Abstract
Background
The use of dried blood spots (DBS) for the assay of lysosomal enzymes has facilitated the implementation of pilot studies for newborn screening for lysosomal storage disorders in various developed countries. The aim of the study was to determine the influence of ambient temperature during DBS preparation and storage on lysosomal enzyme activity in a developing, tropical country.
Methods
Blood samples from 12 healthy subjects collected on a S&S 903 filter paper were dried and stored at different temperatures for different periods of time. Activities of five lysosomal enzymes (acid α‐glucosidase, acid α‐galactosidase, acid β‐glucocerebrosidase, acid sphingomyelinase, and galactocerebrosidase) were determined by tandem mass spectrometric and fluorimetric (acid α‐glucosidase and acid β‐glucocerebrosidase only) assays.
Results
The mean activities of all five enzymes decreased significantly when DBS was dried at temperatures above 24°C (P<.0001). DBS stored at 4°C, 24°C, 30°C, 37°C, and 45°C for 10 days and more, also showed significant reduction in activities of all five enzymes (P<.0001).
Conclusion
The results highlight the importance of maintaining the correct ambient temperature during DBS preparation and storage to avoid false positive results when screening for lysosomal storage disorders.
Keywords: ambient temperature, dried blood spot, fluorimetry, lysosomal enzymes, tandem mass spectrometry
1. Introduction
Lysosomal storage disorders (LSDs), a varied group of inherited metabolic disorders caused by defects of lysosomal function, are individually rare, but collectively common with the prevalence reported to range from 1 in 1500‐7000 live births.1, 2 Their clinical presentation varies widely and some of the common clinical findings include skeletal abnormalities, visceromegaly, and often, features of central nervous system dysfunction, ranging from behavioral abnormalities to severe mental retardation.1, 3 Recently, the availability of effective treatment options has encouraged the implementation of pilot newborn screening programs for selected LSDs in several developed countries.4, 5, 6, 7 Enzyme replacement therapy,1, 8 bone marrow or hematopoietic stem cell transplant,9 and substrate reduction therapy10 are some of the available treatment options for these disorders. Early commencement of the appropriate treatment strategy is crucial to prevent irreversible organ damage.
The diagnosis of LSDs is usually performed by assaying the lysosomal enzyme of interest by means of a synthetic or natural substrate, in serum, leukocytes and fibroblasts. Chamoles et al.11 first demonstrated enzymatic diagnosis of LSDs using dried blood spots (DBS) collected on a filter paper. Later, Li et al.12 developed a multiplexed MS/MS assay to measure the activities of five lysosomal enzymes in DBS:acid α‐glucosidase (GAA), acid α‐galactosidase (GLA), acid β‐glucocerebrosidase (GBA), acid sphingomyelinase (ASM), and galactocerebrosidase (GALC). Zhang and co‐workers reported the assay for measurement of lysosomal enzymes in DBS in a high‐throughput manner suitable for newborn screening laboratories.13 Given the easy sampling, shipping, and stability of samples, DBS has evident advantages over other sample types.14 Several pilot studies on newborn screening for single diseases or multiple LSDs with fluorimetric and mass spectrometric methods have been carried out using the DBS technology.15, 16, 17, 18, 19
When conducting tests on DBS, the integrity of the sample is very important as samples may be collected in diverse locations under different conditions and transported by various means. Few studies have shown that the enzyme activity in DBS depends on storage temperature, heat and the method of DBS preparation.20, 21, 22, 23 However, there are no published literature from tropical countries with limited resources, on the effect of ambient temperature on sample preparation and storage. Since the ambient temperature in certain regions of India is over 45°C during summer months, enzyme activities could be affected during DBS preparation and postal transport. Therefore, the aim of our present study was to elucidate the effect of high ambient temperatures during DBS preparation and the effect of DBS storage time and temperature on the activities of five lysosomal enzymes (GAA, GLA, GBA, ASM, and GALC) measured by tandem mass spectrometry and fluorimetric method. The deficiency of GAA, GLA, GBA, ASM, and GALC, results in Pompe disease, Fabry disease, Gaucher disease, Niemann‐Pick disease types A and B, and Krabbe disease, respectively.2
2. Materials and Methods
2.1. DBS sample preparation
The study was approved by the Ethics Committee of the National Institute of Mental Health and Neuro Sciences (NIMHANS), Bangalore, India, and the work was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Written informed consent was obtained from all the participants. A total of 12 clinically normal subjects, aged between 25 and 30 years, with no known genetic or chronic disorders, were selected for the study. Venous blood samples were collected in ethylenediaminetetraacetic acid (EDTA) vials and immediately mixed by inverting 5‐10 times. Blood samples were spotted onto Whatman S&S (Schleicher & Schuell) 903 sample collection cards.
2.2. Effect of drying temperature and storage conditions
In order to test the effect of drying temperature of the DBS on lysosomal enzyme activities, one set of DBS samples from the 12 subjects were placed in racks and dried for 4 hours at 24°C, which is the ambient temperature of an air‐conditioned room. This was used as the control. The other DBS samples were kept at 30°C, 37°C, and 45°C for 4 hours each.
To evaluate the effect of DBS storage temperature on stability of enzyme activities, DBS were stored at −80°C, 4°C, 20°C, 30°C, 37°C, and 45°C for 10, 20, 30, 40, and 60 days each. The lysosomal enzymes, GAA, GLA, GBA, ASM, and GALC, were estimated in all DBS specimens by a multiplex assay using electrospray ionization‐tandem mass spectrometer (ESI‐MS/MS).
2.3. LSD MS/MS Multiplex assay cocktail preparation
Vials containing substrate and internal standard for assaying lysosomal enzymes were obtained from the Centers for Disease Control and Prevention (CDC) foundation (Atlanta, GA, USA). Appropriate buffer and detergent solutions were added to known lyophilized amounts of substrates and internal standards to create specific cocktails. On mixing, aliquots of the cocktails were then stored at −20°C until use. Quality control samples with low, medium, and high enzyme activities were obtained from CDC foundation.
2.4. Enzyme activity by MS/MS assay
Five enzyme activities were measured using the modification of Li's multiplex assay by MS/MS as described by Zhang et al.13 Two 3.2 mm diameter spots were punched from each DBS sample and blank filter paper. The first spot was extracted in 20 mmol/L sodium phosphate buffer for 1 hour at 37°C with shaking on a rocking platform at ~1000 rpm. The DBS extract was then added to GAA, GLA, GBA, or ASM assay cocktail in separate wells. The second spot was added to a well containing GALC assay cocktail. The plates were then sealed with aluminum foil and incubated in a thermostated orbital shaker at ~225 rpm and 37°C for 20‐24 hours. The enzymatic reactions were stopped with 1:1 ethyl acetate:methanol. After quenching, the five assay reactions were then combined into a single deep well plate, where ethyl acetate and HPLC grade water (liquid extraction) were added and mixed. The top layer was transferred to a new deep well plate and dried under a stream of nitrogen. The dried plate was reconstituted with 19:1 ethyl acetate:methanol. After reconstitution, the samples were subjected to a solid phase extraction step performed with 100 mg silica gel and washing with ethyl acetate:methanol. The samples were dried under nitrogen, sealed and stored at −20°C. Prior to MS/MS analysis, plates were thawed and reconstituted with acetonitrile:water containing formic acid. QC samples were run on each plate. All analytes were monitored by Selected Reaction Monitoring (SRM) using the Waters MS/MS Quattro Micro Research System. The enzyme activity of each sample was calculated from the ion abundance ratio of product to internal standard measured by the MS/MS. All enzyme activities were blank subtracted. Activity was expressed as micromoles of product per liter of whole blood per hour (μmol/L/h).
2.5. Fluorescent GAA activity assay
Acid α‐glucosidase activity in DBS was measured using a modification of an assay described by Nestor Chamoles et al. One 3.2 mm spot was punched from each DBS sample and extracted in water for 1 hour at 10°C shaking at 300 rpm. Substrate solution (1.4 mmol 4‐methylumbelliferyl‐α‐d‐glucopyranoside) was prepared by adding 70 mmol/L 4‐MUG in DMSO solution to 40 mmol/L sodium acetate (pH 3.8). Substrate solution and 8μmol/L acarbose in water was added to DBS extract in new black 96‐well plates. The samples were incubated for 20 hours at 37°C covered with aluminum seal. The reaction was terminated by addition of 150 mmol/L EDTA. 4‐Methylumbelliferone (4‐MU) standard curve (0‐2.5 mol/L) was prepared in duplicate by diluting 25 mmol/L of 4‐MU in DMSO in water. A quantity of 150 mmol (pH‐11.5) EDTA was added to stop the reaction. The plate was read on a fluorimeter at 355 nm excitation and 460 nm emission wavelengths. From the standard curve, molar product quantities were calculated by linear regression. GAA activity of the sample is calculated by subtracting the individual blank activity.
2.6. Fluorescent GBA activity assay
Acid β‐glucocerebrosidase activity in DBS was measured using a modification of an assay described by Nestor Chamoles et al. One 3.2 mm spot was punched from each DBS sample and extracted in 0.2 mol/L citrate‐phosphate buffer with 1% sodium taurodeoxycholate and 1% triton X‐100 (pH 5.2) for 1 hour at RT shaking at 300 rpm. The substrate solution was prepared by adding 1 mol/L 4‐MUG in DMSO to water and to 0.26 mol/L conduritol B epoxide solution (CBE). The final reaction mixture in one well contained substrate solution in water and DBS extract and in another well substrate solution in water with CBE and DBS extract. The samples were incubated for 20 hours at 37°C covered with aluminum seal. The reaction was terminated by addition of 0.5 mol/L EDTA (pH‐11.5). 4‐MU standard curve (0‐2.5 mol/L) was prepared in duplicate by diluting 4M of 4‐MU in DMSO in water. A quantity of 0.5 mL (pH‐11.5) EDTA was added to stop the reaction. The plate was read on a fluorimeter at 355 nm excitation and 460 nm emission wavelengths. From the standard curve, molar product quantities were calculated by linear regression. GBA activity of the sample is calculated by subtracting individual activity with CBE from the activity generated without CBE.
3. Results
3.1. Effect of DBS drying temperatures on enzyme activity
Enzyme activities in DBS dried at 30°C, 37°C, and 45°C for 4 hours were compared with control DBS enzyme activities dried at 24°C. The mean activities of all five enzymes significantly decreased when DBS was dried at temperatures above 24°C (P<.0001). While the mean activities in DBS dried at 30°C measured between 66% (GAA) and 83.51% (GALC) of the mean control DBS activities, the mean activities in DBS dried at 37°C measured between 49.25% and 67.6% of the mean control DBS activities. The activities decreased even more when the DBS were exposed to 45°C (31.5%‐48.3%). Activities of GAA, GBA, GLA, ASM, and GALC in DBS measured using the MS/MS are shown in Figure 1.
Figure 1.
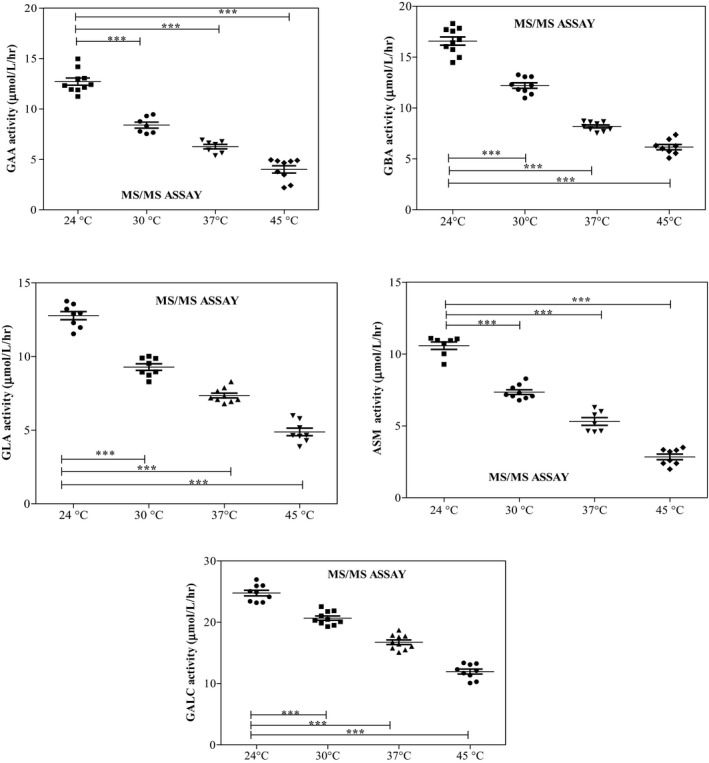
Enzyme activities of acid α‐glucosidase, acid β‐glucocerebrosidase, acid α‐galactosidase, acid sphingomyelinase, and galactocerebrosidase in dried blood spots dried at different temperatures
3.2. Effect of storage conditions on enzyme activity
Effect of different storage conditions on the enzyme activities have been depicted in Figure 2. Enzyme activities in DBS stored at 4°C, 24°C, 30°C, 37°C, and 45°C for 10, 20, 30, 40, and 60 days were compared with the enzyme activities in DBS samples stored at −80°C. The activity of GAA decreased significantly when stored for 10 days at 4°C (P=.02). It decreased further when kept at higher temperatures for 10 days (P<.0001). Decrease in the activity became highly significant when stored for more than 10 days at temperatures higher than −80°C (P<.0001). Similarly, GBA activity also decreased when stored for 10 days at 4°C (P=.0011). Activity decreased even more at higher temperatures (P<.0001). The decrease in activity was significantly higher when stored for longer duration at higher temperatures (P<.0001). Least activity was observed when kept for 60 days.
Figure 2.
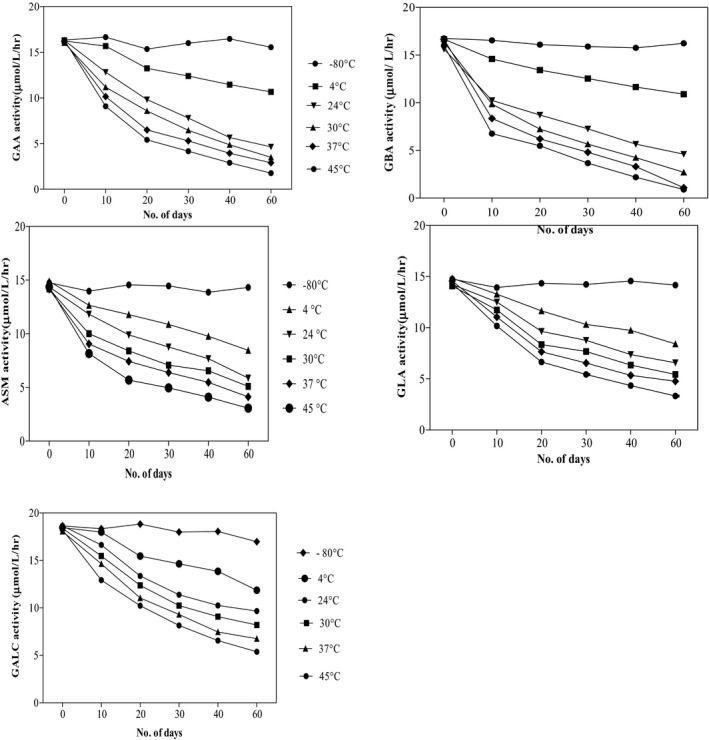
Effect of different storage temperatures on the enzyme activity
Acid sphingomyelinase activity showed comparable results. Lower activity was observed at 4°C, when stored for 10 days (P=.0007). Activity decreased significantly at higher temperatures (P<.0001), with further decrease when stored for more than 10 days (P<.0001).
Difference in GLA activity was not significant (P=.15) when stored at 4°C for 10 days, compared to activity at −80°C. However, difference was significant when stored at 24°C (P=.03) and 30°C (P=.0003), for 10 days. Lower activity was observed at 37°C and 45°C (P<.0001). Activity decreased even more when stored for longer duration (P<.0001). Likewise, GALC activity did not show significant difference (P=.35) at 4°C, but it decreased significantly when kept at 24°C and higher temperature (P<.0001), when stored for 10 days. Least activity was observed when stored for longer duration (P<.0001).
Acid α‐glucosidase and GBA activities in DBS were also measured using the fluorimetric assay, and the results were compared with that of MS/MS assay (Figure 3). We observed no difference in the results of the two methods.
Figure 3.
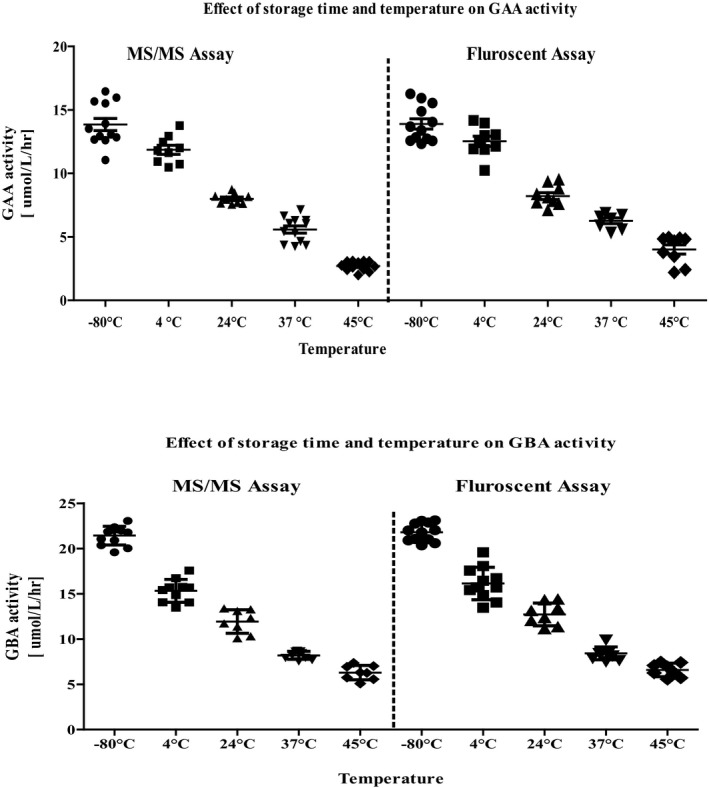
Comparison of MS/MS and fluorimetric methods for determining acid α‐glucosidase and acid β‐glucocerebrosidase in dried blood spots stored at various temperatures
4. Discussion
Despite the increasing acceptance of using DBS for analysis of lysosomal enzymes for newborn screening for LSDs, the method has its limitations. Enzyme activities in DBS can be affected by many factors related to blood collection and sample preparation.7, 24, 25 During DBS preparation, it is vital to dry blood spots completely before transportation. Specifically, a minimum of 3‐4 hours drying in an open space at room temperature is recommended.26, 27 In India, the ambient temperature is over 30°C during several months in summer. Our study showed that enzyme activities decreased significantly when DBS was dried at temperatures above 24°C (P<.0001). Our results are consistent with the finding of Elbin et al., who observed decreased mean enzyme activities in DBS dried at 37°C (measured between 55.3% and 80.7% of the mean control DBS activities), and sufficient loss of activities for all five enzymes in DBS dried at 45°C.23 These findings emphasize the need for blood sample collection and DBS preparation in an air‐conditioned environment where the temperature is maintained at 24°C, to reduce the false positive rates. In India, where a large number of deliveries occur at home, these factors need to be taken into account, especially in regions with high ambient temperatures, for obtaining the right specimen during newborn screening.
After collection, the DBS samples need to be transported to a central laboratory where the samples are pooled for analysis. The stability of the samples during transport under adverse environmental conditions and the temperature at which the samples are stored after reaching the laboratory is also an important concern. The high temperature and humidity of the DBS environment may contribute to changes in the concentrations of enzymes. De Jesus et al.21 observed loss of enzyme activity in DBS stored at 37°C and 45°C ranging from 4.5% to 61% of day 0 activities over the 187 days studied, while samples stored at −20°C and 4°C were shown to be stable for 6 months, with minimal loss of enzyme activity. Our study demonstrated that DBS samples stored at temperatures 4°C and above, for 10 days and more, lead to marked loss of enzyme activities. The shorter enzyme stability found in our study could be due to the exposure of the samples to higher relative humidity. We have not employed measures to control humidity when collecting and storing samples, and this is a major limitation of our current study. In India and other humid tropical countries, where the ambient temperature is high, and transport to a central laboratory for analysis may take time, this aspect needs to be taken into account to avoid false positive results. However, storing and transporting the DBS sample in sealed plastic bags with desiccant may preserve the enzyme activity if delay in analysis is anticipated.
Since tandem mass spectrometry and fluorimetry are the widely accepted analytical techniques used in newborn screening,19 we compared both the methods by determining GAA and GBA activity. We observed no difference in the results of the two methods.
In conclusion, if large scale screening for LSDs is introduced in India or other tropical, developing countries, it is essential to collect and dry blood spots in an air‐conditioned room to maintain an ambient temperature of 24°C or less. In addition, the samples need to be transported to the laboratory and analysis carried out within 10 days for obtaining accurate results.
Acknowledgments
The authors thank the Department of Biotechnology (DBT), Government of India, for funding this research (Grant ref no. BT/PR/14313/MED/30/472/2010).
Supriya M, De T, Christopher R. Effect of temperature on lysosomal enzyme activity during preparation and storage of dried blood spots. J Clin Lab Anal. 2018;32:e22220 10.1002/jcla.22220
References
- 1. Wraith JE. The clinical presentation of lysosomal storage disorders. Acta Neurol Taiwan. 2004;13:101‐106. [PubMed] [Google Scholar]
- 2. Staretz‐Chacham O, Lang TC, LaMarca ME, Krasnewich D, Sidransky E. Lysosomal storage disorders in the newborn. Pediatrics. 2009;123:1191‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vellodi A. Lysosomal storage disorders. Br J Haematol. 2005;128:413‐431. [DOI] [PubMed] [Google Scholar]
- 4. Giugliani R. Newborn screening for lysosomal diseases: current status and potential interface with population medical genetics in Latin America. J Inherit Metab Dis. 2012;35:871‐877. [DOI] [PubMed] [Google Scholar]
- 5. Matern D, Oglesbee D, Tortorelli S. Newborn screening for lysosomal storage disorders and other neuronopathic conditions. Dev Disabil Res Rev. 2013;17:247‐253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pass K, Green NS, Lorey F, Sherwin J, Comeau AM. Pilot programs in newborn screening. Ment Retard Dev Disabil Res Rev. 2006;12:293‐300. [DOI] [PubMed] [Google Scholar]
- 7. Gelb MH, Turecek F, Scott CR, Chamoles NA. Direct multiplex assay of enzymes in dried blood spots by tandem mass spectrometry for the newborn screening of lysosomal storage disorders. J Inherit Metab Dis. 2006;29:397‐404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Desnick RJ. Enzyme replacement and beyond. J Inherit Metab Dis. 2001;24:251‐265. [DOI] [PubMed] [Google Scholar]
- 9. Prasad VK, Kurtzberg J. Transplant outcomes in mucopolysaccharidoses. Semin Hematol. 2010;47:59‐69. [DOI] [PubMed] [Google Scholar]
- 10. Muro S. New biotechnological and nanomedicine strategies for treatment of lysosomal storage disorders. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:189‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chamoles NA, Blanco MB, Gaggioli D, Casentini C. Hurler‐like phenotype: enzymatic diagnosis in dried blood spots on filter paper. Clin Chem. 2001;47:2098‐2102. [PubMed] [Google Scholar]
- 12. Li Y, Scott CR, Chamoles NA, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang XK, Elbin CS, Chuang WL, et al. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008;54:1725‐1728. [DOI] [PubMed] [Google Scholar]
- 14. Li W, Tse FL. Dried blood spot sampling in combination with LC‐MS/MS for quantitative analysis of small molecules. Biomed Chromatogr. 2010;24:49‐65. [DOI] [PubMed] [Google Scholar]
- 15. Chien YH, Chiang SC, Zhang XK, et al. Early detection of pompe disease by newborn screening is feasible: results from the Taiwan screening program. Pediatrics. 2008;122:e39‐e45. [DOI] [PubMed] [Google Scholar]
- 16. Chien YH, Lee NC, Thurberg BL, et al. Pompe disease in infants: improving the prognosis by newborn screening and early treatment. Pediatrics. 2009;124:e1116‐e1125. [DOI] [PubMed] [Google Scholar]
- 17. Dajnoki A, Muhl A, Fekete G, et al. Newborn screening for pompe disease by measuring acid alpha‐glucosidase activity using tandem mass spectrometry. Clin Chem. 2008;54:1624‐1629. [DOI] [PubMed] [Google Scholar]
- 18. Dajnoki A, Fekete G, Keutzer J, et al. Newborn screening for fabry disease by measuring gla activity using tandem mass spectrometry. Clin Chim Acta. 2010;411:1428‐1431. [DOI] [PubMed] [Google Scholar]
- 19. Gelb MH, Scott CR, Turecek F. Newborn screening for lysosomal storage diseases. Clin Chem. 2015;61:335‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freer DE. Observations on heat/humidity denaturation of enzymes in filter‐paper blood spots from newborns. Clin Chem. 2005;51:1060‐1062. [DOI] [PubMed] [Google Scholar]
- 21. De Jesus VR, Zhang XK, Keutzer J, et al. Development and evaluation of quality control dried blood spot materials in newborn screening for lysosomal storage disorders. Clin Chem. 2009;55:158‐164. [DOI] [PubMed] [Google Scholar]
- 22. de Castilhos CD, Mezzalira J, Goldim MP, Werlang FG, Coelho JC. Effect of sample collection, temperature and time of storage on beta‐galactosidase and total hexosaminidase activities in dried blood collected on filter paper. Clin Chem Lab Med. 2011;49:1299‐1302. [DOI] [PubMed] [Google Scholar]
- 23. Elbin CS, Olivova P, Marashio CA, et al. The effect of preparation, storage and shipping of dried blood spots on the activity of five lysosomal enzymes. Clin Chim Acta. 2011;412:1207‐1212. [DOI] [PubMed] [Google Scholar]
- 24. Olivova P, van der Veen K, Cullen E, et al. Effect of sample collection on alpha‐galactosidase a enzyme activity measurements in dried blood spots on filter paper. Clin Chim Acta. 2009;403:159‐162. [DOI] [PubMed] [Google Scholar]
- 25. Reuser AJ, Verheijen FW, Bali D, et al. The use of dried blood spot samples in the diagnosis of lysosomal storage disorders–current status and perspectives. Mol Genet Metab. 2011;104:144‐148. [DOI] [PubMed] [Google Scholar]
- 26. Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr. 2001;131:1631S‐1636S. [DOI] [PubMed] [Google Scholar]
- 27. Edelbroek PM, van der Heijden J, Stolk LM. Dried blood spot methods in therapeutic drug monitoring: methods, assays, and pitfalls. Ther Drug Monit. 2009;31:327‐336. [DOI] [PubMed] [Google Scholar]


