Abstract
Background
Adipose tissue is an important endocrine organ that secretes a number of adipokines, like Resistin (RETN); it's an adipocytes‐secreted cytokine and has been proposed as a link between obesity and diabetes. Many resistin gene polymorphisms were described and their implication in obesity was controversial. This study was to investigate the prevalence of single nucleotide polymorphisms (SNPs) in RETN gene 420C/G; 44G/A; 62G/A; 394C/G and 299 G/A and their association with Resistin level and obesity in Tunisian volunteers.
Methods
We recruited 169 nonobese (mean age=42.16‐14.26 years; mean body mass index [BMI]=24.51‐3.69 kg/m2) and 160 obese (mean age=47.86‐11.17 years; mean BMI=36‐4.78 kg/m2). Genotyping was performed using polymerase chain reaction–restriction fragment length polymorphism. Anthropometric parameters, lipid levels, Glycemia and insulinemia were measured, BMI was calculated and insulinresistance was evaluated with the homeostasis model assessment insulin resistance (HOMA‐IR) and resistin level was measured by ELISA. Statistical analyses were performed by SPSS19.0.
Results
After adjustment for confounding parameters; the Odds Ratio (OR) of obesity associated with mutated genotypes at 420C/G compared with normal genotype was as: OR=2.17; 95% CI [1.28‐3.68], P=.004. The serum Resistin levels present no significant association with all RETN polymorphisms and it was significantly associated with BMI (P=.047). In our haplotype analysis, one haplotype seems to be protective and one other seems to be the highest risk to obesity.
Conclusion
The 420 C/G Polymorphism were associated with obesity and Leptin concentration in our population.
Keywords: levels, obesity, polymorphisms, resistin, tunisian volunteers
1. Introduction
Obesity arises from a complex interaction between genetic variance, environment, and lifestyle changes. Fat excess is an important predisposing factor for serious medical conditions such as type 2 diabetes, cardiovascular disease, stroke and cancer.1 Adipose tissue is an important endocrine organ that secretes a number of factors, adipocytokines or adipokines,2 several protein produced by adipose tissue have been discovered which may provide the link between insulin resistance, obesity and development of diabetes.3, 4
One of the most controversial adipokines is Resistin (RETN), is a macrophage‐derived signaling polypeptide hormone5 which belongs to cysteine‐rich proteins family.6
In mice, RETN is expressed predominantly in adipose tissue and its circulating concentration is markedly increased in genetic and diet‐induced mouse models of obesity7 but in human, RETN is expressed predominantly in monocytes and macrophages, being rarely expressed in adipose tissue.8
RETN is also considered to be a biomarker of metabolic and inflammatory diseases, with increased resistin levels having been associated with metabolic disorders such as obesity,9, 10 insulin resistance,11, 12, 13 type 2 diabetes14 and atherosclerotic cardiovascular disease.15, 16
Resistin levels have been associated with variations in lipid levels in several adult populations.17, 18, 19 A link between this cytokine and obesity has also been reported.20, 21
But controversial studies have found no significant association of the circulating RETN level with obesity, insulin resistance and cardiovascular diseases.22, 23, 24
The gene encoding RETN is located on chromosome 19p13 and a high heritability of serum resistin levels has been evaluated.25 Several single‐nucleotide polymorphisms (SNPs) described in the resistin gene have been associated with RETN levels.26, 27, 28
Based on conflicting results of RETN polymorphisms association with obesity and RETN level in different populations, we aimed to study the relationship between five SNPs in RETN 420C/G; 44G/A; 62G/A;394C/G and 299 G/A with Resistin level and obesity in Tunisian volunteers.
2. Materials and Methods
2.1. Study subjects
As descripted previously by Boumaiza et al.29, this study was composed by two groups (Obese/Nonobese). Obese group was composed of 160 Tunisian unrelated subjects, on the basis of Body mass index (BMI (kg/m2)) ≥30 kg/m2, who are volunteers from consultations at Sahloul University Hospital (the governorate of Sousse, Tunisia). The mean age was 47.86±11.17 years and their mean BMI was 36±4.78 kg/m2. The Nonobese group was composed of 169 unrelated subjects, who are volunteers from the staff of the hospital (BMI<30 kg/m2) (mean age=42.16±14.26 years; mean BMI=24.51±3.69 kg/m2). In both groups we excluded subjects taking lipid‐lowering drugs and all those having renal failure, thyroid disease and hepatic pathology.
A structured questionnaire was completed to the entire members of study. Sociodemographic characteristics, family and personal history, smoking habits, drug intake if any were collected.
The participants underwent physical examinations and laboratory tests. The examiners undertook training in the questionnaire collections and measures. The study was approved by the Hospital Medical Ethic Committee and informed consent was obtained from all study subjects.
2.2. Anthropometric parameters and blood pressure measurements
Weight and height were measured on the subjects barefooted and lightly clothed. Waist circumference (WC) was measured by trained examiner from the narrowest point between the lower borders of the rib cage and the iliac crest. BMI was calculated as body weight (kg)/height2 (m2) and obesity was defined as BMI≥30 kg/m2.30 Blood pressure was measured three times from the left arm of seated subjects with a blood pressure monitor after 20 minutes of rest.
2.3. Biochemical measurements
Blood was collected for laboratory testing after a 12 hours overnight fast. All biochemical parameters were performed on the Synckrom CX7 Clinical System using the Beckman reagents (Beckman, Fullerton, CA, USA). Serum total cholesterol (TC) and triglycerides (TG) were determined by enzymatic assays. High‐density lipoprotein‐cholesterol (HDL‐C) was measured by direct enzymatic assay. Low‐density lipoprotein‐cholesterol (LDL‐C) were measured by direct assay and were calculated with the Friedewald formula.31 Insulin resistance was evaluated with the homeostasis model assessment (HOMA) using the following equation: HOMA‐IR=(Fasting insulin [μU/mL]×fasting glucose[mmol/L])/22.5.32 Fasting glucose was measured by the glucose oxidase method. Insulin concentration was measured by microparticle immunoassay (MEIA) on AxSym Abbott (Abbott Laboratories, Abbott Park, IL, USA). Serum level measurement of Resistin was performed using enzyme‐linked immunosorbent assay (ELISA) according to the manufacturer's instructions (BioVendor Research and Diagnostic Products, Cairo, Egypt, REF; RD191016100; Lot No: E16‐O26) results were expressed in ng/mL.
2.4. Definitions of risk factors
Dyslipidemia was defined as LDL‐C concentration ≥4.1 mmol/L and/or HDL‐C concentration ≤1 mmol/L and/or TG concentration 1.71 mmol/L.33, 34 Hypertension was defined as more than 140/90 mmHg or actually receiving antihypertensive medication.35 Diabetes mellitus was defined as fasting glucose superior a 7 mmol/L or currently receiving anti diabetic treatment.
2.5. DNA analysis
Genomic DNA was isolated from peripheral blood leucocytes by the salting out method.36 Genotyping of polymorphisms were determined by polymerase chain reaction restriction fragment length polymorphism (PCR‐RFLP) and the digest product were resolved by 2% agarose gel electrophoresis and visualized by ethidium bromide staining.
2.6. Statistical analysis
Statistical analysis performed via SPSS19.0 (IBM Company, Armonk, NY, USA). The quantitative parameters were compared by student's test and reported as means±Standard deviations if they were in Guaussian distribution and compared by U Mann Whitney test and reported as median [min‐max] if not. Categorical variables were analyzed by the chi‐square test. We used SNP analyzer 2 program to checked were in Hardy‐Weinberg equilibrium for both test genotype frequencies and haplotype frequencies.37 Correlation between LEP and other biological and anthropometric parameters was studied by Spearmen test. The biological parameter value were compared by Student's t test Odds ratios (ORs), two‐tailed P‐values, and 95% confidence interval (CI) were calculated as a measure of the association of the SNPs and haplotype with presence of obesity. A P‐value of <.05 was considered statistically significant for all tests.
3. Results
3.1. Patient characteristics
The clinical and biological characteristics of our study were presented in Table 1 (modified from table 1 by Boumaiza et al.29). The prevalence of hypertension, diabetes, Metabolic Syndrome and cardiovascular disease was higher in obese group (P<.001). Compared with nonobese subjects, the obese subjects had higher weight, waist circumference, TG, TC, HOMA‐IR, insulin and Resistin levels but lower HDL‐C concentration. Dyslipidemia frequencies (P=.923), Daily energy intake (P=.636) and LDL‐C concentrations (P=.262) did not showed significant differences between the two groups.
Table 1.
Clinical and biochemical characteristics of the study population
| Variables | Population | P | |
|---|---|---|---|
| Nonobese (n=169) | Obese (n=160) | ||
| Age (y) | 43.25±13.12 | 48.41±10.92 | <.001 |
| Sex‐ratio (men/women) | 0.594 | 0.221 | .001 |
| Smoking n (%) | 29 (17.2) | 11 (6.9) | .004 |
| Weight (kg) | 66.94±11.29 | 93.60±12.97 | <.001 |
| BMI (kg/m2) | 24.73±3.50 | 36.6±4.8 | <.001 |
| WC (cm) | 89.09±13.67 | 117.85±12.61 | <.001 |
| Hypertension n (%) | 23 (13.6) | 72 (45.3) | <.001 |
| Diabetes n (%) | 32 (18.9) | 54 (34) | .002 |
| Cardiovascular disease n (%) | 0 (0) | 22 (14) | <.001 |
| Dyslipidemia n (%) | 82 (48.5) | 78 (49.1) | .923 |
| MetS n (%) | 20 (12) | 77 (48.1) | <.001 |
| Fasting insulin (μU/mL) | 6.27 [0.7‐27.3] | 8.2 [0.4‐81.7] | <.001 |
| HOMA‐IR | 1 [0.14‐5.08] | 2,32 [0.11‐40.6] | <.001 |
| TC (mmol/L) | 4.75±1.1 | 5.17±1.25 | <.001 |
| TG (mmol/L) | 0,86 [0.24‐4.69] | 1,2 [0.23‐3.95] | <.001 |
| HDL‐C (mmol/L) | 1.31±0.49 | 1.13±0.34 | .009 |
| LDL‐C (mmol/L) | 3.27±0.94 | 3.24±0.99 | .262 |
| Coffee consumption n (%) | 125 (74.2) | 98 (61.5) | .009 |
| Daily energy intake (kcal) | 3092±1541 | 3204±1333 | .636 |
| Resistin level (ng/mL) | 11.07 [0.1‐28.5] | 14.34 [0.1‐96] | .019 |
BMI, body mass index; HOMA‐IR, homeostasis assessment model insulin resistance; TC, total cholesterol; TG, triglyceride; HDL‐C, high density lipoprotein‐cholesterol; LDL, low density lipoprotein‐cholesterol; MetS, metabolic syndrome; WC, waist circumference.
Mean±standard deviation or n (%).
3.2. Association between Resistin polymorphisms and Resistin level
Results given in Table 2 revaluated that the serum Resistin levels present no significant association with all RETN polymorphisms.
Table 2.
Association between Resistin polymorphisms and Resistin level
| [Resistin] ng/mL | P | Global P | |
|---|---|---|---|
| 420 C/G | |||
| CC | 13.69 [0.1‐53.67] | 1 | .137 |
| CG | 12.43 [0.1‐96] | .744a | |
| GG | 15.72 [0.1‐84] | .038a | |
| 44G/A | |||
| GG | 13.68 [0.1‐96] | 1 | .899 |
| GA | 12.84 [2.48‐84] | .701a | |
| AA | 19.72 [4.25‐25.2] | .082a | |
| 62G/A | |||
| GG | 13.95 [0.1‐96] | 1 | .683 |
| GA | 12.08 [0.1‐53.67] | .971a | |
| AA | 28.55 | .388a | |
| 394 C/G | |||
| CC | 14.31 [.1‐69.27] | 1 | .277 |
| CG | 12.55 [0.1‐96] | .701a | |
| GG | 15.05 [8.64‐84] | .082a | |
| 299 G/A | |||
| GG | 14.63 [0.1‐96] | 1 | .594 |
| GA | 12.78 [0.1‐63.46] | .589a | |
| AA | 13.54 [0.1‐84] | .613a | |
Comparison vs normal genotype.
3.3. Association between Resistin polymorphisms and obesity in all studied population
After adjustment for confounding parameters (age, gender, smoking status, HTA, diabetes, dyslipidemia, and cardiovascular disease), the OR of obesity associated with mutated genotypes at 420C/G compared with normal genotype was as: OR=2.17; 95% CI [1.28‐3.68], P=.004.
There was no significant association between 44G/A, 394 C/G, 62 G/A and 299 G/A.
3.4. Correlation between serum Resistin levels and biological and anthropometric parameters
Results given in Table 3 revealed that the serum Resistin concentration was significantly associated with BMI (P=.047).
Table 3.
Association between resistin polymorphisms and obesity in all studied population
| Polymorphisms | OR crude | CI | P | ORa adjusted | CI | P |
|---|---|---|---|---|---|---|
|
420C/G CG+GG/CC |
2.22 | 1.39‐3.56 | .001 | 2.17 | 1.28‐3.68 | .004 |
|
44 G/A GA+AA/GG |
1.41 | 0.874‐2.291 | .178 | 1.61 | 0.948‐2.74 | .078 |
|
62 G/A GA+AA/GG |
1.41 | 0.773‐2.590 | .284 | 1.38 | 0.742‐2.58 | .307 |
|
394 C/G CG+GG/CC |
1.45 | 0.938‐2.240 | .098 | 1.3 | 0.926‐2.03 | .115 |
|
299 G/A AA+GA/GG |
0.90 | 0.571‐1.442 | .681 | 0.681 | 0.424‐1.520 | .496 |
OR, odds ratio; CI, confidence interval; HTA, hypertension.
The bold values are significant (P<.05).
OR adjusted to age, gender, smoking status, HTA, diabetes, dyslipidemia, and cardiovascular disease.
Whereas no statistical differences were observed for HOMA‐IR, WC, TG and HDL‐C.
3.5. ROC curve, sensibility and specificity of Resistin level
Figure 1 show the ROC curve plotted from the sensitivity and specificity values found for each Resistin level measured in the study sample, with obesity as the outcome. The best cutoff is denoted by the intersection of the dotted lines and its Leptin value of 11.55 ng/mL (Sensitivity=61.5%; Specificity=52.6%) corresponded to the shoulder of the curve. with Positive predictive value (PPV)=82.1% and Negative predictive value (NPV)=27.7%. The area under the curve represents the overall accuracy of the test (0.617).
Figure 1.
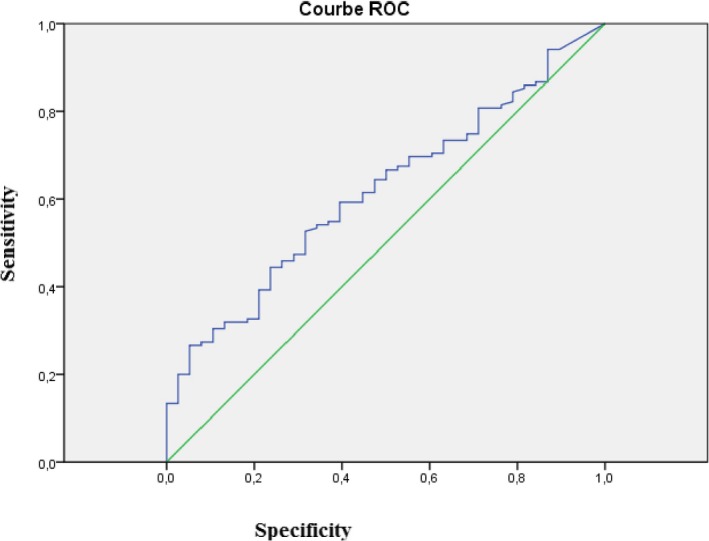
Sensibility and specificity of resistin level in study population. Cut off of resistin=11.55 ng/mL; Sensitivity=61.5%; Specificity=52.6%; PPV (Positive predictive value)=82.1%; NPV (Negative predictive value)=27.7%; air under the curve=0.617 [P=.027]
3.6. Haplotype frequency distribution and Resistin level association
SNP analyzer showed 22 haplotypes (H), two of them seem to be significantly associated with obesity (Table 4).
Table 4.
Correlation between serum Resistin levels and biochemical and anthropometric parameters
| HOMA‐IR | WC | BMI | TG | HDL‐C | |
|---|---|---|---|---|---|
| Resistin level (ng/mL) | |||||
| Coefficient of correlation R | 0.100 | 0.109 | −0.155 | 0.135 | 0.035 |
| P | .192 | .144 | .047 | .070 | .639 |
BMI, body mass index; HOMA‐IR, homeostasis assessment model insulin resistance; TG, triglyceride; HDL‐C, high density lipoprotein‐cholesterol; WC, waist circumference.
Haplotypes follow this order; 420C/G, 44G/A, 62G/A, 394 C/G and 299G/A (Table 5).
Table 5.
Comparison of haplotype frequencies
| Haplotypes (H) | Total population | Frequency | ||
|---|---|---|---|---|
| Nonobese | Obese | |||
| H1 | CGGCG | 0.25238 | 0.26155 | 0.24746 |
| H2 | GGGCG | 0.12320 | 0.09325 | 0.11970 |
| H3 | GGGCA | 0.10610 | 0.08075 | 0.12777 |
| H4 | CGGCA | 0.09959 | 0.16549 | 0.06312 |
| H5 | GGGGA | 0.09490 | 0.02497 | 0.11679 |
| H6 | CGGGG | 0.08288 | 0.10422 | 0.07575 |
| H7 | CAGCG | 0.04951 | 0.04789 | 0.05892 |
| H8 | CGGGA | 0.02435 | 0.03564 | 0.02180 |
| H9 | GAGGA | 0.02146 | 0.02411 | 0.01931 |
| H10 | GGGGG | 0.02064 | 0.03643 | 0.01111 |
| H11 | CAGCA | 0.01458 | 0.04789 | 0.01290 |
| H12 | GGACA | 0.01383 | 0.03930 | 0.00472 |
| H13 | CAACG | 0.01358 | 0 | 0.01648 |
| H14 | GAGCA | 0.01346 | 0.03547 | 0 |
| H15 | GAAGA | 0.01218 | 0.02411 | 0.00876 |
| H16 | GAACG | 0.01150 | 0.01407 | 0.00476 |
| H17 | GGAGA | 0.00998 | 0.01461 | 0.00739 |
| H18 | CAGGG | 0.00919 | 0.00739 | 0 |
| H19 | GAAGG | 0.00851 | 0 | 0.01232 |
| H20 | CAAGA | 0.00571 | 0 | 0.00981 |
| H21 | CGACA | 0.00386 | 0 | 0.00583 |
| H22 | GGAGG | 0.00268 | 0 | 0.00314 |
Haplotypes follow this order; 420C/G, 44G/A, 62G/A, 394 C/G and 299G/A. Bold values indicate haptoype with frequency <1.
The more protective haplotype seems to be H4 “CGGCA”; OR=0.328 [0.151‐0.711] (P=.030) and the highest risk seems to be associated to H5 “GGGGA”; OR=3.299 [0.982‐11.08] (P=.020) (Table 6).
Table 6.
Haplotypes associated with obesity and resistin level
| Haplotypes (H) | H* | OR | CI | P | RETN level | Global P |
|---|---|---|---|---|---|---|
| H4 | CGGCA | 0.328 | 0.151‐0.711 | .030 | 10.03 (0.11‐6.51) | <.001 |
| H5 | GGGGA | 3.299 | 0.982‐11.08 | .020 | 33.3 (6.06‐69.2) |
P, comparison between obese and nonobese groups.
H*, Haplotypes follow this order; 420C/G, 44G/A, 62G/A, 394 C/G and 299G/A.
Comparison of Resistin level between these two haplotypes showed that the potential protective haplotype carriers (H4) had lower Resistin level 10.03 [0.11‐6.51] then the potential carriers of H5 33.3 [6.06‐69.2] with P<.001 (Table 6).
4. Discussion
Obesity is multifactor disease which many factors interact together like genetic, metabolic and environmental factors. There is some urgency in identifying specific genetic influences on overweight and obesity and their interactions with concrete environmental exposures. Indeed, in the Tunisian population, obesity became rather frequent pathology.
In adult Tunisian women aged 20‐59, prevalence of obesity and abdominal obesity were, respectively, 22.6% and 29.2%.38 Several association studies revealed the contribution of RETN gene variants to the pathogenesis of obesity; however, they were inconclusive or inconsistent. These discrepancies may be attributed to differences in sample size, ethnicity, disease status and probably these studies did not analyze the whole gene.39
In our study we found a significant difference between obese/non‐obese subjects and Resistin levels, it is clear that obesity is characterized by high levels of Resistin with cut‐off value of 11.55 ng/mL to separate normal weight from overweight was observed (Sensitivity=61.5%; Specificity=52.6%).
In agreement with our study, Amal et al.39 in Egyptian population reported that RETN levels were measured respectively as controls (1.33±0.27 ng/mL) and obese patients (2.43±1.5 ng/mL). In India, Kumar et al.13 found a significant difference in serum Resistin levels between 305 women with metabolic syndrome (14.63±11.02 ng/mL) and 310 women without metabolic syndrome (9.61±6.28 ng/mL).
In Iranian study, Takhshid et al.40 revealed that Serum Resistin level was correlated with serum triglyceride, total and low density lipoprotein (LDL) cholesterol (P<.05) in diabetes patients. But, controversial results was found also by Yamunah DA et al. in 469 non‐obese and 162 obese Malaysian subjects (P=.729).26 Contrarily, Han et al.23 he did not observe significant differences in serum Resistin levels between the metabolic syndrome and non metabolic syndrome groups.
Menzaghi et al.25 estimated that up to 70% of the variation in circulating resistin levels can be explained by genetic factors but this divergence in results may be explained also by ethnic origin, life style, food composition and the variations in adipose tissue of the body distribution.
In our study we found significant negative correlation between Resistin serum levels and BMI R=−0.155 (P=.045). In agreement with our study results, Yannakoulia et al.41 studied the dynamic between the RETN level and body fat mass in healthy subjects, reported that serum RETN levels were negatively correlated with BMI and body fat in young participants. Several studies confirmed this result.42, 43, 44
Booth et al.45 reported that the relation between subcutaneous adipose tissue and RETN was stronger than the relation of other measures of obesity to RETN and this is may be explained by expression mode of RETN.
In fact, RETN is not expressed by adipocytes but is secreted by macrophages located within adipose tissue depots.8 So, circulating RETN is not directly related to adiposity levels but to the degree of inflammation within the adipose tissue depots.45
In our population we aimed to study not only the relationship between Resistin level and obesity but also to exanimate this relation with some polymorphisms in RETN gene.
Our study revaluated that the serum RETN levels present no significant association with all RETN polymorphisms except for 420 C/G.
For 420C/G SNP we noted an increase in serum resistin in GG carriers vs CC and CG. This is association between G allele and serum resistin was controversies in same studies25, 46 but reported in the Koreans population47 and Malaysians study.26
In other hand, RETN 420C/G has been studied extensively in regards to obesity risk but with conflicting reports and that the GG genotype have been reported similarity to our results to have a higher prevalence of obesity48, 49 and the G allele has been associated with increased BMI, weight, body fat mass, and WC.9, 50
The effect of the 420 C/G SNP can be explained by his localization wish appears to have a key functional consequence and to increase the resistin promoter activity observed with G allele.14, 47 In fact, the DNA element of SNP −420C and SNP −420G of RETN gene had different binding affinities for stimulatory protein 1 (Sp1) and stimulatory protein 3 (Sp3), Sp1 and Sp3 transcription factors specifically bound to the DNA element of SNP −420G with high affinity and enhanced expression of the RETN gene.52
For the 44G/A polymorphism our results were not significant but, Boumaiza et al.53 found a association between 44AA and Metabolic syndrome risk after adjustment to confounding parameters and in a Turkish population, the 44G/A polymorphism is associated with obesity and insulin‐related phenotypes.54 But, Sentinelli et al.55 could not found any significant differences.
Several SNPs in the 3′ UTR of RETN (like 44 G/A and 62 G/A) had been associated with resistin levels in Caucasians and Japanese population.19, 46
The molecular mechanisms of the 44 G/A and 62 G/A are not clearly identified, but these SNPs are present in this critical region 3′ UTR56 and this position of the 3′ end of mRNA may regulate gene expression by several mechanisms57 like regulation of the stability, subcellular localization and mRNA translation.58 Also, for the 44G/A polymorphism is located 60 bp 3′ from the stop codon and 8 bp 5′ from the AATAAA polyadenylation signal and may be therefore affect polyadenylation of RETN mRNA.19
A regards to 394 C/G (promoter 5′ flanking region) and 62 G/A, there is no association between these SNPs and obesity and the lack of association was also reported in many study19, 46, 53, 55, 59 and this may be explained by differential distributions of risk factors, genetic predisposition and environmental conditions. However, 62 G/A it has been reported to be associated with hypertension60, 61 and type 2 diabetes61 and this may explain by he's localization in a critical region (3′UTR). Nambiar et al.62 reported a positive correlation between BMI and resistin levels but no significant correlation was found between the genotypes analyzed of 62 G/A polymorphism and RETN levels. Similar findings were reported in the study from Spain.63
As regards to 299 G/A SNP located at intron 2 of resistin gene, we have report any significant association of serum resistin with obesity and our results was also reported by Miyamoto et al.49 and Suriyaprom et al.64 Also, Wang et al.65 added that RETN gene variants may influence insulin sensitivity interaction in obese subjects. On the contrary, Osawa et al.66 he did not found this association with RETN +299 G/A with obesity.
Chung et al.67 found significant association between +299 G/A SNP and diabetes and also. Demonstrated that this SNP was in moderate linkage disequilibrium (LD) with rs1862513 (r 2=.45, D′=0.71 in Han Chinese in Beijing and Japanese in Tokyo combined samples). So, It is hard to assess by only genetic syndrome studies exactly which SNP is involved; especially when a gene is very polymorphic with several SNPs in LD.53
The lack of association between 394 C/G, 299 G/A and 62 G/A SNPs and obesity risk in our study may be explained by different genetic backgrounds or environmental conditions of the studied population than by the SNP itself.
In other hand, this is divergence in results may be explained by different genetic backgrounds or environmental conditions, lifestyle characteristics and ethnic origin of distinct population groups rather than by the SNP itself. Obesity it's a polygenic disease, with a strong environmental influence, genotype is just one factor in the causal pathway to the disease, and gene‐gene and gene‐environment interactions can influence the final association between genotype and disease.
Also, this dissimilarity might be results too many interaction factors like a limited number of subjects, the ethnic specificity of population or also the variation in the entry criteria of subjects.
In our study population, comparison of the haplotype frequencies between obese and non‐obese groups; showed significant difference for two haplotypes H4 “CGGCA” which seems to be protective and it occurred more frequently in the non‐obese than in obese group (P=.030) but H5 “GGGGA” seems to be the most Haplotype associated to obesity risk with OR=3.299 [0.982‐11.08] (P=.020).
Concerning the Resistin level, H5 showed higher value 33.3 [6.06‐69.2] vs the protective haplotype H4=10.03 [0.11‐6.51].
In conclusion, this study showed that only RETN 420 C/G Polymorphism were associated with obesity and Leptin concentration in our population.
Also, when combined in haplotypes a synergic effect was observed in Resistin levels and obesity risk.
Acknowledgments
This study was supported by grants from the Tunisian Ministry of Higher Education, Scientific Research and Technology and the Tunisian Ministry of Health (LR12SP11); without their extremely generous and strong support, this study could not have been undertaken. The authors are especially grateful to the study participants. We acknowledge general director of Sahloul University Hospital and the excellent echnical assistance of members of the Biochemistry MDepartment of Sahloul University Hospital.
Zayani N, Hamdouni H, Boumaiza I, et al. Resistin polymorphims, plasma resistin levels and obesity in Tunisian volunteers. J Clin Lab Anal. 2018;32:e22227 10.1002/jcla.22227
References
- 1. Nguyen DM, El‐Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inadera H. The usefulness of circulating adipokine levels for the assessment of obesity‐related health problems. Int J Med Sci. 2008;5:248‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conneely KN, Silander K, Scott LJ, et al. Variation in the resistin gene is associated with obesity and insulin‐related phenotypes in Finnish subjects. Diabetologia. 2004;47:1782‐1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol. 2006;17:170‐175. [DOI] [PubMed] [Google Scholar]
- 5. Nogueiras R, Novelle M, Vazquez M, Lopez M, Dieguez C. Resistin: regulation of food intake, glucose homeostasis and lipid metabolism. Endocr Dev. 2010;17:175‐184. [DOI] [PubMed] [Google Scholar]
- 6. McTernan PG, Kusminski CM, Kumar S. Resistin. Curr Opin Lipidol. 2006;17:170‐175. [DOI] [PubMed] [Google Scholar]
- 7. Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307‐312. [DOI] [PubMed] [Google Scholar]
- 8. Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPARγ activators. Biochem Biophys Res Commun. 2003;300:472‐476. [DOI] [PubMed] [Google Scholar]
- 9. Engert JC, Vohl M‐C, Williams SM, et al. 5′ flanking variants of resistin are associated with obesity. Diabetes. 2002;51:1629‐1634. [DOI] [PubMed] [Google Scholar]
- 10. Mattevi VS, Zembrzuski VM, Hutz MH. A resistin gene polymorphism is associated with body mass index in women. Hum Genet. 2004;115:208‐212. [DOI] [PubMed] [Google Scholar]
- 11. Bouchard L, Weisnagel S, Engert J, et al. Human resistin gene polymorphism is associated with visceral obesity and fasting and oral glucose stimulated C‐peptide in the Quebec Family Study. J Endocrinol Invest. 2004;27:1003‐1009. [DOI] [PubMed] [Google Scholar]
- 12. Pizzuti A, Argiolas A, Di Paola R, et al. An ATG repeat in the 3′‐untranslated region of the human resistin gene is associated with a decreased risk of insulin resistance. J Clin Endocrinol Metab. 2002;87:4403‐4406. [DOI] [PubMed] [Google Scholar]
- 13. Kumar S, Gupta V, Srivastava N, et al. Resistin 420C/G gene polymorphism on circulating resistin, metabolic risk factors and insulin resistance in adult women. Immunol Lett. 2014;162:287‐291. [DOI] [PubMed] [Google Scholar]
- 14. Osawa H, Yamada K, Onuma H, et al. The G/G genotype of a resistin single‐nucleotide polymorphism at −420 increases type 2 diabetes mellitus susceptibility by inducing promoter activity through specific binding of Sp1/3. Am J Hum Genet. 2004;75:678‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932‐939. [DOI] [PubMed] [Google Scholar]
- 16. Muse ED, Feldman DI, Blaha MJ, et al. The association of resistin with cardiovascular disease in the Multi‐Ethnic Study of Atherosclerosis. Atherosclerosis. 2015;239:101‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Owecki M, Nikisch E, Miczke A, Pupek‐Musialik D, Sowiński J. Serum resistin is related to plasma HDL cholesterol and inversely correlated with LDL cholesterol in diabetic and obese humans. Neuro Endocrinol Lett. 2009;31:673‐678. [PubMed] [Google Scholar]
- 18. Uslu S, Kebapçı N, Kara M, Bal C. Relationship between adipocytokines and cardiovascular risk factors in patients with type 2 diabetes mellitus. Exp Ther Med. 2012;4:113‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asano H, Izawa H, Nagata K, et al. Plasma resistin concentration determined by common variants in the resistin gene and associated with metabolic traits in an aged Japanese population. Diabetologia. 2010;53:234‐246. [DOI] [PubMed] [Google Scholar]
- 20. Maggio AB, Wacker J, Montecucco F, et al. Serum resistin and inflammatory and endothelial activation markers in obese adolescents. J Pediatr. 2012;161:1022‐1027 e1021. [DOI] [PubMed] [Google Scholar]
- 21. Gherlan I, Vladoiu S, Alexiu F, et al. Adipocytokine profile and insulin resistance in childhood obesity. Maedica (Buchar). 2012;7:205‐213. [PMC free article] [PubMed] [Google Scholar]
- 22. Utzschneider K, Carr D, Tong J, et al. Resistin is not associated with insulin sensitivity or the metabolic syndrome in humans. Diabetologia. 2005;48:2330‐2333. [DOI] [PubMed] [Google Scholar]
- 23. Han J, Yakup K, Yuan Q, et al. Relationship between Serum Resistin Level of Xinjiang Uygur and Han subjects with metabolic syndrome. Clin Lab. 2015;61:1941‐1946. [DOI] [PubMed] [Google Scholar]
- 24. Lee JH, Chan JL, Yiannakouris N, et al. Circulating resistin levels are not associated with obesity or insulin resistance in humans and are not regulated by fasting or leptin administration: cross‐sectional and interventional studies in normal, insulin‐resistant, and diabetic subjects. J Clin Endocrinol Metab. 2003;88:4848‐4856. [DOI] [PubMed] [Google Scholar]
- 25. Menzaghi C, Coco A, Salvemini L, et al. Heritability of serum resistin and its genetic correlation with insulin resistance‐related features in nondiabetic Caucasians. J Clin Endocrinol Metab. 2006;91:2792‐2795. [DOI] [PubMed] [Google Scholar]
- 26. Apalasamy YD, Rampal S, Salim A, et al. Polymorphisms of the resistin gene and their association with obesity and resistin levels in Malaysian Malays. Biochem Genet. 2015;53:120‐131. [DOI] [PubMed] [Google Scholar]
- 27. Kumar S, Gupta V, Srivastava N, et al. Resistin 420C/G gene polymorphism on circulating resistin, metabolic risk factors and insulin resistance in adult women. Immunol Lett. 2014;162(2 Pt B):287‐291. [DOI] [PubMed] [Google Scholar]
- 28. Zhang LY, Jin YJ, Jin QS, Lin LY, Zhang DD, Kong LL. Association between resistin +299A/A genotype and nonalcoholic fatty liver disease in Chinese patients with type 2 diabetes mellitus. Gene. 2013;529:340‐344. [DOI] [PubMed] [Google Scholar]
- 29. Boumaiza I, Omezzine A, Rejeb J, et al. Relationship between leptin G2548A and leptin receptor Q223R gene polymorphisms and obesity and metabolic syndrome risk in Tunisian volunteers. Genet Test Mol Biomarkers. 2012;16:726‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Organization WH . Physical status: the use of and interpretation of anthropometry, Report of a WHO Expert Committee; 1995. [PubMed]
- 31. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499‐502. [PubMed] [Google Scholar]
- 32. Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R. Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412‐419. [DOI] [PubMed] [Google Scholar]
- 33. Expert Panel on Detection E . Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001;285:2486. [DOI] [PubMed] [Google Scholar]
- 34. Alberti KGMM, Zimmet PF. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15:539‐553. [DOI] [PubMed] [Google Scholar]
- 35. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560‐2571. [DOI] [PubMed] [Google Scholar]
- 36. Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lewontin RC. The interaction of selection and linkage. I. General considerations; heterotic models. Genetics. 1964;49:49‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beltaifa L, Traissac P, El Ati J, Lefevre P, Romdhane HB, Delpeuch F. Prevalence of obesity and associated socioeconomic factors among Tunisian women from different living environments. Obes Rev. 2009;10:145‐153. [DOI] [PubMed] [Google Scholar]
- 39. Amal S, Pasha HF, Rashad NM. Association of resistin gene polymorphisms with insulin resistance in Egyptian obese patients. Gene. 2013;515:233‐238. [DOI] [PubMed] [Google Scholar]
- 40. Takhshid MA, Zare Z. Resistin ‐ 420 C/G polymorphism and serum resistin level in Iranian patients with gestational diabetes mellitus. J Diabetes Metab Disord. 2015;14:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yannakoulia M, Yiannakouris N, Blüher S, Matalas A‐L, Klimis‐Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88:1730‐1736. [DOI] [PubMed] [Google Scholar]
- 42. Briffa JF, McAinch AJ, Poronnik P, Hryciw DH. Adipokines as a link between obesity and chronic kidney disease. Am J Physiol‐Renal Physiol. 2013;305:F1629‐F1636. [DOI] [PubMed] [Google Scholar]
- 43. Dan S, Aditya P, Banerjee P, Bal C, Roy H, Banerjee I. Effect of chronic kidney disease on serum resistin level. Niger J Clin Pract. 2014;17:735‐738. [DOI] [PubMed] [Google Scholar]
- 44. Youn B‐S, Yu K‐Y, Park HJ, et al. Plasma resistin concentrations measured by enzyme‐linked immunosorbent assay using a newly developed monoclonal antibody are elevated in individuals with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:150‐156. [DOI] [PubMed] [Google Scholar]
- 45. Booth A, Magnuson A, Foster M. Detrimental and protective fat: body fat distribution and its relation to metabolic disease. Horm Mol Biol Clin Investig. 2014;17:13‐27. [DOI] [PubMed] [Google Scholar]
- 46. Hivert M‐F, Manning AK, McAteer JB, et al. Association of variants in RETN with plasma resistin levels and diabetes‐related traits in the Framingham Offspring Study. Diabetes. 2009;58:750‐756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cho YM, Youn B‐S, Chung SS, et al. Common genetic polymorphisms in the promoter of resistin gene are major determinants of plasma resistin concentrations in humans. Diabetologia. 2004;47:559‐565. [DOI] [PubMed] [Google Scholar]
- 48. Chen Y‐H, Hung P‐F, Kao Y‐H. IGF‐I downregulates resistin gene expression and protein secretion. Am J Physiol‐Endocrinol Metab. 2005;288:E1019‐E1027. [DOI] [PubMed] [Google Scholar]
- 49. Miyamoto Y, Morisaki H, Kokubo Y, et al. Resistin gene variations are associated with the metabolic syndrome in Japanese men. Obes Res Clin Pract. 2009;3:I‐ii. [DOI] [PubMed] [Google Scholar]
- 50. Zayani N, Omezzine A, Boumaiza I, et al. Association of ADIPOQ, leptin, LEPR, and resistin polymorphisms with obesity parameters in Hammam Sousse Sahloul Heart Study. J Clin Lab Anal. 2017. doi: 10.1002/jcla.22148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chung S, Choi H, Kim K, Cho Y, Lee H, Park K. Regulation of human resistin gene expression in cell systems: an important role of stimulatory protein 1 interaction with a common promoter polymorphic site. Diabetologia. 2005;48:1150‐1158. [DOI] [PubMed] [Google Scholar]
- 52. Sato N, Kobayashi K, Inoguchi T, et al. Adenovirus‐mediated high expression of resistin causes dyslipidemia in mice. Endocrinology. 2005;146:273‐279. [DOI] [PubMed] [Google Scholar]
- 53. Boumaiza I, Omezzine A, Rejeb J, et al. Association between four resistin polymorphisms, obesity, and metabolic syndrome parameters in Tunisian volunteers. Genet Test Mol Biomarkers. 2012;16:1356‐1362. [DOI] [PubMed] [Google Scholar]
- 54. Duman BS, Cagatay P, Hatemi H, Ozturk M. Association of Resistin gene 3′‐untranslated region EX4‐44G–> a polymorphism with obesity‐and insulin‐related phenotypes in Turkish type 2 diabetes patients. Rev Diabet Stud. 2007;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sentinelli F, Romeo S, Arca M, et al. Human resistin gene, obesity, and type 2 diabetes mutation analysis and population study. Diabetes. 2002;51:860‐862. [DOI] [PubMed] [Google Scholar]
- 56. Savage DB, Sewter CP, Klenk ES, et al. Resistin/Fizz3 expression in relation to obesity and peroxisome proliferator–activated receptor‐γ action in humans. Diabetes. 2001;50:2199‐2202. [DOI] [PubMed] [Google Scholar]
- 57. Conne B, Stutz A, Vassalli J‐D. The 3′ untranslated region of messenger RNA: a molecular ‘hotspot'for pathology? Nat Med. 2000;6:637‐641. [DOI] [PubMed] [Google Scholar]
- 58. Chen J‐M, Férec C, Cooper DN. A systematic analysis of disease‐associated variants in the 3′ regulatory regions of human protein‐coding genes II: the importance of mRNA secondary structure in assessing the functionality of 3′ UTR variants. Hum Genet. 2006;120:301‐333. [DOI] [PubMed] [Google Scholar]
- 59. Chi S, Lan C, Zhang S, et al. Association of ‐394C>G and ‐420C>G polymorphisms in the RETN gene with T2DM and CHD and a new potential SNP might be exist in exon 3 of RETN gene in Chinese. Mol Cell Biochem. 2009;330:31‐38. [DOI] [PubMed] [Google Scholar]
- 60. Gouni‐Berthold I, Giannakidou E, Faust M, Kratzsch J, Berthold H, Krone W. Resistin gene 3′‐untranslated region+ 62G→ A polymorphism is associated with hypertension but not diabetes mellitus type 2 in a German population. J Intern Med. 2005;258:518‐526. [DOI] [PubMed] [Google Scholar]
- 61. Tan M‐S, Chang S‐Y, Chang D‐M, Tsai JC‐R, Lee Y‐J. Association of resistin gene 3′‐untranslated region+ 62G→ A polymorphism with type 2 diabetes and hypertension in a Chinese population. J Clin Endocrinol Metab. 2003;88:1258‐1263. [DOI] [PubMed] [Google Scholar]
- 62. Nambiar V, Vijesh VV, Lakshmanan P, Sukumaran S, Suganthi R. Association of adiponectin and resistin gene polymorphisms in South Indian women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2016;200:82‐88. [DOI] [PubMed] [Google Scholar]
- 63. Escobar‐Morreale H, Villuendas G, Botella‐Carretero J, et al. Adiponectin and resistin in PCOS: a clinical, biochemical and molecular genetic study. Hum Reprod. 2006;21:2257‐2265. [DOI] [PubMed] [Google Scholar]
- 64. Suriyaprom K, Phonrat B, Namjuntra P, Chanchay S, Tungtrongchitr R. The +299(G>A) resistin gene polymorphism and susceptibility to type 2 diabetes in Thais. J Clin Biochem Nutr. 2009;44:104‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang H, Chu WS, Hemphill C, Elbein SC. Human resistin gene: molecular scanning and evaluation of association with insulin sensitivity and type 2 diabetes in Caucasians. J Clin Endocrinol Metab. 2002;87:2520‐2524. [DOI] [PubMed] [Google Scholar]
- 66. Osawa H, Onuma H, Murakami A, et al. Systematic search for single nucleotide polymorphisms in the Resistin gene the absence of evidence for the association of three identified single nucleotide polymorphisms with Japanese type 2 diabetes. Diabetes. 2002;51:863‐866. [DOI] [PubMed] [Google Scholar]
- 67. Chung CM, Lin TH, Chen JW, et al. Common quantitative trait locus downstream of RETN gene identified by genome‐wide association study is associated with risk of type 2 diabetes mellitus in Han Chinese: a Mendelian randomization effect. Diabetes Metab Res Rev. 2014;30:232‐240. [DOI] [PubMed] [Google Scholar]


