Abstract
Background
Organizing work flow is a major task of laboratory management. Recently, clinical laboratories have started to adopt methodologies such as Lean Six Sigma and some successful implementations have been reported. This study used Lean Six Sigma to simplify the laboratory work process and decrease the turnaround time by eliminating non‐value‐adding steps.
Methods
The five‐stage Six Sigma system known as define, measure, analyze, improve, and control (DMAIC) is used to identify and solve problems. The laboratory turnaround time for individual tests, total delay time in the sample reception area, and percentage of steps involving risks of medical errors and biological hazards in the overall process are measured.
Results
The pre‐analytical process in the reception area was improved by eliminating 3 h and 22.5 min of non‐value‐adding work. Turnaround time also improved for stat samples from 68 to 59 min after applying Lean. Steps prone to medical errors and posing potential biological hazards to receptionists were reduced from 30% to 3%.
Conclusion
Successful implementation of Lean Six Sigma significantly improved all of the selected performance metrics. This quality‐improvement methodology has the potential to significantly improve clinical laboratories.
Keywords: medical error, pre‐analytical, process flow, quality improvement, quality management, workflow
1. Introduction
The major role of a clinical laboratory is to produce reliable, reproducible, accurate, timely, and correctly interpreted test results to aid clinical decision‐making; the ultimate goal must be to assure desirable clinical outcomes. To achieve this goal, laboratories must establish and maintain quality in all laboratory processes, while focusing on cost‐effectiveness. Currently, clinical laboratories must handle increasing workloads with a broader spectrum of parameters with the same (or fewer) number of staff and must still deliver consistent results with improved turnaround times (TATs) and with utmost quality. Laboratories influence more than 70% of critical medical decisions, such as admitting, treating, and discharging patients, but make up <5% of all health costs.1 Nevertheless, clinical laboratories are under increasing pressure to reduce costs while maintaining or even improving quality standards. The ongoing expansion of diagnostic laboratory testing within given budgetary constraints calls for continued efforts to keep the costs affordable.2, 3 One way to achieve this goal is to simplify the entire laboratory process and eliminate “waste,” not just from the analytical phase but also from the pre‐ and post‐analytical phases. The Lean concept is a quality‐improvement tool that focuses on providing “value” and improving performance by systematically eliminating waste, which we define as anything that does not add value to the final product or service.4
Six Sigma is a quality management strategy that makes efforts to improve the quality of processes and focuses on the identification and removal of defects. A defect is considered to be anything that causes dissatisfaction including unnecessary processes and services. It uses a structured methodology of problem solving described by an acronym “DMAIC” which stands for Define, Measure, Analyze, Improve, and Control. Therefore, Lean Six Sigma (LSS) is a marriage of two different strategies that reduce inefficiencies and increase quality.5, 6
In our central laboratory, we performed a lean‐mapping exercise to identify the sources of sample delays, potential biological hazards, and stages prone to medical errors within the sample reception area and main laboratory. It is critical to have a good understanding of the entire process of sample collection, transportation to the laboratory, sample preparation, analytical procedures, post‐analytical sample handling, and validation of results, to devise appropriate solutions for the laboratory. Our main goals were to reduce TATs by simplifying the process, increase quality by reducing errors, and protect our staff from potential biological hazards. We diagnosed problems during the workflow, eliminated steps that do not add value to the final product, improved sample flow, and reduced sample transit time according to DMAIC.
2. Materials and Methods
Each year, our central laboratory handles over 650 000 samples and produces roughly 6.5 million test results, with workloads showing a fairly consistent 5‐6% increase each year. Because it is a central laboratory, it conducts specialized analyses in clinical chemistry, endocrinology, microbiology, serology, and immunology. The peak period during which samples arrive at the laboratory is at 09:00 AM and the load continues unabated into the early evening (Figure 1).
Figure 1.
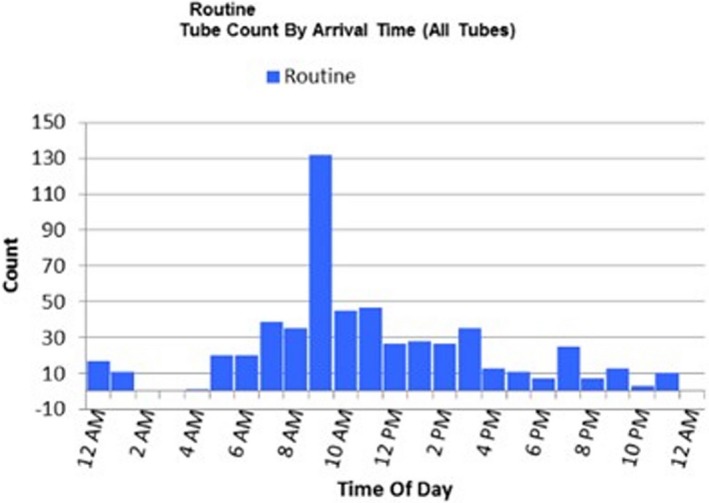
Routine tube count by arrival time
2.1. Study design
This study was a longitudinal, before–after analysis of process improvements in the central laboratory of a teaching university hospital. A quality‐improvement team collected data on delays in the reception area and extracted turnaround times from the laboratory information system.
2.2. Study setting
The study was performed in the central laboratory of Çukurova University Teaching Hospital, Adana, Turkey, which has approximately 1000 beds. The laboratory has been accredited by the Joint Commission International since 2006, making it the first accredited clinical laboratory in a university hospital in Turkey.
2.3. Intervention
2.3.1. Pre‐intervention
For inpatients, samples are collected in the wards, mostly by nurses and interns, and then barcoded and sent to the central laboratory reception unit via a pneumatic tube system. For outpatients, samples are collected by an experienced seven‐nurse phlebotomy team in the sample‐collection area, and sent to the sample‐reception area via the pneumatic tube system. Appropriate, quantitatively adequate samples are accepted, sorted, and sent to the main laboratory where the tests are performed. The results of tests are validated and interpreted (if necessary), and then distributed via the electronic hospital information system.
2.3.2. Intervention
The intervention was a focused Lean‐based reorganization of the flow of the laboratory process. The main goal was to preserve steps that provide value and eliminate sources of waste. Because the Lean concept is complementary to Six Sigma and they can be combined to create LSS, the five‐stage system of Six Sigma known as DMAIC was used.
2.4. Methods of measurement
The primary outcomes measured were the laboratory TATs for individual tests, defined as the time interval between sample collection and the final result; the total delay time in the sample‐reception area, and the percentage of steps prone to medical errors or that could expose staff to biological hazards in the overall process.
2.5. Data collection and analysis
Data were extracted from the laboratory information system for both the pre‐ and post‐intervention periods. Delay times in the reception area were measured on different days of the week and the average delay time was calculated. Steps that require sample handling (sorting, aliquoting, and so forth) are considered potential biological hazards and those that need re‐barcoding for any reason (e.g., low‐quality barcodes, erroneously labeled tubes) are considered potential sources of medical errors. Data analysis was performed with the statistical software Minitab Version 17 (Minitab, Ltd., Coventry, UK). Differences between two proportions were estimated by Fisher's Exact test and P<.05 was considered to be statistically significant.
3. Results
In the reception area, 250 to 300 tubes a day or 25‐30% of all samples were erroneously labeled. This was due to a lack of training and low‐quality barcodes. Samples from the outpatient unit were barcoded correctly because the phlebotomy team was well‐trained, although there were still problems because of low‐quality barcodes. Each faulty barcode needed to be replaced with a new printed barcode, and this step was time consuming, increased the risk of exposure to biological hazards for the staff, and was prone to mislabeling errors. The need to replace the barcodes for 300 of 1000 specimens on average means that 30% of the samples were associated with medical errors and potential biological hazards. The average time to change a barcode recorded by the Lean team was 45 s (the staff had to check the patient in the hospital information system, remove the faulty barcode, print a new one, and re‐label the sample). For 300 erroneously labeled samples, that represented 3 h and 45 min of wasted time.
First, we retrained ward personnel, including nurses and interns, using written and visual material, and then conducted on‐site practical training emphasizing the importance of correct barcoding for patient safety (Figure 2). The low‐quality barcodes were discarded and new high‐quality barcodes were purchased. During this training period, the Lean team evaluated the impact of the training by counting the incorrectly labeled barcodes, and observed a decline. Three months later, we repeated the study and found that the number of incorrectly labeled barcodes had dropped to 25 to 30 per day (Figure 3). This reduced the wasted time from 3 h and 45 min to 22.5 min, saving 3 h and 22.5 min per day. In addition, the percentage of samples associated with medical errors and potential biological risk dropped from 30% to 3% (P=.0000).
Figure 2.
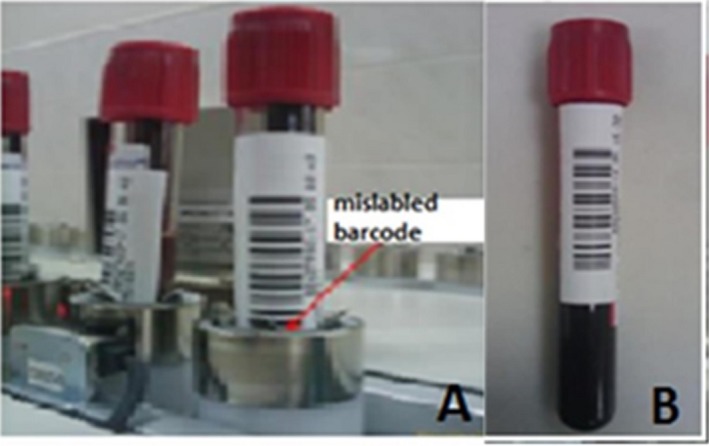
A mislabeled tube that cannot be read by the autoanalyzer (A). A correctly labeled tube (B), published in the training documents and laboratory information system
Figure 3.
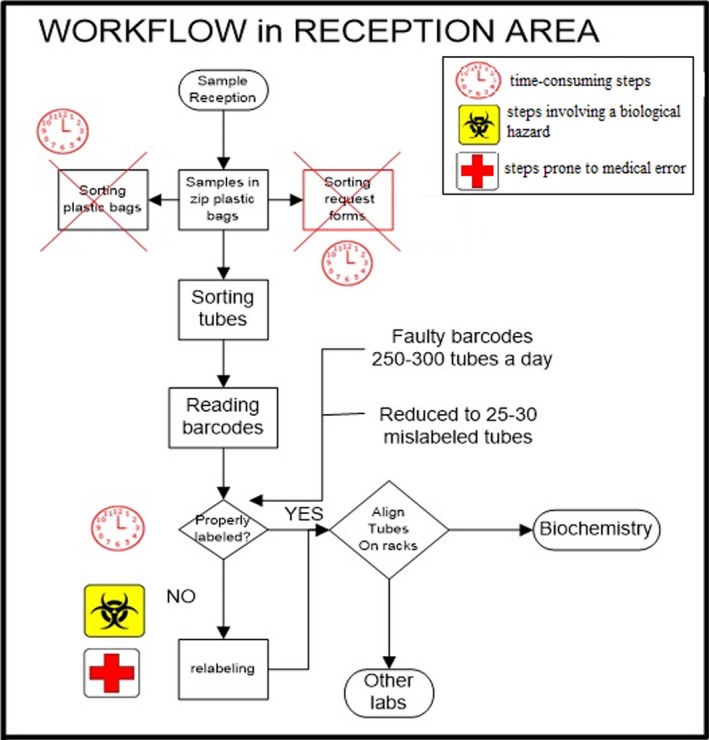
Workflow analysis after intervention. Steps adding no value are shown with crosses.
Although the laboratory has been receiving electronic orders since 2006, written request forms are still used and one individual was responsible for sorting these forms. These non‐value‐adding steps were considered waste; we eliminated written forms, which saved about 1 h per day (Figure 3).
This Lean analysis also improved TATs for urgent samples, which were subject to the same problems described above. By the end of the study, the average TAT had improved by 9 min (99% CI: 8‐11 min), and this achievement was maintained in the following months (Figure 4).
Figure 4.
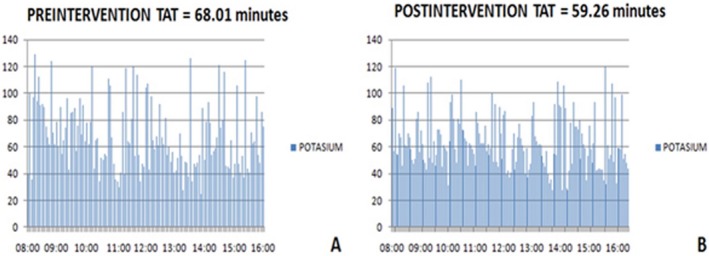
TATs before (A) and after (B) intervention
4. Discussion
The main task of a clinical laboratory is to improve clinical outcomes by providing accurate results in a timely manner. Although the workflow processes in most accredited laboratories are well designed, there are still inefficiencies that can affect quality, such as unnecessary duplication of services, long waiting times, and delays. At our hospital, patient satisfaction is highly dependent on the turnaround times of test results, and long waiting times have been identified as a major cause of patient discontent. Lean is a viable methodology for improving the efficiency and effectiveness of clinical laboratory procedures.7, 8, 9 Six Sigma quality‐management methodologies complement Lean, and can easily be applied to any clinical laboratory.10
A before–after study conducted by Moron et al.11 showed that patient satisfaction was improved and the number of complaints regarding delays was reduced after the Lean methodology was applied to their laboratory. Other studies have reviewed the importance of Lean in anatomy and surgical pathology laboratories for improving the accuracy of diagnostic and molecular testing, reducing TATs, and increasing physician and patient satisfaction.12, 13 Even avoiding unnecessary walking in the laboratory can increase satisfaction and quality of care.14, 15
The application of Lean concepts to the laboratory environment can be used to examine its normal operation, highlighting where typical problems occur and ultimately improving processes and quality of care. Phlebotomy units are one of the most important parts of a clinical laboratory, and any mistakes and delays in this part of the lab affect the other parts. Improved timeliness of blood draws and decreased error rates can be achieved using Lean principles.16 Clinical laboratories are very complex, dynamic organizations that always need to improve the quality of testing and meet stringent guidelines, while trying to decrease costs. It is obvious that a fundamental change in service delivery methodology is necessary to cope with declining health reimbursements.
Simultaneously, laboratory staff satisfaction is as important as patient satisfaction. It is critical to involve all levels of staff in the process to produce better results. Our laboratory staff, particularly those who work in the reception area, are involved in the “Lean Team” approach, to understand the need for change better. Their feedback was positive, which proves the importance of their involvement in each and every step of the Lean process.
After initiating and implementing the Lean process throughout the laboratory, a significant improvement in TAT was observed. The steps prone to medical errors and associated with potential risk of exposure to biological hazards were reduced. There are other reports of the adoption of Lean principles producing desirable outcomes, including a reduction in operational expenses and improved work–life balance for laboratory personnel.17 Lean principles could even be used to improve analytical methods. In a study designed to improve selenium analysis, an LSS approach provided more reliable results, a greatly reduced cycle time, and superior control features.18 Damato19 applied LSS to reduce hemolytic samples in an emergency care center and reduced hemolysis from 9.8% to 0.88%.
There were some limitations to our study. The most important is that this study was performed at a single institution, and the findings might not be generalizable to other clinical laboratories with markedly different laboratory process flows. However, our findings and implementation of Lean should be of value to other laboratories at some level. In addition, we did not assess changes in patient satisfaction due to process improvement initiatives. However, it is likely that shortened waiting times and improved TATs would increase patient satisfaction. Lastly, determining the cost‐effectiveness of implementing the process improvements was beyond the scope of this study.
In conclusion, laboratory management is required to decrease costs, increase efficiency, and promote user satisfaction by emphasizing quality. After the successful implementation of quality‐improvement strategies, all selected performance metrics showed significant improvements and sustainability in the subsequent 3 years. There seems to be no ideal solution or single concept that suits all clinical laboratories, but each organizational model has different impacts according to different types of waste in a process. The LSS approach has found its way into the healthcare sector, and more studies on this subject will improve process flows to provide more efficient and productive services, which definitely affect overall patient care.
Acknowledgments
The authors would like to acknowledge the contributions that Umut Uçan, a Laboratory Workflow Consultant employed by Beckman Coulter, made to designing the study and collecting data.
Inal TC, Goruroglu Ozturk O, Kibar F, et al. Lean six sigma methodologies improve clinical laboratory efficiency and reduce turnaround times. J Clin Lab Anal. 2018;32:e22180 10.1002/jcla.22180
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/byHA7N
References
- 1. Busby J, Schroeder K, Woltersdorf W, et al. Temporal growth and geographic variation in the use of laboratory tests by NHS general practices: using routine data to identify research priorities. Br J Gen Pract. 2013;63:pE256–pE266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fryer AA, Smellie WS. Managing demand for laboratory tests: a laboratory toolkit. J Clin Pathol. 2013;66:62–72. [DOI] [PubMed] [Google Scholar]
- 3. Janssens PM. Managing the demand for laboratory testing: options and opportunities. Clin Chim Acta. 2010;411:1596–1602. [DOI] [PubMed] [Google Scholar]
- 4. Villa D. Automation, lean, six sigma: synergies for improving laboratory efficiency. J Med Biochem. 2010;29:339–348. [Google Scholar]
- 5. Nevalainen D, Berte L, Kraft C, Leigh E, Picaso L, Morgan T. Evaluating laboratory performance on quality indicators with the six sigma scale. Arch Pathol Lab Med. 2000;124:516–519. [DOI] [PubMed] [Google Scholar]
- 6. Coskun A, Inal TC, Serteser M, eds. Six Sigma Projects and Personal Experiences. Croatia: InTech; 2011. [Google Scholar]
- 7. Samuel L, Novak‐Weekley S. The role of the clinical laboratory in the future of health care: lean microbiology. J Clin Microbiol. 2014;52:1812–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Clark DM, Silvester K, Knowles S. Lean management systems: creating a culture of continuous quality improvement. J Clin Pathol. 2013;66:638–643. [DOI] [PubMed] [Google Scholar]
- 9. Knowles S, Barnes I. Lean laboratories: laboratory medicine needs to learn from other industries how to deliver more for less. J Clin Pathol. 2013;66:635–637. [DOI] [PubMed] [Google Scholar]
- 10. Coskun A, Unsal I, Serteser M, Inal T. Six Sigma as a Quality Management Tool: Evaluation of Performance in Laboratory Medicine, Quality Management and Six Sigma. 2010. Abdurrahman Coskun (Ed.), InTech, DOI: 10.5772/9928. Available from: http://www.intechopen.com/books/quality-management-and-six-sigma/six-sigma-as-a-quality-management-tool-evaluation-of-performance-in-laboratory-medicine [DOI]
- 11. Morón‐Castañeda LH, Useche‐Bernal A, Morales‐Reyes OL, et al. Impact of Lean methodology to improve care processes and levels of satisfaction in patient care in a clinical laboratory. Rev Calid Asist. 2015;30:289–296. [DOI] [PubMed] [Google Scholar]
- 12. Visinoni F. Towards the lean lab: the industry challenge. Recent Results Cancer Res. 2015;199:119–133. [DOI] [PubMed] [Google Scholar]
- 13. Smith ML, Wilkerson T, Grzybicki DM, Raab SS. The effect of a Lean quality improvement implementation program on surgical pathology specimen accessioning and gross preparation error frequency. Am J Clin Pathol. 2012;138:367–373. [DOI] [PubMed] [Google Scholar]
- 14. Hayes KJ, Reed N, Fitzgerald A, Watt V. Applying lean flows in pathology laboratory remodeling. J Health Organ Manag. 2014;28:229–246. [DOI] [PubMed] [Google Scholar]
- 15. Yerian LM, Seestadt JA, Gomez ER, Marchant KK. A collaborative approach to lean laboratory workstation design reduces wasted technologist travel. Am J Clin Pathol. 2012;138:273–280. [DOI] [PubMed] [Google Scholar]
- 16. Le RD, Melanson SE, Santos KS, et al. Using Lean principles to optimise inpatient phlebotomy services. J Clin Pathol. 2014;67:724–730. [DOI] [PubMed] [Google Scholar]
- 17. Mitchell PS, Mandrekar JN, Yao JD. Adoption of lean principles in a high‐volume molecular diagnostic microbiology laboratory. J Clin Microbiol. 2014;52:2689–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cloete BC, Bester A. A Lean Six Sigma approach to the improvement of the selenium analysis method. Onderstepoort J Vet Res. 2012;79:E1–E13. [DOI] [PubMed] [Google Scholar]
- 19. Damato C, Rickard D. Using Lean‐Six Sigma to reduce hemolysis in the emergency care center in a collaborative quality improvement project with the hospital laboratory. Jt Comm J Qual Patient Saf. 2015;41:99–107. [DOI] [PubMed] [Google Scholar]


