Abstract
Background
The aim of this study was to evaluate salivary amylase in patients with primary oral cancer undergoing radiotherapy as the main modality of treatment.
Materials/methods
The study was conducted on ten histologically proven cases of oral cancer undergoing radiotherapy. Stimulated whole saliva was collected at three stages of radiotherapy—0, 3, and 6 weeks. Salivary amylase was estimated using Henry‐Chiamori method and comparison was made with appropriate age‐ and gender‐matched controls.
Results
Salivary amylase levels showed significant decrease in healthy subjects when compared to oral cancer patients (P < 0.001). The latter group also showed changing trend with initial decrease from 0 to 3 weeks followed by increase from 3 to 6 weeks following radiotherapy (P < 0.0528).
Conclusions
The trend in changes in the levels of salivary amylase could be used as a surrogate marker of salivary gland function in patients with oral cancer undergoing radiotherapy as primary treatment.
Keywords: Henry‐Chiamori method, oral cancer, salivary amylase
Bio‐molecular targets are important in diagnosis and aid in prognostication of various diseases. The annual incidence of 300,000 oro‐pharyngeal cancers worldwide is alarming as only 30–40% of the patients are cured at early stages of disease. Studies reported variable 5‐year survival rates based on choice of the treatment as 54% for combined therapy (surgery followed by radiotherapy), 32% for radiotherapy alone, and 48% for patients treated by surgery only 1. Therefore, differentiation of oral squamous cell carcinoma (OSCC) from non‐cancerous lesions with the judicious use of tumor markers is essential for appropriate treatment strategies that may enhance prognosis.
Tumor markers are biochemical substances produced by the tumor cells due to a cause or as a result of malignant process. An ideal tumor marker exhibits a high degree of sensitivity/specificity, high positive/negative predictive value, 100% accuracy in screening, predicting the recurrence and prognosis of OSCC cases. This study focuses on the variation in the levels of salivary amylase due to the alterations seen in the salivary gland parenchyma either due to the tumor or treatment associated with tumor. Thus, altered salivary amylase may be used as an indicator of the progression of oral cancer and its treatment.
Saliva is considered to be the “Mirror of the Oral Cavity and Systemic Health”. This maintains the lubrication, antimicrobial activity, speech, digestive activity, and protection of mucosal integrity 2. Altered salivary gland function along with its composition is observed in various pathological conditions ranging from mild inflammatory conditions to malignancies. Although serum analysis acts as an adjuvant to the established gold standard of histopathological examination for diagnosis of oral cancer, there exist various properties for consideration of saliva as the alternate medium of choice. Collection of saliva is non‐invasive, economical, and more accessible especially in elderly patients and children, thus making salivary analysis the emerging area for further research in cancer studies. Salivary testing in ovarian and breast carcinomas has been proven as a legitimate alternate to serum testing. As salivary amylase acts as a good indicator in predicting the function of salivary glands, it is hypothesized that assessing the levels of salivary amylase could potentially act as surrogate biomarker in screening and monitoring the prognosis of patients with OSCC undergoing radiotherapy 3. Most cases of head and neck cancer are treated by customized variation in radiation dosage depending on clinical and pathological presentation of the lesion. Studies have proven significant acinar cell atrophy in salivary glands of patients with head and neck cancer undergoing radiotherapy resulting in qualitative changes such as altered viscosity, pH, immunoglobulin, flow rate (reduction by 57% and 67% after first and 6 weeks after radiation and 97% after 3 years of treatment) and quantitative changes in the salivary composition 4, 5.
The present study attempted to quantitate the levels of salivary amylase in ten patients with histologically proven oral cancer receiving 2 Gray/week for 6 weeks (total 60 Gy) radiation therapy as the only modality of treatment and appropriate age/gender‐matched healthy controls at the Department of Radiotherapy, Shirdi Sai Baba Institute of Oncology, Manipal. This study also aimed to evaluate the efficacy of salivary amylase as an effective monitoring tool for the course of treatment of primary oral cancer.
Institutional Ethics Committee clearance and patient's consent were obtained prior to the study. Stimulated whole saliva was collected from patients at three stages during the schedule of radiotherapy; viz. at 0 weeks (prior to start of radiotherapy), 3 weeks following the radiotherapy and finally, 6 weeks after the start of radiotherapy (i.e., on completion of radiotherapy). However, patients who have undergone chemotherapy and suffering from other systemic diseases were excluded from the study.
Stimulated saliva (4–5 ml) from the patients and healthy subjects was collected in wide‐mouthed graduated tubes over a 30‐min period according to the “Spitting method” as illustrated by Navazesh et al. 6. Initially, patients were instructed to rinse their mouth with de‐ionized water and the samples were collected before breakfast. The subjects were asked to produce stimulated saliva following 5–10 min of chewing an inert gum base/lemon (provided by the investigator) at 45 chews per minute. The first 2‐min saliva sample was discarded. Patients were not allowed to smoke/chew tobacco and consume alcohol throughout the course of radiotherapy. The subjects were also instructed to refrain from eating food 30 min prior to the salivary collection. Saliva collected from the patients was immediately centrifuged at 1200 × g for ten minutes at 40°C. Centrifuged saliva was transferred into Eppendorf vials and stored at −20°C until analysis. Saliva samples from oral cancer patients and healthy controls were subjected to biochemical Henri Chiamori Method of analysis for salivary amylase levels 7, 8. The parameters were statistically analyzed using Mann–Whitney ‘U’ test and Repeated measures analysis of variance (ANOVA) in OSCC patients on radiotherapy and P value of <0.05 was considered statistically significant.
Amylase is synthesized primarily in the acinar cells and less consistently in the proximal cells of the intercalated ducts. Parotid glands which produce amylase have been considered to be highly radiosensitive and the amount of serous secretion is directly proportional to the amylase produced. In our study, Mann–Whitney ‘U’ test revealed a significant higher α‐amylase activity in the OSCC patients with a median of 335.3 units/ml as compared to 146.56 units/ml in controls with a P value of <0.001. This could be attributed to the increase in the number of acinar cells in the parotid gland as quoted by Chitra et al. 4 and also a significant fall in the volume of secreted saliva rather than by the rate of protein synthesis in the salivary glands 9. Studies proved that the irreversible decrease in the flow rate may be related to a loss of tissue integrity and alterations in the intra‐lobular nerve endings and not due to reduced secretory potential of the glandular tissue.
Second, Repeated measures ANOVA revealed significant decrease in the levels of salivary amylase from baseline to 3 weeks of radiotherapy, followed by an increase in quantity at 6 weeks of radiotherapy (P < 0.028) [Fig. 1]. The decrease in salivary α‐amylase activity as observed after 3 weeks of radiation treatment may probably be due to a reduction in the number of acinar cells, incomplete tissue regeneration and radiation‐induced late stromal vascular changes. The increased levels at 6 weeks could be attributed to the qualitative changes observed in irradiated salivary glands like hyperamylasemia (10–80 fold increase in 24–48 hr) due to marked immediate cellular changes associated with cell death such as disruption of cell membrane leading to leakage of cell constituents into extracellular space, sharp decrease in saliva flow rate with parotitis followed by other qualitative changes like atrophy/loss of parenchyma, fibrosis, squamous metaplasia, and chronic inflammation.
Figure 1.
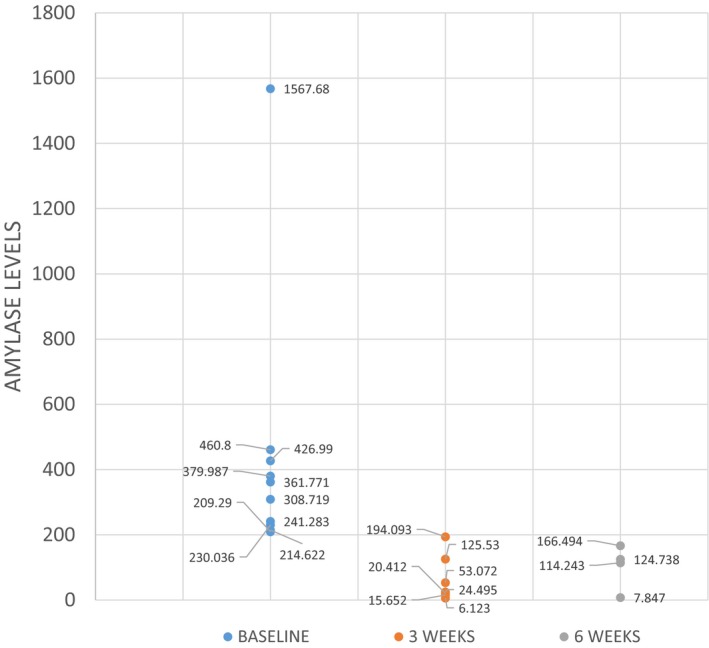
The scatter plot shows the distribution of salivary amylase values in the test cases at baseline, 3 weeks and 6 weeks of radiation therapy.
Therefore, salivary amylase is a good indicator of the function of salivary glands, and hence, of the general health of an individual 10. At best, the results of this study can use the quantitation of salivary amylase as a surrogate marker of damage to salivary gland function due to radiotherapy. Further studies with increased sample size will be needed to obtain a definite cut‐off value to conclusively prove the same.
References
- 1. Sankaranarayanan R. Oral cancer in India: An epidemiologic and clinical review. Oral Surg Oral Med Oral Pathol 1990;69:325–330. [DOI] [PubMed] [Google Scholar]
- 2. Farnaud SJ, Kosti O, Getting SJ, Renshaw D. Saliva: Physiology and diagnostic potential in health and disease, review. Sci World J 2010;10:434–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martí‐Álamo Silvia, Mancheño‐Franch Aisha. Cristina Marzal‐Gamarra Saliva as a diagnostic fluid. J Clin Exp Dent 2012;4:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chitra S, Shyamala Devi CS. Effects of radiation and α‐tocopherol on saliva flow rate, amylase activity, total protein and electrolyte levels in oral cavity cancer. Indian J Dent Res 2008;19:213–218. [DOI] [PubMed] [Google Scholar]
- 5. Dose AM. The symptom experience of mucositis, stomatitis and xerostomia. Semin Oncol Nurs 1995;11:248–255. [DOI] [PubMed] [Google Scholar]
- 6. Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res 1992;71:1363–1369. [DOI] [PubMed] [Google Scholar]
- 7. Bernfeld P. Amylase alpha and beta. Methods Enzymol 1955;1:1149–1158. [Google Scholar]
- 8. Gowenlock AH. Varley's Practical Clinical Biochemistry, 4th edn New Delhi: CBS Publishers and Distributers, p 402–403. [Google Scholar]
- 9. Ashok L, Sujatha GP, Hema G. Estimation of salivary amylase and total proteins in leukemia patients and its correlation with clinical feature and radiographic finding. Indian J Dent Res 2010;21:486–490. [DOI] [PubMed] [Google Scholar]
- 10. Nagler RM. Ionizing irradiation and the salivary gland sequelae. Biomed Rev 1998;9:121–129. [Google Scholar]


