Abstract
Background
For analysis of urine samples during abstinence control for driving ability assessment (medical and psychological assessment, MPA), a reliable screening method for ethyl glucuronide and drugs of abuse (cannabinoids, opiates, cocaine, amphetamines, methadone, and benzodiazepines) is needed.
Methods
In this study CEDIA and DRI immunoassays were applied on a Thermo Fisher Scientific Indiko Plus analyzer. Precision and accuracy as well as sensitivity and specificity at the required cut‐offs for the MPA were evaluated.
Results
The specificity was satisfactory and ranged from 91% for methamphetamine to 100% for opiates, cocaine metabolite, amphetamine, EDDP, and benzodiazepines. Moreover, sensitivity was 100% for all assays except for cannabinoids (91%).
Conclusion
The presented method can therefore be recommended for abstinence control.
Keywords: abstinence control, diagnostic tests, ethyl glucuronide, forensic toxicology
Introduction
Abstinence control for drugs of abuse and ethanol is often demanded for driving license regranting during medical and psychological assessment (MPA). The MPA guidelines require sensitive polytoxicological analyses of urine samples including the parameters and the quantitative MPA cut‐offs given in Table 1 1.
Table 1.
Parameters and MPA Cut‐Offs in Urine for Abstinence Control 1
| Substance classTarget analyte | Urine[ng/ml] |
|---|---|
| Cannabinoids | |
| THC‐COOH | 10 (after hydrolysis) |
| Opiates | |
| Morphine (codeine, dihydrocodeine) | 25 (after hydrolysis) |
| Cocaine (metabolite) | |
| Benzoylecgonine | 30 |
| Amphetamines | |
| Amphetamine, methamphetamine, MDMA, MDEA | 50 |
| Methadone | |
| EDDP | 50 |
| Benzodiazepines | |
| Nordiazepam, oxazepam, hydroxy‐alprazolam, hydroxy‐bromazepam, 7‐amino‐flunitrazepam, lorazepam | 50 |
| Ethyl glucuronide | 100 |
At the MPA cut‐offs, the sensitivity of the applied screening method should be close to 100%, and preferably, positive samples with concentrations slightly below the MPA cut‐off should be detected as well. According to the MPA guidelines these samples have to be reported as positives because the consumption of the drug is proven. In addition, false‐positive samples should be avoided for economic reasons, as samples with a positive screening result have to be reanalyzed by chromatographic‐mass spectrometric methods. Typically, immunoassays are used for screening purposes. Thus, at the defined immunoassay cut‐offs, a reasonable compromise has to be achieved regarding sensitivity and specificity. Immunoassays applied for forensic analysis in the context of abstinence control have to be validated by measuring authentic samples to show that the method is capable of detecting the target analytes in at least 9 of 10 samples with concentrations in the range of the MPA cut‐off 2.
Most of the urine samples collected during abstinence control for MPA are negative, so an efficient analysis procedure usually consists of a fast and easy screening method followed by confirmation of positive screening results using a quantitative method. Musshoff et al. 3 evaluated CEDIA and DRI drugs of abuse immunoassays for abstinence control in the context of MPA. The tests were carried out on an Olympus AU 400. ELISA manual microtiter tests were also evaluated for this purpose 4, 5.
The aim of this study was to evaluate the performance of CEDIA and DRI drugs of abuse and ethyl glucuronide tests on the Thermo Scientific Indiko Plus Analyzer, which is a fully automated bench‐top analyzer for clinical chemistry analytics for the purpose of abstinence control according to the MPA and German society of Toxicological and Forensic Chemistry (GTFCh) guidelines. The main requirement is sufficient sensitivity of the assays to reliably detect the low concentrations required in the context of the MPA. In addition, the precision of the assays was assessed.
Materials and Methods
Samples
Drug and ethyl glucuronide (EtG)‐positive urine samples obtained in our laboratory for routine drug‐testing were selected. Only positive samples with concentrations up to twice the MPA cut‐off were used (for opiates up to triple the MPA cut‐off). If necessary, the samples were diluted with blank urine samples. For some analytes spiked urine samples were used additionally to increase the number of positive samples (analytes and respective numbers of spiked samples: methamphetamine: 15; MDMA: 15; benzoylecgonine: 9; morphine: 10). For benzodiazepines, positive samples of each of the target analytes according to the MPA parameters were included. As expected, all of the oxazepam‐positive samples included also nordiazepam and temazepam. Two of the lorazepam‐positive samples included also less than 2 ng/ml of oxazepam. One of the two alprazolam‐positive samples included also 17 ng/ml nordiazepam and 28 ng/ml oxazepam. One bromazepam positive sample was used. For opiates, all authentic samples contained mainly morphine next to low concentrations of normorphine and codeine. The blank urine samples were tested negative with confirmatory methods. Sample numbers and concentration ranges are given in Table 2.
Table 2.
Overview of Authentic Samples Used for Evaluation of Cut‐Offs, Sensitivity, and Specificity
| Immunoassay | Number of samples | Concentration range [ng/ml] | |
|---|---|---|---|
| Negative | Positive | ||
| Cannabinoids | 28 | 28 | 2.0–19 |
| Opiates (morphine) | 22 | 26 | 1.0–75 |
| Cocaine (metabolite) | 27 | 42 | 2.0–40 |
| Amphetamine | 67 | 32 | 2.0–92 |
| Methamphetamine | 22 | 25 | 2.0–55 |
| Ecstasy | 23 | 33 | 2.0–61 |
| EDDP | 23 | 16 | 1.0–85 |
| Benzodiazepines | 31 | 48 | 5.0–100 |
| Nordiazepam | (31) | 17 | 6.5–26 |
| Oxazepam | (31) | 33 | 1.7–100 |
| Hydroxy‐alprazolam | (31) | 2 | 18–35 |
| 7‐Aminoflunitrazepam | (31) | 4 | 30–55 |
| Lorazepam | (31) | 10 | 7.9–66 |
| Ethyl glucuronide | 34 | 27 | 80–190 |
Positive samples show concentrations above the limit of detection of the confirmative method. The concentration ranges of respective target analytes in positive samples are given.
Chemicals and Reagents
Analytical standards were purchased from LGC Standards GmbH (Wesel, Germany). All solvents and chemicals were at least of analytical or HPLC grade. Reagents, calibration, and control materials for the immunoassay testing were obtained from Thermo Fisher Scientific (Passau, Germany). The following CEDIA tests were used: THC (cannabinoids), Opiate, Cocaine, Amphetamine OFT, Methamphetamine OFT, EDDP (methadone metabolite), and Benzodiazepine. The Benzodiazepine CEDIA included a beta‐glucuronidase protocol. The following DRI tests were used: XTC (ecstasy) and EtG (ethyl glucuronide). Beta‐glucuronidase/arylsulfatase (E. coli) was obtained from Roche (Mannheim, Germany).
Immunoassay Testing
Four calibration points were applied for the CEDIA tests and three calibration points were used for the DRI tests. For all tests, a calibration point of zero (CEDIA‐Negative Cal or DRI‐Negative Cal, respectively) was applied. The calibration samples were prepared by dilution of the following standard calibration materials with CEDIA‐ or DRI‐Negative Cal (matrix: urine), if necessary: cannabinoids: THC 25 ng/ml Cal, THC 50 ng/ml Cal; opiates, cocaine (metabolite), methamphetamine, EDDP, and benzodiazepines: Multidrug Optional CutOff Cal; amphetamine: Amphetamine 200 ng/ml Serum Cal (matrix: serum); ecstasy: Ecstasy 250 ng/ml Cal; and ethyl glucuronide: DRI EtG CE calibrator. The following concentrations (ng/ml) were used for calibration: cannabinoids: 12.5, 25, and 50; opiates: 37.5, 100, and 300; cocaine: 37.5, 100, and 300; amphetamine: 25, 67, and 200; methamphetamine: 37.5, 100, and 300; EDDP: 25, 50, and 100; benzodiazepines: 50, 100, and 200; ecstasy: 50 and 250; and ethyl glucuronide: 100 and 500. The calibration matrix was urine for all parameters except for amphetamine, where a mixture of serum and urine was applied. All calibration samples were analyzed in duplicate. A point‐to‐point calibration model was applied. Calibration was performed on every working day.
The analyses were performed using standard settings as recommended by the manufacturer. Shortly, the volume of reagents 1 and 2 was 55 μl, the first incubation time was 300 sec, and the second incubation time was 240 sec for CEDIA tests and 90 sec for DRI tests. The main detection wavelength was 575 nm for CEDIA tests and 340 nm for DRI tests. The following sample volumes were used: THC 9 μl, Opiate 6 μl, Cocaine 9 μl, Amphetamine OFT 6 μl, Methamphetamine OFT 8 μl, EDDP 5 μl, Benzodiazepine 6 μl, XTC 22 μl, and EtG 23 μl.
Quality controls (QCs) in the low, the middle, and the high concentration range were used. The QC samples were prepared by dilution of standard control or calibration material with CEDIA‐ or DRI‐Negative Cal (matrix: urine), if necessary. Different lots were used for dilution of QC and calibration samples. Concentrations and dilutions are given in Table 3.
Table 3.
Quality Control Target Concentrations and Respective Standard Material Dilution
| Immunoassay and QC | Target concentration | Material and dilution | |
|---|---|---|---|
| Cannabinoids | |||
| Low | 9.4 | THC 25 Ctrl Low | 1 + 1 |
| Med | 12.5 | THC 25 Cal | 1 + 1 |
| High | 16 | THC 25 Ctrl High | 1 + 1 |
| Opiates | |||
| Low | 28 | MD Optional Ctrl Low | 1 + 7 |
| Med | 37.5 | MD Optional CutOff Cal | 1 + 7 |
| High | 47 | MD Optional Ctrl High | 1 + 7 |
| Cocaine (metabolite) | |||
| Low | 28 | MD Optional Ctrl Low | 1 + 7 |
| Med | 37.5 | MD Optional CutOff Cal | 1 + 7 |
| High | 47 | MD Optional Ctrl High | 1 + 7 |
| Amphetamine | |||
| Low | 20 | Amphetamine 200 Serum Cal | 1 + 9 |
| Med | 25 | Amphetamine 200 Serum Cal | 1 + 7 |
| High | 50 | Amphetamine 200 Serum Cal | 1 + 3 |
| Methamphetamine | |||
| Low | 28 | MD Optional Ctrl Low | 1 + 7 |
| Med | 37.5 | MD Optional CutOff Cal | 1 + 7 |
| High | 47 | MD Optional Ctrl High | 1 + 7 |
| Ecstasy | |||
| Low | 37.5 | MD Select Ctrl Low | 1 + 9 |
| Med | 50 | Ecstasy 250 Cal | 1 + 4 |
| High | 75 | MD Select Ctrl High | 1 + 7 |
| EDDP | |||
| Low | 37.5 | MD Optional Ctrl Low | 1 + 1 |
| Med | 50 | MD Optional CutOff Cal | 1 + 3 |
| High | 75 | MD Optional Ctrl High | 1 + 1 |
| Benzodiazepines | |||
| Low | 38 | MD Optional Ctrl Low | 1 + 3 |
| Med | 50 | MD Optional CutOff Cal | 1 + 3 |
| High | 63 | MD Optional Ctrl High | 1 + 3 |
| Ethyl glucuronide | |||
| Low | 75 | EtG 375 Ctrl | 1 + 4 |
| Med | 100 | EtG 100 Cal | Undiluted |
| High | 375 | EtG 375 Ctrl | Undiluted |
The matrix of the standard control material was urine for all parameters except for amphetamine, where a serum standard was applied and diluted with Negative Cal (matrix: urine), to reach the desired concentrations of 20, 25, and 50 ng/ml. One of the QC concentrations per parameter was in the range of the MPA cut‐off. A negative urine control (material: DAU Neg Ctrl) was applied after each calibration. The intra‐day precision and accuracy were calculated for all three QC levels with 20 samples. Inter‐day precision and accuracy were calculated for the low and the high QCs with 7–13 samples. For calculating the accuracy, the manufacturer's stated concentrations and respective dilutions were applied as target concentrations. For amphetamine and methamphetamine, OFT assays, which were originally designed for the analysis of oral fluid samples, were applied, as their sensitivity is higher than the sensitivity of the respective standard clinical urine assays.
Confirmation Analysis
All urine samples were confirmed with standard chromatographic‐mass spectrometric methods, which were fully validated according to the guidelines of the German society of Toxicological and Forensic Chemistry (GTFCh; 2) and accredited according to DIN EN ISO 17025. For THC‐carboxylic acid (THC‐COOH), 1 ml urine per sample was spiked with deuterated THC‐COOH and hydrolyzed with 10 M sodium hydroxide (NaOH) for 15 min at 60°C. The sample was extracted via automated solid phase extraction (SPE) on Chromabond C18 cartridges, derivatized with N‐methyl‐N‐trimethylsilyltrifluoroacetamide (MSTFA), and analyzed with gas chromatography‐mass spectrometry (GC‐MS) (GC: 6890N GC Agilent, Waldbronn, Germany, GC‐column: J&W HP5‐MS, Agilent, Waldbronn, Germany, MS: 5973N MSD, Agilent, Waldbronn, Germany). For ethyl glucuronide, 100 μl urine per sample was spiked with deuterated ethyl glucuronide, diluted with methanol, and analyzed with liquid chromatography‐tandem mass spectrometry (LC‐MS/MS) (LC: LC20AD, Shimadzu, Duisburg, Germany, LC‐column: Hypercarb, Thermo Scientific, Dreieich, Germany, MS: QTrap 4000, ABSciex, Toronto, Kanada). For all other drugs, sulfates or glucuronides in the samples were cleaved with beta‐glucuronidase/arylsulfatase for 2 hr at 45°C. For benzodiazepines, 1 ml urine per sample was spiked with a mixture of deuterated analytes, extracted with 1‐chlorobutane (liquid–liquid extraction) at pH 9, and analyzed with LC‐MS/MS (LC: G1312, Agilent, Waldbronn, Germany, LC‐column: Polar RP, Phenomenex, Aschaffenburg, Germany, MS: QTrap2000, ABSciex, Toronto, Canada).
For the remaining analytes (basic drugs), 1 ml urine per sample was spiked with a mixture of deuterated analytes, extracted via automated SPE on Chromabond Drug cartridges, and analyzed with LC‐MS/MS (LC: G1312, Agilent, Waldbronn, Germany, LC‐column: Luna PFP, Phenomenex, Aschaffenburg, Germany, MS: QTrap2000, ABSciex, Toronto, Canada). The limits of detection (LOD) and limits of quantification (LOQ) are given in Table 4.
Table 4.
Limits of Quantification and Limits of Detection in Urine of the Confirmatory Methods
| Target analyte | Limit of detection [ng/ml] | Limit of quantification [ng/ml] |
|---|---|---|
| THC‐COOH | 2.0 | 5.0 |
| Morphine (opiates) | 1.0 | 2.5 |
| Benzoylecgonine | 2.0 | 5.0 |
| Amphetamine | 2.0 | 2.0 |
| Methamphetamine | 2.0 | 2.0 |
| MDMA, MDA (ecstasy) | 2.0 | 2.0 |
| EDDP | 1.0 | 2.5 |
| Benzodiazepines | 0.5–5.0 | 0.5–5.0 |
| Ethyl glucuronide | 75 | 75 |
Data Analysis
Receiver operating characteristic (ROC) plots 6 were generated by SPSS software (version 22, IBM, Armonk, NY). Sensitivity and specificity were calculated from the ROCs with Microsoft Excel 2010 (Redmont, WA).
Sensitivity and Specificity
The sensitivity of an assay was defined as the ability of the assay to correctly identify samples with analyte concentrations above the MPA cut‐off. The sensitivity was calculated as the number of immunoassay‐positive samples above the respective assay cut‐off minus the number of false‐positive samples then divided by the number of samples with analyte concentrations above the MPA cut‐off. A high sensitivity therefore indicates a low rate of false‐negative samples. For calculating the sensitivity, the result of the mass spectrometric analysis was regarded as positive, if the measured concentration reached or exceeded the required MPA cut‐off, thus samples with a negative immunoassay result and a concentration between the MPA cut‐off and the LOD are not counted as false negatives. According to the MPA guidelines, drug‐positive samples with concentrations below the MPA cut‐off have to be reported as well because the consumption of the respective drug is proven. This has to be taken into account when evaluating the sensitivity of an assay. Samples with concentrations below the MPA cut‐off, which are identified as positive by the respective immunoassay, are therefore regarded as true‐positive samples.
The specificity of an assay was defined as the ability of the assay to correctly identify samples with analyte concentrations below the LOD of the confirmation method. The specificity was calculated as the number of immunoassay‐negative samples below the respective assay cut‐off minus the number of false‐negative samples then divided by the number of samples with analyte concentrations below the LOD of the confirmation method. A high specificity therefore indicates a low rate of false‐positive samples. For calculating the specificity, the result of the mass spectrometric analysis was regarded as positive, if the measured concentration exceeded the LOD of the method. Thus, samples with a positive immunoassay result and a concentration between the MPA cut‐off and the LOD are not counted as false positives but true positives and, according to the MPA guidelines, they have to be reported as positive.
Results and Discussion
Precision and Accuracy
Precision, accuracy, and target concentrations of the control samples are given in Table 4. The relative standard deviation (RSD %) was 11% for the low control of EDDP and below 10% for all other concentrations and parameters. Thus, the precision is high in the low and the high concentration range as well (Table 5).
Table 5.
Precision and Accuracy of Quality Controls
| Immunoassay | MPA | Target concentration [ng/ml] | Precision (RSD %) | Accuracy (bias, %) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intra‐day | Inter‐day | Intra‐day | Inter‐day | |||||||||||
| Cut‐off | Low | Middle | High | Low | Middle | High | Low | High | Low | Middle | High | Low | High | |
| Cannabinoids | 10 | 9.4 | 12.5 | 16 | 2.4 | 1.0 | 0.9 | 5.2 | 5.1 | −1 | 13 | 1 | −12 | 0 |
| Opiates | 25 | 28 | 37.5 | 47 | 2.0 | 1.4 | 1.4 | 6.0 | 3.5 | 2 | 4 | 7 | 1 | 7 |
| Cocaine (metabolite) | 30 | 28 | 37.5 | 47 | 1.1 | 2.9 | 1.5 | 3.1 | 3.2 | 18 | 17 | 25 | 15 | 21 |
| Amphetamine | 50 | 20 | 25 | 50 | 3.8 | 6.5 | 2.5 | 9.9 | 6.6 | 13 | 10 | 11 | 10 | 8 |
| Methamphetamine | 50 | 28 | 37.5 | 47 | 4.4 | 6.4 | 4.3 | 5.3 | 4.5 | 25 | 26 | 32 | 17 | 23 |
| Ecstasy | 50 | 37.5 | 50 | 75 | 7.6 | 1.5 | 0.7 | 4.4 | 3.3 | −19 | 3 | 2 | −21 | −2 |
| EDDP | 50 | 37.5 | 50 | 75 | 6.1 | 4.1 | 2.8 | 11 | 3.6 | 3 | −8 | 7 | 10 | 11 |
| Benzodiazepines | 50 | 38 | 50 | 63 | 2.4 | 2.3 | 3.5 | 4.7 | 4.5a | 3 | 0 | 1 | 16 | 13a |
| Ethyl glucuronide | 100 | 75 | 100 | 375 | 4.8 | 4.5 | 0.9 | 9.7 | 2.0 | −7 | 3 | −6 | −1 | −6 |
N = 4.
Methamphetamine showed the highest intra‐day and inter‐day bias of all parameters (25–32 % and 17–23%, respectively), the bias for cocaine, ecstasy, and benzodiazepines was in the ±25% range. The bias for cannabinoids, opiates, amphetamine, EDDP, and ethyl glucuronide was in the ±15% range.
Sensitivity and Specificity
For evaluating the sensitivity and specificity, only samples with concentrations up to twice (opiates: triple) the MPA cut‐off were applied (see Table 2) because these are the crucial concentrations for the evaluation of the performance of an immunoassay used for abstinence screening.
Sensitivity and specificity diagrams are given in Figure 1. Possible assay cut‐offs and respective sensitivities and specificities are given in Table 6. The assays for opiates, cocaine (metabolite), EDDP, and benzodiazepines showed 100% specificity and sensitivity, simultaneously. At these assays the cut‐off could also be varied in a certain range without generating false negatives, e.g., for opiates from 8 to 16, for cocaine (metabolite) from 10 to 32, for EDDP from 9 to 46, and for benzodiazepines from 13 to 30.
Figure 1.
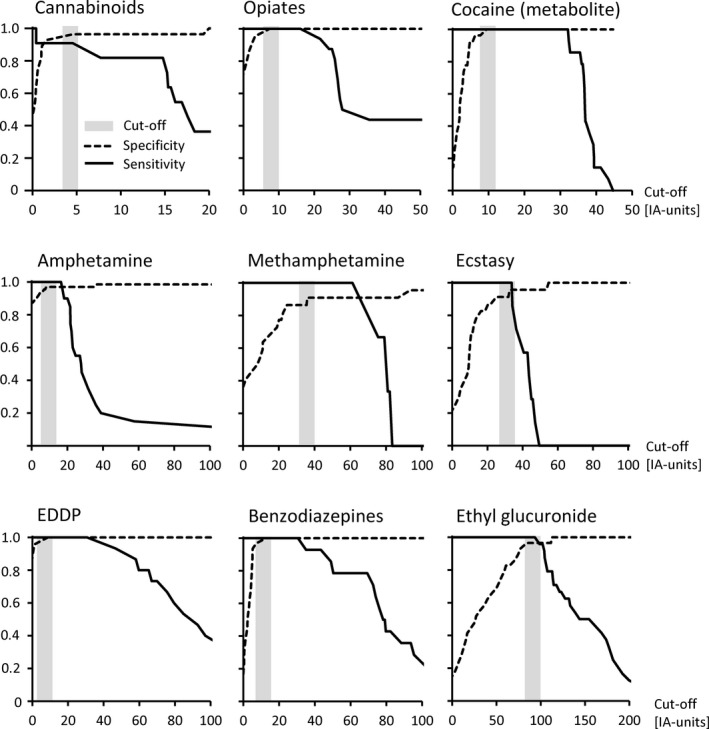
Specificity and sensitivity diagrams calculated from ROC data.
Table 6.
Assay Cut‐Offs and Respective Sensitivity and Specificity
| Immunoassay | Assay cut‐off [IA‐units] | Sensitivity [%] | Specificity [%] |
|---|---|---|---|
| Cannabinoids | 4.0 | 91 | 96 |
| Opiates | 8 | 100 | 100 |
| Cocaine (metabolite) | 10 | 100 | 100 |
| Amphetamine | 8 | 100 | 97 |
| Methamphetamine | 36 | 100 | 91 |
| Ecstasy | 33 | 100 | 96 |
| EDDP | 9 | 100 | 100 |
| Benzodiazepines | 13 | 100 | 100 |
| Ethyl glucuronide | 90 | 100 | 97 |
For cannabinoids, amphetamine, methamphetamine, ecstasy, and ethyl glucuronide, the specificity declines with increasing assay cut‐off before the sensitivity reaches 100%. For those assays several false‐positive samples have to be accepted at the low necessary cut‐off required to fulfill the MPA guidelines. The lack of specificity of the methamphetamine and ecstasy assays at low cut‐offs results from cross‐reactivity with endogenous phenethylamines which can be present in urine samples. Only the cannabinoids assay showed a sensitivity below 100% as there was one sample with a confirmed concentration of 13 ng/ml THC‐COOH and a cannabinoid immunoassay value of 0.4 (assay cut‐off: 4.0). This sample can be regarded as an outlier, resulting from a potential adulteration of the sample with, e.g., an oxidant during collection (leading to a reduction in enzyme activity). The resulting sensitivity for cannabinoids of 91% is acceptable as the GTFCh guidelines 2 demand a rate of detection of at least 9 of 10 positive samples (sensitivity ≥90%) for the validation of immunoassays for MPA cut‐offs.
Conclusion
The evaluation of CEDIA and DRI drugs of abuse and ethyl glucuronide immunoassays for abstinence control on the Thermo Indiko Plus Analyzer showed good results. The method showed sufficient precision and accuracy for urine screening. The sensitivity and specificity are mostly 100% at the assay cut‐offs required to meet the MPA criteria. For the majority of analytes false‐positive immunoassay results are rare, thus only few unnecessary confirmation analyses have to be carried out. More importantly, the only false‐negative result occurred at the cannabinoids assay. So the presence of a drug or metabolite in urine in the concentration range of the MPA cut‐off would be revealed in almost every case. The presented method can therefore be recommended for urine screening for abstinence control on drugs of abuse or ethanol.
Acknowledgements
The authors would like to thank Thermo Fisher Scientific for providing reagents and equipment as well as technical and scientific support. The Institute for Clinical Chemistry and Laboratory Medicine, Medical Center – University of Freiburg is acknowledged for their collaboration.
References
- 1. Schubert W, Dittmann V, Brenner‐Hartmann J. 2013. Beurteilungskriterien: Urteilsbildung in der Fahreignungsbegutachtung, 3rd edn Bonn: Kirschbaum Verlag. [Google Scholar]
- 2. Peters FT, Hartung M, Herbold M, Schmitt G, Daldrup T, Musshoff F. Anhang B zu den Richtlinien der GTFCh zur Qualitätssicherung bei forensisch‐toxikologischen Untersuchungen; Anforderungen an die Validierung von Analysenmethoden. Toxichem Krimtech 2009;76:185–199. [Google Scholar]
- 3. Musshoff F, Wolters T, Lott S, Ippisch J, Gradl S, Madea B. Optimization and validation of CEDIA drugs of abuse immunoassay tests in serum and urine on an Olympus AU 400. Drug Test Anal 2013;5:366–371. [DOI] [PubMed] [Google Scholar]
- 4. Kirschbaum KM, Musshoff F, Wilbert A, Röhrich J, Madea B. Direct ELISA kits as a sensitive and selective screening method for abstinence control in urine. Forensic Sci Int 2011;207:66–69. [DOI] [PubMed] [Google Scholar]
- 5. Agius R, Nadulski T, Moore C. Validation of LUCIO((R))‐Direct‐ELISA kits for the detection of drugs of abuse in urine: Application to the new German driving licence re‐granting guidelines. Forensic Sci Int 2012;215:38–45. [DOI] [PubMed] [Google Scholar]
- 6. Zweig MH, Campbell G. Receiver‐operating characteristic (ROC) plots: A fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561–577. [PubMed] [Google Scholar]


