identified 20 potential TEP RNA biomarkers in NSCLC for diagnosis by integrated bioinformatical analysis, and alternations in TEP RNA profile may be related to the post‐transcriptional regulation and the splicing metabolisms of spliceosome.
Keywords: biomarker, integrated bioinformatical analysis, RNA, spliceosome, tumor‐educated platelets
1. INTRODUCTION
Non‐small‐cell lung cancer (NSCLC), the most common cause of deaths from cancer worldwide, encompasses different subtypes, among which adenocarcinomas and squamous cell carcinomas account for almost 40% and 30% of all cases, respectively.1 The disease is diagnosed at early stage, and surgically resected may reduce the mortality.2 Nowadays, the common molecular biomarkers for lung cancer diagnosis include carcinoembryonic antigen (CEA), carbohydrate antigen 125 (CA125), cytokeratin‐19 fragment 21‐1 (CYFRA 21‐1), and New York esophageal cancer‐1 antibody (NY‐ESO‐1), but these biomarkers are more accurate in late‐stage disease than in early‐stage disease.3 Therefore, new early diagnostic biomarkers are urgently needed for NSCLC.
Non‐small‐cell lung cancer is characterized by a diverse collection of genomic alterations, and many pathogenetically important changes have already been identified in a substantial proportion of patients and classified as disease biomarkers.4 Interpretation of the molecular profiles of NSCLC is crucial for early detection and effective personalized treatment approaches. At present, multiple blood‐based biomolecules and biosources for cancer have been the subject of research, including cell‐free (cf) DNA, cfRNA, proteins, metabolic products, extracellular vesicles, and circulating tumor cells (CTCs); blood platelets have also joined this array of blood‐based biosources.5 Platelets have important functions in hemostasis, immunity, and inflammation and also play a key role in cancer growth and metastasis.6 The functions of tumor‐educated platelets (TEPs) have changed significantly, indicating the alterations in platelets RNA profile.7 The combination of specific splice events in response to external signals and the capacity of platelets to sequester tumor‐associated biomolecules can provide TEPs with a highly dynamic mRNA repertoire, with potential applicability to cancer diagnosis.8, 9 In addition, miRNAs also play an important role in altering TEP RNA profile as post‐transcriptional regulators.
We believe that blood platelets could provide an eligible biomaterial source for RNA biomarkers of NSCLC diagnosis. The high‐throughput platforms for analysis of gene expression such as next‐generation sequencing (NGS) are increasingly valued and common in medical oncology with great clinical applications, allowing testing of multiple genetic variants from a single sample,10 which have led to the identification of genetic variation and molecular biomarkers serving for personalized medicine.11 The research group of Best et al demonstrated that TEPs with altered RNA profiles which were able to distinguish 55 healthy individuals from 228 cancer patients with 96% accuracy using RNA‐seq, and also developed a particle‐swarm optimization (PSO)‐driven thromboSeq algorithm which resulted in TEP‐based detection of early‐stage NSCLC with accuracy 81%.
In this study, we downloaded the NGS datasets from several studies which had identified panels of differential mRNAs for cancer diagnosis, and aimed to analyze these datasets in deep and explore the causes of changes in TEP RNA profile utilizing integrated bioinformatics analysis, and finally to conclude the biological functions and key pathways of these common differential mRNAs, thereby proposing an insight in molecular regulatory mechanisms and potential candidate biomarkers for early diagnosis and individualized therapy of NSCLC.
2. MATERIALS AND METHODS
2.1. NGS datasets and differentially expressed mRNAs/miRNAs between NSCLC patients and health donors
The platelet mRNA expression profile of GSE68086 and GSE89843 from Gene Expression Omnibus (GEO) DataSets (https://www.ncbi.nlm.nih.gov/gds/) was obtained, both of which were collected from high‐throughput sequencing, based on GPL16791 platform (Illumina Hiseq 2500). Exosomal miRNAs data were obtained from previous research which was implemented by miRNA‐seq using Illumina HiSeq4000 analyzer.12
2.2. Gene ontology and pathway enrichment analysis of differentially expressed mRNAs in platelets
Gene ontology analysis (GO) consists of three domains: cellular component (CC), molecular function (MF), and biological process (BP). Kyoto Encyclopedia of Genes and Genomes (KEGG) is a knowledge database resource for understanding high‐level functions and utilities of the biological system, from molecular datasets which is generated by genome sequencing and other high‐throughput experimental technologies. We used the Database for Annotation Visualization and Integrated Discovery13, 14(DAVID 6.8 online, https://david.ncifcrf.gov/home.jsp) to analyze the GO enrichment and KEGG pathway of differentially expressed mRNAs. Significance was established at P < .05.
2.3. MiRNA targets prediction and construction of miRNA‐mRNA network
MiRanda15 (http://34.236.212.39/microrna/getMirnaForm.do) and miRDB16 (http://mirdb.org/miRDB/index.html) are online database for the prediction of potential miRNA targets. We chose the conserved predictions with mirSVR scores≤−0.1 in miRanda, as well as those with target score >80 in miRDB.
The miRNA‐mRNA regulatory network was visualized via Cytoscape software (version 3.5.1; www.cytoscape.org).
3. RESULTS
3.1. Identification of differentially expressed mRNAs in platelets between NSCLC patients and health donors
The NGS data of GSE89843 had 263 platelet samples, including 159 NSCLCs and 104 individuals without reported cancers, but not excluding individuals with inflammatory conditions. There were 1625 differentially expressed mRNAs, among which 698 were up‐regulated vs 927 were down‐regulated. The NGS data of GSE68086 had 115 blood platelet samples, including 60 TEP samples collected from NSCLCs and 55 healthy individuals. This differentially expressed mRNAs listed 1215 up‐regulated mRNAs and 1163 down‐regulated mRNAs.
We identified 951 commonly altered differential mRNAs from above platelets RNA‐sequencing datasets, among them only 2 were consistently up‐regulated and 18 were consistently down‐regulated (Figure 1 and Table 1).
Figure 1.
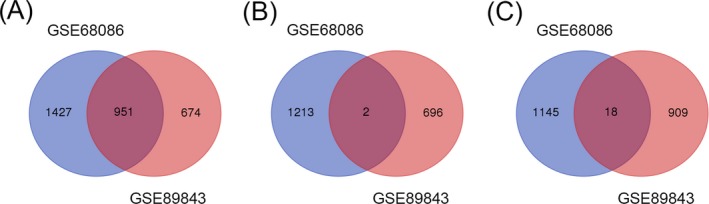
Identification of differential mRNAs. A, 951 commonly altered differentially expressed mRNAs between GSE68086 and GSE89843. B, Identification of up‐regulated differentially expressed mRNAs. C, Identification of down‐regulated differentially expressed mRNAs
Table 1.
The genes list of 2 consistently up‐regulated and 18 consistently down‐regulated differentially expressed mRNAs was showed
| Ensembl gene ID | HGNC symbol | Description |
|---|---|---|
| Up‐regulated differentially expressed mRNAs | ||
| ENSG00000114857 | NKTR | Natural killer‐tumor recognition sequence |
| ENSG00000163386 | NBPF10 | Neuroblastoma breakpoint family, member 10 |
| Down‐regulated differentially expressed mRNAs | ||
| ENSG00000010404 | IDS | Iduronate 2‐sulfatase |
| ENSG00000112245 | PTP4A1 | Protein tyrosine phosphatase type IVA, member 1 |
| ENSG00000138449 | SLC40A1 | Solute carrier family 40 (iron‐regulated transporter), member 1 |
| ENSG00000124491 | F13A1 | Coagulation factor XIII, A1 polypeptide |
| ENSG00000114573 | ATP6V1A | ATPase, H+ transporting, lysosomal 70 kDa, V1 subunit A |
| ENSG00000181104 | F2R | Coagulation factor II (thrombin) receptor |
| ENSG00000155096 | AZIN1 | Antizyme inhibitor 1 |
| ENSG00000135821 | GLUL | Glutamate‐ammonia ligase |
| ENSG00000150712 | MTMR12 | Myotubularin‐related protein 12 |
| ENSG00000122218 | COPA | Coatomer protein complex, subunit alpha |
| ENSG00000065615 | CYB5R4 | Cytochrome b5 reductase 4 |
| ENSG00000164181 | ELOVL7 | ELOVL fatty acid elongase 7 |
| ENSG00000168175 | MAPK1IP1L | Mitogen‐activated protein kinase 1 interacting protein 1‐like |
| ENSG00000175582 | RAB6A | RAB6A, member RAS oncogene family |
| ENSG00000162521 | RBBP4 | Retinoblastoma binding protein 4 |
| ENSG00000187800 | PEAR1 | Platelet endothelial aggregation receptor 1 |
| ENSG00000073849 | ST6GAL1 | ST6 beta‐galactosamide alpha‐2,6‐sialyltransferase 1 |
| ENSG00000109332 | UBE2D3 | Ubiquitin‐conjugating enzyme E2D3 |
3.2. GO enrichment and KEGG pathway analysis of differentially expressed mRNAs in platelets between NSCLC patients and health donors
Above 20 consistently altered mRNAs were mapped and subjected to relevant biological annotation analysis in the DAVID database. GO analysis results showed that differentially expressed mRNAs were significantly enriched in several biological processes, including transport, establishment of localization, localization, single‐organism transport and single‐organism localization. For cell component, the 20 consistently altered mRNAs were significantly enriched in cytoplasm, intracellular membrane‐bounded organelle, cytoplasmic part, endomembrane system and membrane‐bounded vesicle. In addition, molecular function displayed that they were enriched in catalytic activity (Table 2). The most significantly enriched pathways of the 20 consistently altered mRNAs analyzed by KEGG analysis. They were enriched in complement and coagulation cascades but showed no significant difference, and the main differentially expressed mRNAs involved in the functions and pathways were down‐regulated (Table 2).
Table 2.
Gene ontology and KEGG pathway analysis of 20 consistently altered differentially expressed mRNAs
| Category | Term | Count | % | P Value | mRNAs |
|---|---|---|---|---|---|
| GOTERM_BP_ALL | GO:0006810~transport | 12 | 66.67 | 7.63E‐04 | MTMR12, ATP6V1A, COPA, CYB5R4, UBE2D3, GLUL, PEAR1, F13A1, AZIN1, RAB6A, SLC40A1, F2R |
| GOTERM_BP_ALL | GO:0051234~establishment of localization | 12 | 66.67 | 9.80E‐04 | MTMR12, ATP6V1A, COPA, CYB5R4, UBE2D3, GLUL, PEAR1, F13A1, AZIN1, RAB6A, SLC40A1, F2R |
| GOTERM_BP_ALL | GO:0051179~localization | 13 | 72.22 | .00128 | COPA, CYB5R4, PEAR1, F13A1, AZIN1, MTMR12, ATP6V1A, UBE2D3, GLUL, PTP4A1, RAB6A, SLC40A1, F2R |
| GOTERM_BP_ALL | GO:0044765~single‐organism transport | 10 | 55.56 | .00166 | ATP6V1A, COPA, CYB5R4, GLUL, PEAR1, F13A1, AZIN1, RAB6A, SLC40A1, F2R |
| GOTERM_BP_ALL | GO:1902578~single‐organism localization | 10 | 55.56 | .00253 | ATP6V1A, COPA, CYB5R4, GLUL, PEAR1, F13A1, AZIN1, RAB6A, SLC40A1, F2R |
| GOTERM_CC_ALL | GO:0005737~cytoplasm | 15 | 83.33 | .01545 | COPA, CYB5R4, ST6GAL1, F13A1, AZIN1, MTMR12, ATP6V1A, GLUL, UBE2D3, IDS, PTP4A1, ELOVL7, RAB6A, SLC40A1, F2R |
| GOTERM_CC_ALL | GO:0043231~intracellular membrane‐bounded organelle | 15 | 83.33 | .02032 | COPA, CYB5R4, ST6GAL1, RBBP4, F13A1, AZIN1, ATP6V1A, GLUL, UBE2D3, IDS, PTP4A1, ELOVL7, RAB6A, SLC40A1, F2R |
| GOTERM_CC_ALL | GO:0044444~cytoplasmic part | 14 | 77.78 | .00297 | COPA, CYB5R4, ST6GAL1, F13A1, AZIN1, ATP6V1A, UBE2D3, GLUL, IDS, PTP4A1, ELOVL7, RAB6A, SLC40A1, F2R |
| GOTERM_CC_ALL | GO:0012505~endomembrane system | 11 | 61.11 | 4.20E‐04 | COPA, CYB5R4, UBE2D3, GLUL, ST6GAL1, F13A1, PTP4A1, ELOVL7, RAB6A, SLC40A1, F2R |
| GOTERM_CC_ALL | GO:0031988~membrane‐bounded vesicle | 9 | 50.00 | .00658 | ATP6V1A, COPA, UBE2D3, GLUL, ST6GAL1, F13A1, PTP4A1, RAB6A, SLC40A1 |
| GOTERM_MF_ALL | GO:0003824~catalytic activity | 12 | 66.67 | .00724 | ATP6V1A, CYB5R4, UBE2D3, GLUL, ST6GAL1, RBBP4, IDS, F13A1, PTP4A1, ELOVL7, AZIN1, RAB6A |
| KEGG_PATHWAY | hsa04610:Complement and coagulation cascades | 2 | 11.11 | .08641 | F13A1, F2R |
3.3. The prediction of tumor‐derived exosomal miRNA targets in NSCLC
In recent years, accumulating evidence had demonstrated that deregulated miRNA expression was involved in primary tumorigenesis and metastasis.17 MiRNAs play important roles in gene post‐transcriptional regulation, thus influencing on TEP RNA profiles. In 26 (16 with adenocarcinoma and 10 with squamous cell carcinoma) stage I NSCLC samples compared with 12 healthy individuals matched for sex and age, more than 200 specific tumor‐derived exosomal miRNA were identified by miRNA‐seq using an Illumina HiSeq4000 analyzer.12 The total differentially expressed miRNAs were composed of 98 up‐regulated miRNAs and 108 down‐regulated miRNAs.
Next, we predicted miRNA which regulated above 20 consistently altered mRNA using miRNA targets prediction tools as described in Materials and methods. Sixty‐nine miRNAs that regulated 2 consistently increased mRNAs as well as 225 miRNAs that regulated 18 consistently decreased mRNAs were identified. Twelve identical miRNAs were found between exosomal miRNA‐seq dataset and 229 predicted miRNAs that regulated 20 consistently altered mRNAs in platelets, among them only 1 overlap was selected between 108 down‐regulated exosomal miRNAs and above 69 predicted miRNAs, as well as 7 overlaps between 98 up‐regulated exosomal miRNAs and above 225 predicted miRNAs (Figure 2).
Figure 2.
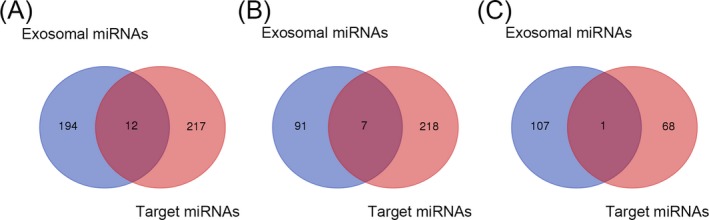
Identification of differential miRNAs. A, Twelve identical miRNAs were found between two datasets. B, Identification of up‐regulated exosomal miRNAs and miRNAs that regulated consistently down‐regulated differentially expressed mRNAs. C, Identification of down‐regulated exosomal miRNAs and miRNAs that regulated 2 consistently up‐regulated differentially expressed platelets mRNAs
The target mRNA of 1 overlap was NKTR, and the target mRNAs of 7 overlaps were PTP4A1, F13A1, ATP6V1A, F2R, AZIN1, MTMR12, COPA, CYB5R4, ELOVL7, MAPK1IP1L, RAB6A, and UBE2D3. Subsequently, miRNA‐mRNA network about consistently up‐regulated exosomal miRNAs and consistently down‐regulated mRNAs in platelets was constructed (Figure 3).
Figure 3.
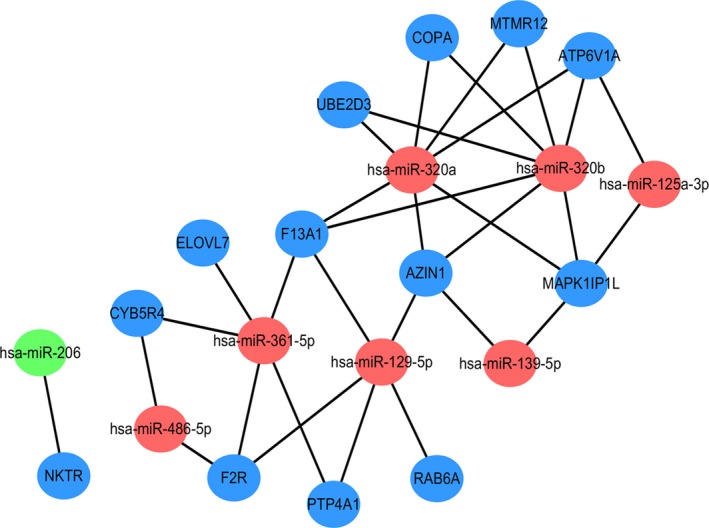
miRNA‐mRNA network. Red nodes stand for up‐regulated miRNAs, while green nodes stand for down‐regulated miRNAs. Blue nodes stand for mRNAs. The lines stand for the regulation relationship between miRNAs and mRNAs
We found miRNAs that regulated consistently altered mRNAs did not exactly matched the differentially expressed miRNAs in exosomes, suggesting miRNAs influenced mRNA level by other mechanisms in addition to the direct regulation. We hypothesized that miRNA might influence the expression of mRNAs through regulating the levels of distinct spliceosomal proteins.
3.4. Spliceosomal mRNAs and their miRNA predictions
Platelets were able to escape the nuclear confines and to splice pre‐mRNA by a functional spliceosome which could alter their pool of translatable messages in response to external stimulation.18 We selected 13 distinct spliceosomal proteins (Table 3) according to previous study18 and predicted 338 miRNAs that regulated their mRNAs. Then, we compared 206 differentially expressed exosomal miRNAs with 338 miRNAs that regulated the coding mRNAs of 13 distinct spliceosomal proteins. There were 27 common miRNAs between them, 17 up‐regulated in tumor‐derived exosomal miRNAs which regulated SRSF1, SNRPD1, SRRM1, SRSF3, SRSF7, SRSF2, and SRSF5. These 7 mRNAs that were regulated by 17 up‐regulated tumor‐derived exosomal miRNAs were not included in the 18 consistently down‐regulated differentially expressed mRNAs; SRSF2 was down‐regulated in GSE68086 while SRSF7, SNRPD1, SRRM1, and SRSF5 were down‐regulated in GSE89843 and 10 down‐regulated in tumor‐derived exosomal miRNAs which regulated SNRNP70, U2AF2, SRSF1, SRRM1, SRSF3, SRSF9, SRSF7, and SRSF6. (Figure 4).
Table 3.
The information of 13 distinct spliceosomal proteins was presented
| Protein name | Ensembl gene ID | HGNC symbol(gene) | Official full name |
|---|---|---|---|
| U1‐70kD | ENSG00000104852 | SNRNP70 | Small nuclear ribonucleoprotein U1 subunit 70 |
| U2 AF65 | ENSG00000063244 | U2AF2 | U2 small nuclear RNA auxiliary factor 2 |
| U2AF35 | ENSG00000160201 | U2AF1 | U2 small nuclear RNA auxiliary factor 1 |
| ASF/SF2 | ENSG00000136450 | SRSF1 | Serine and arginine‐rich splicing factor 1 |
| Sm B/B’ | ENSG00000125835 | SNRPB | Small nuclear ribonucleoprotein polypeptides B and B1 |
| Sm D1 | ENSG00000167088 | SNRPD1 | Small nuclear ribonucleoprotein D1 polypeptide |
| SRm160 | ENSG00000133226 | SRRM1 | Serine and arginine repetitive matrix 1 |
| SRp20 | ENSG00000112081 | SRSF3 | Serine and arginine‐rich splicing factor 3 |
| SRp30c | ENSG00000111786 | SRSF9 | Serine and arginine‐rich splicing factor 9 |
| 9G8 | ENSG00000115875 | SRSF7 | Serine and arginine‐rich splicing factor 7 |
| SC35 | ENSG00000161547 | SRSF2 | Serine and arginine‐rich splicing factor 2 |
| SRp55 | ENSG00000124193 | SRSF6 | Serine and arginine‐rich splicing factor 6 |
| SRp40 | ENSG00000100650 | SRSF5 | Serine and arginine‐rich splicing factor 5 |
Figure 4.

Spliceosomal mRNAs and their miRNA predictions. A, There were 27 common miRNAs between 206 differentially expressed exosomal miRNAs and 338 miRNAs dataset that regulated the mRNAs which encoded 13 distinct spliceosomal proteins. B, miRNA‐mRNA network. Red nodes stand for up‐regulated miRNAs, while green nodes stand for down‐regulated miRNAs. Blue nodes stand for mRNA. The lines stand for the regulation relationship between miRNAs and mRNAs
4. DISCUSSION
Lung cancer is a severe disease, and its morbidity and mortality are gradually increasing.19 Compelling preclinical and clinical data demonstrate that platelets have emerged as key players in tumor cell growth, invasion, spread, migration, intravasation, extravasation, and establishment of distant metastasis.20, 21 In 2010, Calverley et al8 discovered 200 altered RNAs in platelets between the healthy individuals and the patients with lung cancer using micro‐array analysis, among which 197 genes were decreased in platelets of patients with lung cancer. Interestingly, prominent tumor‐driving mutations were also accurately reflected in the results obtained from platelet RNA sequencing and bioinformatics processing of the resulting data. This finding could have major implications for future clinical trials and therapy regimens.22
This study identified 951 commonly altered mRNAs in TEPs compared with control samples; however, only 20 consistently altered mRNAs were selected between two mRNA‐seq datasets probably due to the post‐transcriptional regulation and alternative splicing events during genes expression, indicating the great variation of platelet mRNAs. Then, these differentially expressed mRNAs were subjected to GO and KEGG pathway analysis using bioinformatics approaches, demonstrating they were involved in the biological process of transport and localization and the molecular function of catalytic activity. F13A1 and F2R were included in KEGG pathway of complement and coagulation cascades. F2R is a G protein–coupled receptor which could regulate coagulation and cell survival, and the serum levels of F2R might act as the diagnostic marker in lung cancer.23 A unique case reported a highly aggressive angiosarcoma complicated with inherited factor XIII deficiency which was encoded by F13A1.24
Many researchers have reported that miRNAs are important post‐transcriptional regulators in all biological processes virtually.25 MiRNAs are short, single‐strand, noncoding RNAs of approximately 20‐25 nucleotides which bind to complementary sequences mainly located at the 3′‐untranslated region (3′‐UTR) of their target mRNAs for degradation or the induction of translational repression.17 It has been established that tumor cells can release RNA into the circulation via a variety of microvesicles, which could help tumor‐derived RNA transfer into platelets, although microvesicle‐independent mechanisms were also involved in this process.9
In NSCLC, tumor‐derived exosomal miRNAs expression profiles had been identified with remarkably high predictive values including 206 most significant AC‐ and SCC‐specific miRNAs.12 Twenty consistently altered mRNAs in platelets could be regulated by total 229 miRNAs identified using the online bioinformatics tool, as well as by 12 of 206 most significant NSCLC‐specific exosomal miRNAs discovered in miRNAs list. Twelve of 18 decreased differential mRNAs in platelets were the targets of 7 increased exosomal miRNAs which regulated gene expression post‐transcriptionally.26 From the data and network, we knew that 7 miRNAs exerted their functional effects by targeting multiple mRNAs usually in the same pathway,27 suggesting blood platelets not only took up tumor‐derived microvesicle that contained tumor‐associated mRNA but also altered the mRNA profiles by the regulation of miRNAs from exosomes.
Anucleate as platelets are, they contain mRNA, the entire spliceosome machinery as well as a full complement of ribosomes, and have the ability to synthesize de novo proteins upon activation.28 Megakaryocytes can produce large quantities of mRNA and protein which are packaged into platelets prior to their release in to the circulation.29 The TEPs perhaps behave in a “semi‐activational state,” which are able to splice pre‐mRNA with the help of functional spliceosome and several splice factor proteins.30 Moreover, there are very many exon skip splice isoforms in human platelets which are characterized by the peptides spanning the exon‐exon junction of a novel splicing event.31 Calverly et al8 identified a subset of megakaryocyte/platelet‐derived mRNAs that were differentially expressed in lung cancer metastasis. Alternative Splicing and Exon Skipping in TEPs of patients with NSCLC caused specific changes in platelet RNAs.
We took the 13 mRNAs that encoded spliceosomal proteins, 9 of them were referred in GSE68086 and 6 of them were contained in GSE89843. We found that 17 miRNAs which regulated SRSF1, SNRPD1, SRRM1, SRSF3, SRSF7, SRSF2, and SRSF5 were up‐regulated in tumor‐derived exosomes when compared 206 differential exosomal miRNAs with 338 miRNAs that regulated 13 spliceosomal mRNAs. MiRNAs had an impact on degradation and translation of the spliceosomal mRNAs; meanwhile, alterations in spliceosomal mRNAs could influence other RNAs expression. Therefore, the mRNA profiles of platelets could be significantly changed.
These results further demonstrated that the variations of TEP RNA profiles could not be ignored, and the TEP RNA profile not only reflected the RNA changes of tumor tissues, but also showed platelet‐specific RNA variations in individuals who suffered tumor. Therefore, platelets can serve as a potential biomarker source for cancer diagnosis, and platelets contain tumor‐associated RNA that allows the researchers to scan for general molecular traces of cancer by sequencing of TEP RNA, thus providing a strategic opening for cancer surveillance. TEP RNA profiles have the great potential for NSCLC diagnosis and contribute to exploring the mechanisms of tumor metastasis. In addition, our study only took account into two NGS datasets about the platelet RNA profiles that might lead to bias in the data analysis, and further molecular biological experiments are required to confirm the functions of the altered RNAs in NSCLC as well as the mechanisms that cause alternations of TEP RNA profile.
Xue L, Xie L, Song X, Song X. Identification of potential tumor‐educated platelets RNA biomarkers in non‐small‐cell lung cancer by integrated bioinformatical analysis. J Clin Lab Anal. 2018;32:e22450 10.1002/jcla.22450
REFERENCES
- 1. Stella GM, Luisetti M, Pozzi E, Comoglio PM. Oncogenes in non‐small‐cell lung cancer: emerging connections and novel therapeutic dynamics. Lancet Respir Med. 2013;1:251‐261. [DOI] [PubMed] [Google Scholar]
- 2. Leng QX, Lin YL, Jiang FR, et al. A plasma miRNA signature for lung cancer early detection. Oncotarget. 2017;8:111902‐111911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mazzone PJ, Wang XF, Han X, et al. Evaluation of a serum lung cancer biomarker panel. Biomark Insights. 2018;13:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zalcman G, Bergot E, Creveuil C, Levallet G, Lechapt E. Integrating biomarkers into clinical trials: methodological issues for a new paradigm in nonsmall cell lung cancer. Curr Opin Oncol. 2011;23:106‐111. [DOI] [PubMed] [Google Scholar]
- 5. Best MG, Vancura A, Wurdinger T. Platelet RNA as a circulating biomarker trove for cancer diagnostics. J Thromb Haemost. 2017;15:1295‐1306. [DOI] [PubMed] [Google Scholar]
- 6. Sharma D, Brummel‐Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229:1005‐1015. [DOI] [PubMed] [Google Scholar]
- 7. Best MG, Sol N, Kooi I, et al. RNA‐Seq of tumor‐educated platelets enables blood‐based pan‐cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell. 2015;28:666‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calverley DC, Phang TL, Choudhury QG, et al. Significant downregulation of platelet gene expression in metastatic lung cancer. Clin Transl Sci. 2010;3:227‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nilsson RJ, Balaj L, Hulleman E, et al. Blood platelets contain tumor‐derived RNA biomarkers. Blood. 2011;118:3680‐3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hiley CT, Le Quesne J, Santis G, et al. Challenges in molecular testing in non‐small‐cell lung cancer patients with advanced disease. Lancet. 2016;388:1002‐1011. [DOI] [PubMed] [Google Scholar]
- 11. Alama A, Truini A, Coco S, Genova C, Grossi F. Prognostic and predictive relevance of circulating tumor cells in patients with non‐small‐cell lung cancer. Drug Discovery Today. 2014;19:1671‐1676. [DOI] [PubMed] [Google Scholar]
- 12. Jin X, Chen Y, Chen H, et al. Evaluation of tumor‐derived exosomal mirna as potential diagnostic biomarkers for early‐stage non‐small cell lung cancer using next‐generation sequencing. Clin Cancer Res. 2017;23:5311‐5319. [DOI] [PubMed] [Google Scholar]
- 13. Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44‐57. [DOI] [PubMed] [Google Scholar]
- 15. Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biol. 2003;5:R1. Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43:D146‐D152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Behbahani GD, Ghahhari NM, Javidi MA, Molan AF, Feizi N, Babashah S. MicroRNA‐mediated post‐transcriptional regulation of epithelial to mesenchymal transition in cancer. Pathol Oncol Res. 2017;23:1‐12. [DOI] [PubMed] [Google Scholar]
- 18. Denis MM, Tolley ND, Bunting M, et al. Escaping the nuclear confines: signal‐dependent pre‐mRNA splicing in anucleate platelets. Cell. 2005;122:379‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gong L, Mi HJ, Zhu H, Zhou X, Yang H. P‐selectin‐mediated platelet activation promotes adhesion of non‐small cell lung carcinoma cells on vascular endothelial cells under flow. Mol Med Rep. 2012;5:935‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470‐1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelets effects on tumor growth. Semin Oncol. 2014;41:359‐369. [DOI] [PubMed] [Google Scholar]
- 22. Feller SM, Lewitzky M. Hunting for the ultimate liquid cancer biopsy ‐ let the TEP dance begin. Cell Commun Signal. 2016;14:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Erturk K, Tastekin D, Bilgin E, Tas F, Disci R, Duranyildiz D. Clinical significance of serum protease activated receptor1 levels in patients with lung cancer. Eur Rev Med Pharmacol Sci. 2016;20:243‐249. [PubMed] [Google Scholar]
- 24. Ivaškevicius V, Goldmann G, Biswas A, et al. Neoplasm‐induced bleeding in inherited, heterozygous FXIII‐A deficiency. Hämostaseologie. 2015;35:S32‐S35. [PubMed] [Google Scholar]
- 25. Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;319:1785‐1786. [DOI] [PubMed] [Google Scholar]
- 26. Wozniak MB, Scelo G, Muller DC, Mukeria A, Zaridze D, Brennan P. Circulating MicroRNAs as Non‐Invasive Biomarkers for Early Detection of Non‐Small‐Cell Lung Cancer. PLoS ONE. 2015;10:e0125026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gamez B, Rodriguez‐Carballo E, Ventura F. MicroRNAs and post‐transcriptional regulation of skeletal development. J Mol Endocrinol. 2014;52:R179‐R197. [DOI] [PubMed] [Google Scholar]
- 28. Zimmerman GA, Weyrich AS. Signal‐dependent protein synthesis by activated platelets: new pathways to altered phenotype and function. Arterioscler Thromb Vasc Biol. 2008;28:s17‐s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davila J, Manwani D, Vasovic L, et al. A novel inflammatory role for platelets in sickle cell disease. Platelets. 2015;26:726‐729. [DOI] [PubMed] [Google Scholar]
- 30. Best MG, Sol N, In‘t Veld S, et al. Swarm intelligence‐enhanced detection of non‐small‐cell lung cancer using tumor‐educated platelets. Cancer Cell. 2017;32:238‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Karen A, Power JPM, Stefani AD, Gallagher WM, Peadar O. High‐Throughput Proteomics Detection of Novel Splice Isoforms in Human Platelets. PLoS ONE. 2009;4:e5001. [DOI] [PMC free article] [PubMed] [Google Scholar]


