Abstract
Background
Use of total laboratory automation (TLA) system has expanded to microbiology and hemostasis and upgraded to second and third generations. We herein report the first successful upgrades and fusion of different versions of the TLA system, thus improving laboratory turnaround time (TAT).
Methods
A 21‐day schedule was planned from the time of pre‐meeting to installation and clinical sample application. We analyzed the monthly TAT in each menu, distribution of the “out of range for acceptable TAT” samples, and “prolonged time out of acceptable TAT,” before and after the upgrade and fusion.
Results
We installed and customized hardware, middleware, and software. The one‐way CliniLog 2.0 version track, 50.0‐m long, was changed to a 23.2‐m long one‐way 2.0 version and an 18.7‐m long two‐way 4.0 version. The monthly TAT in the outpatient samples, before and after upgrading the TLA system, were uniformly satisfactory in the chemistry and viral marker menus. However, in the tumor marker menu, the target TAT (98.0% of samples ≤60 minutes) was not satisfied during the familiarization period. There was no significant difference in the proportion of “out of acceptable TAT” samples, before and after the TLA system upgrades (7.4‰ and 8.5‰). However, the mean “prolonged time out of acceptable TAT” in the chemistry samples was significantly shortened to 17.4 (±24.0) minutes after the fusion, from 34.5 (±43.4) minutes.
Conclusions
Despite experimental challenges, a fusion of the TLA system shortened the “prolonged time out of acceptable TAT,” indicating a distribution change in overall TAT.
Keywords: laboratory quality, out of turnaround time sample, total automation system, total laboratory automation, turnaround time
1. INTRODUCTION
Total laboratory automation (TLA) systems began to be introduced in the field of clinical chemistry in the mid‐1960s. This caused a major change in the map of clinical laboratories. With respect to healthcare economy, the equipment costs associated with TLA are compensated for by a remarkable decrease in other costs.1 TLA is now expanding into the fields of hematology and microbiology,2, 3, 4, 5, 6 and even into the post‐analytical phase, for the storage and recall of samples.7 In the future, the scope will be extended to the liquid chromatography–mass spectrometry/mass spectrometry fields as well.8 In the 1990s, Korea began introducing the TLA system, mainly in large tertiary‐care hospital laboratories.9 In the early days of TLA introduction, middle‐ to large‐sized hospital laboratories were targeted; however, in recent times, it is also being introduced into small‐ or medium‐sized hospital laboratories and 24‐hour satellite laboratories.10, 11, 12 Hospitals that introduced the TLA system in Korea, in the early stages, are now in the process of replacing the system with its second‐ or third‐generation versions.
There are two concerns for hospital laboratories that are in the process of replacing TLA systems: efficiency and cost.12, 13, 14 In terms of efficiency, it is to be noted that the tracks, modules, and connected devices that make up the TLA system do not age at the same rate. Therefore, replacing the entire system at once is a waste of medical resources. In terms of cost, even for large hospitals with strong financial resources, replacing the entire TLA system of the laboratory at the same time can prove to be costly. This is even more difficult in the healthcare environment of the Republic of Korea, where medical expenses are tightly controlled by the government. Therefore, sequential replacement with time differences seems to be inevitable when upgrading a TLA system in the country.
The National Cancer Center Hospital of South Korea (NCC) is a 571‐bed tertiary cancer hospital. The Department of Laboratory Medicine of the NCC hospital runs two clinical laboratories. One is the main central laboratory, with 9 divisions‐hematology, chemistry, immunology, microbiology, blood bank, flow cytometry, molecular pathology, chromosomal study, and HLA study. The other is a 24/7 stat laboratory. As of 2016, an annual total of 6 272 098 tests was conducted in these laboratories. In our laboratories, TLA was first introduced in 2001, in the chemistry and immunology divisions. The TLA system completed its first‐generation lineup in 2007. Fifteen years later, in 2016, we decided to replace the TLA system. However, faced with the aforementioned problems, we planned a step‐by‐step sequential upgrade. The loading, de‐capping, aliquoting, centrifugation, delivery, and storage systems were changed. In terms of the track system, the authors upgraded to version 4.0 and fused it with pre‐existing version 2.0. There have been no reports in which different versions of TLA systems have been linked and applied to clinical laboratories, and this was an experimental challenge for us.
The purpose of this study was to share the experience of successfully upgrading the TLA system to help laboratories facing similar challenges regarding simultaneous sequential expiration of multiple laboratory auto‐analyzers and a cost burden.
2. MATERIALS AND METHODS
2.1. Laboratory setup
The NCC is a complex comprising a cancer hospital, health promotion center, research institute, and graduate school aimed at the prevention and treatment of cancer. The laboratories are located within a hospital building, and inpatient and outpatient samples are registered and processed in an accessioning area. Samples are then delivered to the testing laboratory and the site of each division, on the same floor. In 2001, first‐generation TLA was established in the main laboratory and initiated to create an integrated automation system, with enhanced sample processing and testing efficiency. The second‐generation TLA, in 2016, aimed to upgrade the systems successfully and to fuse them with the previous version of the tracks.
2.2. Upgrading the total laboratory automation system
Multiple analyzers in clinical chemistry and diagnostic immunology, with various laboratory disciplines, were linked to the track. Table 1 shows the layout along with a brief description of the first‐ and second‐generation TLA systems, and each component that was connected to the track.
Table 1.
Total laboratory automation (TLA) layout and description of the connected modules in the first and second generations, respectively. Function, number of modules, description, and throughput are listed
| Module and function | Number | Description and throughput |
|---|---|---|
| (A) First‐generation TLA system, in 2007 | ||
| Start Stocker/Sample input | 1 | Maximum 600 tubes/once |
| Decapper | 1 | 400 tubes/h |
| Aliquoter | 1 | 450 tubes/h |
| Centrifuge | 2 | 30 tubes per batch, in each centrifuge |
| Rack Buffer | 1 | 70 tubes |
| Terminal Stocker | 1 | Storage capacity, 300 tubes |
| Track‐CliniLog v2.0 | Oneway, total 50 m(Low‐speed line 30 cm/sHigh‐speed line 50 cm/s | |
| ADVIA Centaur XP | 2 | Immunoassay analyzer |
| ARCHITECT i2000 | 3 | Immunoassay analyzer |
| Toshiba TBA‐200FR neo | 3 | Chemistry analyzer |
| (B) Second‐generation TLA system after fusion, in 2016 | ||
| Managed Pre‐Analyzed Module | ||
| Sample Input | 1 | Maximum 200 tubes/once |
| Decapper | 1 | 570 tubes/h |
| Aliquoter | 1 | 540 tubes/h |
| Intelligent Centrifuge Module | ||
| Input centrifuge buffer | 1 | 96 tubes |
| Centrifuge | 1 | 96 tubes/batch |
| Output centrifuge buffer | 1 | 96 tubes |
| Buffer Module | ||
| Buffering samples for run and rerun | 2 | 80 tubes |
| Random Access Archive Module | ||
| Sample storage and recall | 1 | 1200 tubes of storage capacity,Automated storage and recall at request |
| Track | ||
| CliniLog v4.0 | Total 18.7 m | Low speed,‐17 cm/s, High speed‐100 cm/s |
| Connector | 1 | Fusion of different versions of the track |
| CliniLog v2.0 | Total 23.2 m | Low speed‐30 cm/s, High speed‐50 cm/s |
| Automated analyzer | ||
| ADVIA Centaur XP | 2 | Immunoassay analyzer |
| ARCHITECT i2000 | 3 | Immunoassay analyzer |
| Toshiba TBA‐200FR neo | 3 | Chemistry analyzer |
When upgrading the TLA system, we took several areas for considering logistics and handling issues, facilities and space considerations, mapping workflow, and timed workflow. Working group meetings, including laboratory information system (LIS)/hospital information system (HIS) staff and laboratory staff, were conducted to draw the outline, and simulations were carried out.
A 21‐day schedule was planned, from pre‐meeting to installation and clinical sample application: ‐14 days, computer system meeting; ‐7 days, operation meeting; ‐3 days, check the location of the work table; upgrade day 1, disconnect the device from the existing TLA system; day 2 ~ 3, access the floor construction; day 4, install the upgraded TLA system; day 5, install 8 analyzer systems and connect them to the LIS; day 6, virtual system test; and day 7, actual patient sample test.
The data management system receives the orders from the LIS (self‐developed by the NCC) and monitors the state of operations of the associated modules of TLA. Results from the analyzers are then relayed to and managed by the middleware system (Innovative Power, Seoul, Korea) that is interfaced with the LIS.
2.3. Data on turnaround times
During the laboratory test processing at the NCC hospital, four time points were automatically recorded in the LIS: “barcode printing,” when the sample barcode was printed by an autolabeler in the phlebotomy room; “scanning,” when the sample barcode was scanned at the registration desk of central laboratory after delivery from the phlebotomy room; “result to LIS,” when the result was transmitted from the instrument to the LIS after the analysis; and “result to HIS,” when the verified result was transmitted from the LIS to the HIS. In this study, TAT was defined from “scanning” to “result to HIS” to compare the pre‐ and post‐fusion performance of the TLA system. Retrospective data were extracted from the LIS.
The monthly TAT fulfillment rates of the chemistry menu, tumor marker menu, and viral marker menu were examined before and after TLA fusion. Outpatient samples were tested in the TLA system. TAT data were extracted for 2 months, in which the working days were similar and were considered representative, respectively.
To investigate the pattern of TAT prolongation, we focused on “Out of (acceptable) TAT” samples. More than 99.9% of “Out of TAT” samples were obtained on Monday when there is a concentrated request for analysis for outpatients. After applying exclusion criteria, all “out of acceptable TAT” samples were observed only on Monday. Accordingly, TAT data were extracted over 10 Mondays (the day for which “out of acceptable TAT” samples present), before and after TLA fusion. Exclusion criteria included a new sample being requested for hemolytic, lipemic, icteric samples, or insufficient quantity. “Prolonged time out of (acceptable) TAT” was defined as “total TAT”‐ “target TAT. ”
2.4. Statistical analysis
Statistical analyses were performed using Microsoft Excel with R program 3.3.2 free software. A Mann‐Whitney U test and Student t test were used to verify statistically significant differences between the two groups in data with non‐parametric and parametric distributions, respectively. All the probabilities were two‐tailed, and P ≤ .05 was considered significant.
3. RESULTS
3.1. Upgrade and fusion of the total laboratory automation system
The changes in the hardware, before and after upgrade and fusion, are summarized in Table 1 and Figure 1. The biggest feature, after upgrade, was the integrated modular system which also has the control function of the individual module, as shown in Figure 1B. Three separate modules from the previous version—sample injection, de‐capping, and aliquoter (sample input)—were integrated into the Managed Pre‐Analyzed Module (MPAM). Before upgrade, two centrifuge units with 30 tubes/batch‐capacity were manually operated. After upgrade, centrifuge units and two buffering systems, composed of “before‐centrifuge standby” and “after‐centrifuge standby,” were integrated into an Intelligent Centrifuge Module (ICM), resulting in a 96‐tubes‐per‐batch performance. Before the fusion, the track was a one‐way CliniLog 2.0 version, 50.0‐m long. After the fusion and equipment upgrades, a 23.2‐m long one‐way 2.0 version and 18.7‐m long two‐way 4.0 version were linked via a connector. Therefore, if a chemistry assay and other assays are requested with the same tube, a daughter sample is automatically generated and moved to the instrument for assays. The original sample is circulated for the requested assays, and then stored in the Random Access Archive Module (RAAM). In the second generation, sample storage and recall became possible.
Figure 1.
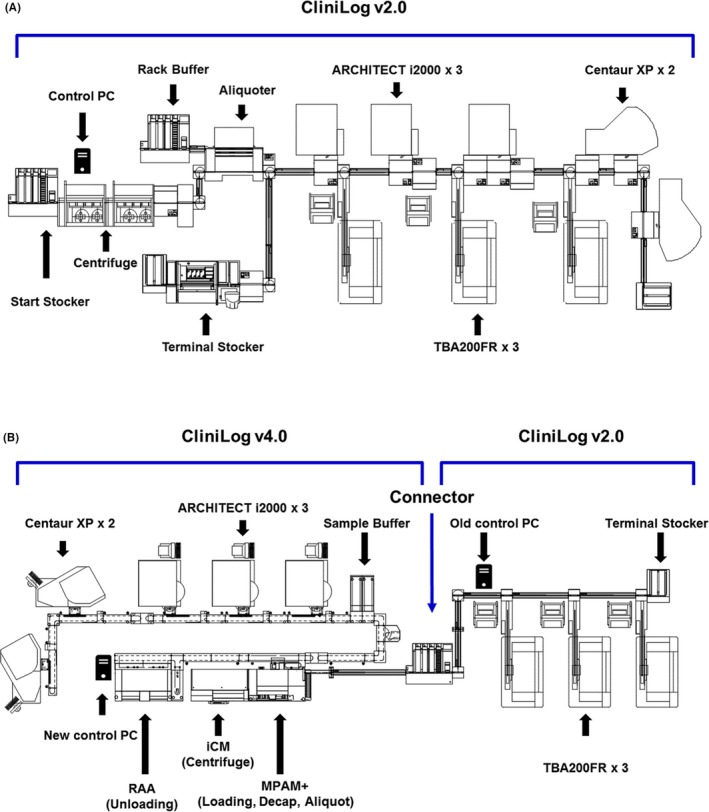
Map of the total laboratory automation (TLA) layout (A) First‐generation TLA system, in 2007: the position of the module included along the automation line, at the National Cancer Center. From the left: Start Stocker/Sample input and centrifuge, aliquoter, and connected test modules. (B) Second‐generation TLA system after fusion, in 2016. From the left: sample input and uploading, centrifuge, MPAM for de‐capping and aliquoting, and connected test modules
3.2. Monthly TAT fulfillment rates
Table 2 reveals the fulfillment rate of the target TAT in the outpatient samples, before and after the TLA system was upgraded. Each menu targeted different goals (time and fulfillment rate). Monthly TAT monitoring showed that both first‐ and second‐generation TLA systems met the target. Just after the upgrade, 97.9% of the samples in the tumor marker menu met the target TAT (≤60 minutes); however, this did not satisfy the target of 98.0%. After familiarization, it increased to over 99.1%, considered satisfactory.
Table 2.
Trends of turnaround time (TAT) in outpatient samples, before and after upgrading the total laboratory automation (TLA) system
| Menu | Laboratory TAT target | Fulfillment rate in the first‐generation TLA (%) | FamiliarizationPeriod | Fulfillment rate in the second‐generation TLA (%) | ||
|---|---|---|---|---|---|---|
| Before 3 mo (n = 16 243) | Before 1 mo (n = 17 899) | After 1 mo (n = 17 214) | After 3 mo (n = 16 580) | After 5 mo (n = 17 489) | ||
| Chemistry | 99.0% of samples ≤60 min | 99.7 | 99.8 | 99.2 | 99.5 | 99.4 |
| Tumor marker | 98.0% of samples ≤60 min | 99.1 | 98.9 | 97.9 | 99.1 | 98.8 |
| Viral marker | 99.0% of samples ≤24 h | 99.8 | 99.8 | 100.0 | 100.0 | 100.0 |
Each menu has a different target TAT and target fulfillment rate. Total requested sample numbers for each month are shown as n. Monthly TAT monitoring results show that both first‐ and second‐generation TLA systems met the target, except in the case of the tumor marker, during the harmonization period. If the target TAT is not met, it is indicated in bold.
3.3. Distribution of the samples “out of acceptable TAT”
The target TAT of the outpatient sample for the chemistry analysis is 60 minutes, and the NCC central laboratory aims to satisfy more than 98.0% of this target. We observed the distribution of specimens which were reported after 60 minutes from the time of sample reception (which is considered an acceptable TAT), and defined these as “out of acceptable TAT” samples. Before the upgrade, a total of 6821 tubes were requested from the outpatient clinic, for 10 days. The 6762 tubes were analyzed, except for 59 tubes which met the exclusion criteria. A total of 50 samples were found to be “out of acceptable TAT” (74/1000 = 7.4‰). After the upgrade of the TLA system, a total of 7079 tubes were requested from the outpatient clinic, for 10 days. The 7036 tubes were analyzed, except for 43 tubes which met the exclusion criteria. A total of 60 samples were “out of acceptable TAT” (8.5‰). After the application of exclusion criteria, it was noted that all “out of acceptable TAT” samples were observed only on Monday.
The sample distribution of the “prolonged time out of TAT (total TAT‐ target TAT)” is shown in Figure 2, as a Violin Plot. The vertical axis represents the time elapsed from the target TAT (60 minutes), and the horizontal axis represents the two groups before and after the TLA upgrade. As shown in the figure, when the first‐generation TLA system was used, the interquartile range (IQR) of the prolonged time from the target TAT (60 minutes) was 50 minutes. The IQR was contracted to 25 minutes, as (half of the first generation) in the case of the second‐generation TLA system. The maximum time elapsed from the target TAT was shortened from 164 minutes in the first‐generation TLA system to 101 minutes in the second generation. The mean ± standard deviations (SDs) of “prolonged time out of acceptable TAT” were 34.5 ± 43.4 minutes in the first generation and 17.4 ± 24.0 minutes in the second generation, showing a statistically significant difference (P = .015).
Figure 2.
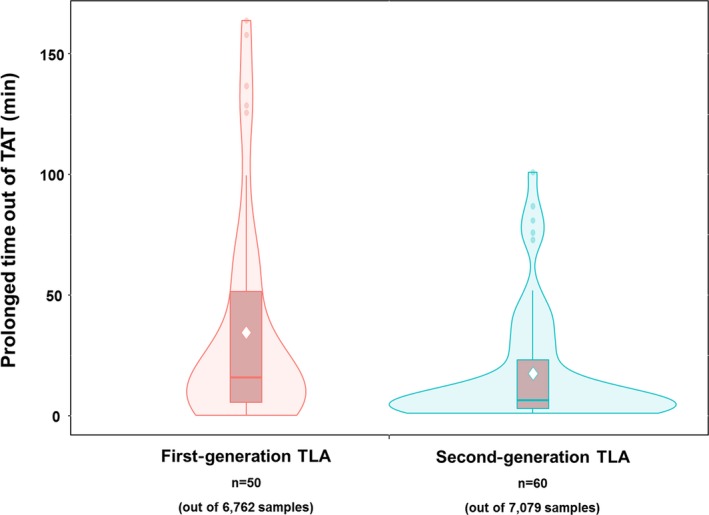
The Violin Plot shows the distribution shape of the out of turnaround time (TAT) samples. In this figure, that the pattern of “prolonged time out of TAT” is more compressed can be recognized. In the density plot, the vertical line indicates the 95% confidence interval of “prolonged time out of TAT,” and the width indicates the frequency. The box indicates the interquartile range, with central horizontal line revealing the 50th percentile. The diamond point in the middle of the box in each group represents the mean value of “prolonged time out of TAT.” The mean “prolonged time out of acceptable TAT” in the chemistry samples was significantly shortened to 17.4 (±24.0) min after the fusion, from 34.5 (±43.4) min
4. DISCUSSION
Our laboratories are part of NCC‐affiliated medical institutions, which have an average of 1700‐2000 daily outpatient visits, and an average of 550 beds are occupied by hospitalized patients. The Department of Laboratory Medicine conducted 6 272 098 tests in 2016 and performed 25 291 tests/d on a working day basis. The TLA system was first introduced into the core laboratory of NCC in 2001, and in 2007, four additional automated analyzers (two ARCHITECT i2000, one ADVIA Centaur XP, and one TBA‐200FR neo) were added and the TLA track was extended. Now, however, due to stepwise equipment expiration, partial replacement and upgrades were inevitable. As in the case of the NCC laboratory, for clinical laboratories across South Korea, TLA systems are no longer new.9 TLA systems have expanded to microbiology field2, 5 and hemostasis.4 The type of automation is also diversified into total automation or sub‐total automation.8, 12, 15 Now, Korean laboratories are faced with the need to upgrade to the second or third generations of the TLA system. We believe that the experiences and results described in this study can help laboratory staffs and directors of clinical laboratories. In our experience, the most important step was customizing (tailoring) the software systems. Even with many simulations, there were errors in the middleware system, in TLA management software, and in connection to LIS. Therefore, to minimize errors and provide the best tailoring, it is essential to obtain accurate and representative laboratory operation data. Multidisciplinary information sharing and co‐operation among LIS staff, IT personnel of TLA systems, and medical technicians are needed. There were no problems in the hardware step, including disconnecting the previous TLA and installing the upgraded TLA and laboratory auto‐analyzer systems.
The NCC laboratory is currently participating in the CAP survey, a nationwide external proficiency testing in Korea (KEQAS, http://www.keqas.org/), and Korean Laboratory Accreditation Program (KLAP) by Laboratory Medicine Foundation (LMF), and makes continuous efforts to improve laboratory quality. As recommended in the Korean KLAP, one of the key laboratory indicators, TAT is under active surveillance to ensure the highest adherence rates. The laboratory director monitors the monthly TAT, and the total TAT is sub‐divided by phase.16 For “out of TAT” samples, the extended phase and reasons behind it are documented every day, and each unit supervisor checks it monthly. During the familiarization period, after the introduction of the fused TLA system, TAT prolongation was observed due to frequent program errors and equipment errors. The biggest challenge was to stabilize this within a short period.
This fusion was the third TLA system change the NCC laboratory underwent. It is worthy to note that a month after the upgrade, which is the time taken for the hardware to stabilize, the TLA was successfully operated without any errors. In terms of TAT, it can be concluded that the familiarization period for both the equipment and users was less than 3 months, from the time of the upgrade.
The department of laboratory medicine of NCC plans to replace one TBA200fr system with a TBA2000fr system every year, for the next 3 years, moving the fusion contacts to the distal part and eventually completely phasing out the previous version of the track. Through this fusion experience, the 2‐year preliminary plan was confirmed.
In this study, for the first time, authors have succeeded in experimenting with other versions of TLA system fusion. After the familiarization period, the TAT of the laboratory, that is tightly supervised, was found to be satisfactory. In addition, the “prolonged time out of TAT” was shortened. The authors' experience paves the way for sequential upgrades in large laboratories.
CONFLICT OF INTEREST
The authors declare no conflict of interests. Only the authors are responsible for the development of the content and writing of the paper.
AUTHOR CONTRIBUTIONS
Hee‐Jung Chung: Conceptualization, Formal analysis, Methodology, Project administration, Software, Supervision, Visualization, Writing original draft, Writing–review, and editing. Yoon Kyung Song: Data curation, Investigation, Resources, Software, Validation. Sang Hyun Hwang: Funding acquisition, Writing–review, and editing. Tetsuro Sugiura: Conceptualization, Writing–review, and editing. Do Hoon Lee: Conceptualization, Funding acquisition, Writing–review, and editing, Supervision.
ACKNOWLEDGMENT
This study was supported by a grant from the National Cancer Center, Republic of Korea (Grant NCC 510100).
Chung H‐J, Song YK, Hwang S‐H, Lee DH, Sugiura T. Experimental fusion of different versions of the total laboratory automation system and improvement of laboratory turnaround time. J Clin Lab Anal. 2018;32:e22400 10.1002/jcla.22400
REFERENCES
- 1. Archetti C, Montanelli A, Finazzi D, Caimi L, Garrafa E. Clinical laboratory automation: a case study. J Public Health Res. 2017;6:881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mutters NT, Hodiamont CJ, de Jong MD, Overmeijer HP, van den Boogaard M, Visser CE. Performance of Kiestra total laboratory automation combined with MS in clinical microbiology practice. Ann Lab Med. 2014;34:111‐117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Da Rin G, Zoppelletto M, Lippi G. Integration of diagnostic microbiology in a model of total laboratory automation. Lab Med. 2016;47:73‐82. [DOI] [PubMed] [Google Scholar]
- 4. Sedille‐Mostafaie N, Engler H, Lutz S, Korte W. Advancing haemostasis automation‐successful implementation of robotic centrifugation and sample processing in a tertiary service hospital. Clin Chem Lab Med. 2013;51:1273‐1278. [DOI] [PubMed] [Google Scholar]
- 5. Novak SM, Marlowe EM. Automation in the clinical microbiology laboratory. Clin Lab Med. 2013;33:567‐588. [DOI] [PubMed] [Google Scholar]
- 6. Burnham CA, Dunne WM Jr, Greub G, Novak SM, Patel R. Automation in the clinical microbiology laboratory. Clin Chem. 2013;59:1696‐1702. [DOI] [PubMed] [Google Scholar]
- 7. Hawker CD. Nonanalytic laboratory automation: a quarter century of progress. Clin Chem. 2017;63:1074‐1082. [DOI] [PubMed] [Google Scholar]
- 8. Armbruster DA, Overcash DR, Reyes J. Clinical chemistry laboratory automation in the 21st century ‐ Amat victoria curam (Victory loves careful preparation). Clin Biochem Rev. 2014;35:143‐153. [PMC free article] [PubMed] [Google Scholar]
- 9. Park H, Kim JW, Min WK, Chi HS, Kim JQ, Kim DW. Implementation of total laboratory automation and its outcome analysis for laboratory productivity. Korean J Clin Pathol. 1998;18:494‐500. [Google Scholar]
- 10. Zaninotto M, Plebani M. The, “hospital central laboratory”: automation, integration and clinical usefulness. Clin Chem Lab Med. 2010;48:911‐917. [DOI] [PubMed] [Google Scholar]
- 11. Ialongo C, Porzio O, Giambini I, Bernardini S. Total automation for the core laboratory: improving the turnaround time helps to reduce the volume of ordered STAT tests. J Lab Autom. 2016;21:451‐458. [DOI] [PubMed] [Google Scholar]
- 12. Hawker CD. Laboratory automation: total and subtotal. Clin Lab Med. 2007;27:749‐770, vi. [DOI] [PubMed] [Google Scholar]
- 13. Tatsumi N, Okuda K, Tsuda I. A new direction in automated laboratory testing in Japan: five years of experience with total laboratory automation system management. Clin Chim Acta. 1999;290:93‐108. [DOI] [PubMed] [Google Scholar]
- 14. Seaberg RS, Stallone RO, Statland BE. The role of total laboratory automation in a consolidated laboratory network. Clin Chem. 2000;46:751‐756. [PubMed] [Google Scholar]
- 15. Kricka LJ, Polsky TG, Park JY, Fortina P. The future of laboratory medicine ‐ a 2014 perspective. Clin Chim Acta. 2015;438:284‐303. [DOI] [PubMed] [Google Scholar]
- 16. Chung HJ, Lee W, Chun S, et al. Analysis of turnaround time by subdividing three phases for outpatient chemistry specimens. Ann Clin Lab Sci. 2009;39:144‐149. [PubMed] [Google Scholar]


