Abstract
Background
Alzheimer's disease (AD) is a neurodegenerative disease, which is associated with malnutrition and hyperhomocysteine. The current study aimed to analyze the relationship between malnutrition and hyperhomocysteine in AD patients, and effects of diet intervention with betaine on the disease.
Methods
The nutritional statuses of the AD patients were assessed by short form mini nutritional assessment (MNA‐SF). The levels of Hcy, tau hyperphosphorylation, synaptic proteins, blood inflammatory factors were measured by enzymatic cycling assay, Western blot and ELISA. The cognitive function was measured by AD assessment scale (ADAS‐cog).
Results
There was a significant difference in mental status between normal people and AD patients (P<.05). Overall, malnutrition was reported in a larger proportion of AD patients and high level of Hcy was closely associated with malnutrition. Betaine decreased the levels of phosphorylated tau, elevated PP2Ac activity and inhibited Aβ accumulation (P<.05). The levels of IL‐lβ and TNF‐α were significantly higher in the untreatment group while much lower in the intervention group (P<.05). After intervention of betaine treatment, the expression level of Hcy can be restored and betaine can effectively suppress inflammation as well as trigger an increase in memory‐related proteins. ADAS‐Cog suggested that significant improvement was found after the intervention of betaine.
Conclusions
AD was associated with both malnutrition and higher levels of Hcy. Betaine could restore Hcy expression to normal level in AD patient, which might ameliorate memory deficits.
Keywords: Alzheimer's disease, betaine, hyperhomocysteine, malnutrition
1. Introduction
Alzheimer's disease (AD), a heterogeneous and multifactorial neurodegenerative disease, is characterized by clinical symptoms of irreversible behavioral alterations as well as learning and memory processes disorders.1, 2 Currently, there are more than 46 million individuals diagnosed with AD in the world, and the number of AD patients is estimated to reach 100 million worldwide by 2050. Therefore, AD is a growing public health concern with great socioeconomic burden.3 Current hypotheses about the pathology of AD include neurodegeneration, which is represented in the form of extraneuronal neuritic plaques, and neuronal deaths due to the excessive production of amyloid‐β (Aβ) peptide.4 In addition, intraneuronal neurofibrillary tangles formed by hyperphosphorylated tau proteins are associated with AD.2 As one of the tau protein phosphatases, Protein phosphatase 2AC (PP2Ac) is able to dephosphorylate tau protein.5 The deactivation of PP2Ac deprives its function of dephosphorylation and leads to the formation of neurofibrillary tangle eventually.6
There are multiple risk factors for AD including age, certain genetic alleles, and some nutritional substances.7 However, pharmacology that can cure AD is limited, therefore, whether diet could alleviate the risk and process of AD is of great interest. Although it is challenging to confirm whether diet is a contributing factor, many epidemiology studies and intervention tests have strongly demonstrated that cognitive function decline is connected to diet.1, 7, 8 For instance, epidemiological studies suggest that a low intake of n‐3 fatty acids (n‐3 FA), B‐vitamins, and antioxidants are associated with an increased risk of AD. Several nutritional compounds that can stabilize neurons at normal levels are believed to play a part in the pathological process of AD.1 For example, the n‐3 long‐chain polyunsaturated fatty acid (n‐3 LC‐PUFA) and docosahexaenoic acid inhibit aberrant Aβ and tau‐protein progression.9 In addition, antioxidants like vitamin E have the function of protecting neurons against Aβ‐induced oxidative stress and stabilizing neuronal membranes.10 Besides lower micronutrient levels, protein/energy malnutrition has been reported to be associated with disease progression in mild AD patients.11 These evidences indicate that malnutrition is a contributing factor for AD.
A number of researchers have reported that patients with cardiovascular, cerebrovascular diseases and AD usually have an elevated level of plasma homocysteine (Hcy).12 Hyperhomocysteine (Hhcy) is closely related to silent brain infarcts, greater cortical atrophy, and longitudinally more severe cognitive decline.13, 14 Hcy is a sulfur‐containing amino acid and a transitional production of the methionine circle, and as a kind of neurotoxin, normal physiological levels of Hcy are maintained through its remethylation to methionine, in which common dietary nutrients vitamin B6, folate and B12 are essential.15 Moreover, several studies have suggested that the uptake of excessive methionine or a deficit of folate or certain enzymes in the methionine cycle due to genetic alterations would increase Hcy content in vivo.16 Another potential association between high Hcy and AD is the alteration of the amyloid precursor protein metabolic pathway(s). A diet‐induced chronic high Hcy results in a substantial increase in brain Aβ levels and deposition in transgenic mouse models of AD‐like amyloidosis.17 Therefore, it is vital to understand the molecular mechanistic relationship between Hcy and AD pathogenesis as it could offer clues for prevention or treatment of AD.
Betaine, a zwitterionic quaternary ammonium compound, also called glycine betaine, trimethylglycine, oxyneurine, and lycine, is a methyl donor that provides the one‐carbon units for Hcy remethylation.18 To date, few studies have investigated the association between betaine and AD caused by Hhcy. In this study, we aim to study the possible relationship between mild‐to‐moderate AD and malnutrition, and restore cognitive functions through betaine diet.
2. Materials and Methods
2.1. Subjects
We recruited 97 AD patients (the mean age was 74.6±9.2 years and the clinical course was 6‐48 months) who consulted in Shandong Provincial Qianfoshan Hospital from January 2013 to January 2015. They were classified as non‐malnutrition group (n=36) and malnutrition group (n=61) according to the result of Short form mini nutritional assessment (MNA‐SF). Then, all patients were randomly assigned into different groups including untreatment group (AD patients without treatment) and intervention group (AD patients were treated with 50, 100, and 200 μg/kg betaine for 1 month).
Another 65 subjects were enrolled in the control group. They were healthy volunteers without any mental diseases or neurological diseases consulted in the same hospital. The MNA‐SF score of each patient in the control group was ≥11.
Inclusion criteria of AD patients were set according to the Diagnostic and Statistical Manual of Mental Disorders IV (DSM‐IV). Individuals who had acute infection, trauma, and myocardial infarction were excluded. No treatment was performed in the control or untreatment group. All of the subjects signed the informed consent form. This study was approved by the ethics committee of Shandong Provincial Qianfoshan Hospital.
2.2. Short form mini nutritional assessment (MNA‐SF) Evaluation and Blood measurement
The nutritional status of the patients with senile dementia was assessed by MNA‐SF. The total score was 14; a score ≥11 was considered normal; malnutrition was defined if the score was <11. All patients had about 2‐3 mL early morning fasting venous blood taken and blood samples were placed in anticoagulation tube. The sample was sent for testing after 30 minutes of mixing. Enzymatic cycling assay was performed to measure the amount of serum Hcy, hemoglobin, urea, cholesterol, and serum albumin via an automatic biochemical analyzer (iChem‐340; icubio Biological Technology Co., Ltd, Shenzhen, China).
2.3. Western blot
We tested the amount of phosphorylated tau proteins including Thr231 (pT231), Thr205 (pT205) and Ser396 (pS396) in the extracted blood samples. Equal amounts of proteins were subjected to polyacrylamide gel electrophoresis. The membrane was incubated in 10 mL of 5% non‐fat milk in m (TBST) solution to block the membrane for 1.5 hours after transferring the gel to polyvinllidence fluoride membrane. The membrane was incubated with the primary antibodies against Tau1, pT205, pT231, pS396, Tau5, PP2Ac, p‐PP2Ac, NR1, NR2A, NR2B, synaptotagmin, synaptophysin, synapsin 1, p‐synapsin 1 (diluted with 1:800, 1:1000, 1:500, 1:500, 1:800, 1:800, 1:500, 1:800, 1:1000, 1:500, 1:500, 1:800, 1:800, 1:500, respectively; Zhongshan Biology Company, Beijing, China). After washing the membrane, the secondary antibodies were used (horseradish peroxidase‐conjugated goat anti‐goat, 1:2000 dilution; Zhongshan Biology Company) to repeat the procedure again. Then ECL chemiluminescence reagent kit was added to monitor development after washing for three times with TBST.
2.4. Enzyme linked immunosorbent assay (ELISA) and ADAS‐Cog analysis
ELISA was used to analyze blood inflammatory factors (IL‐lβ, TNF‐α), Aβ40, and Aβ42. ELISA was performed with IL‐lβ, TNF‐α, Aβ40, and Aβ42 ELISAkits from Bnder MedSystems according to the manufacturer's protocol.
The AD assessment scale (ADAS‐Cog) includes recall of words, naming of items and fingers, following instructions, visual‐spatial capacity, intentional‐practice skills, directional ability, recognition of dual words, recall of test instructions, verbal language skills, word finding difficulty, and attention, and understanding dysfunction. The corresponding score was divided into five grades. Severity level was evaluated using selective domains ranging from 0 to 70. A score of 0 means no dementia while a score of 70 points means no cognitive functions.
2.5. Statistical analysis
Statistical analyses were performed using the SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, USA). Measurement data were presented as mean±standard deviation (SD). Enumeration variables were analyzed by the chi‐square (χ2) test. Continuous variables were analyzed by two‐tailed t‐test or one‐way analysis of variance (ANOVA). A P value <.05 was considered statistically significant.
3. Results
3.1. Subject characteristics
There was no significant difference between the normal people and AD patients in terms of age, sex ratio, education degree, tea or alcohol consumption, smoking, and intake of vitamin B (P>.05). On the contrary, significant difference was observed in the results of Mini‐Mental State examination (P<.05, Table 1).
Table 1.
Baseline characteristics of two groups
| Index | AD (n=97) | Control (n=65) | P |
|---|---|---|---|
| Age (year) | 73.3±5.3 | 74.8±6.3 | .104 |
| Sex | |||
| Male | 52 | 35 | .976 |
| Female | 45 | 30 | |
| Education degree (year) | |||
| <1 | 6 | 4 | .992 |
| <6 | 13 | 9 | |
| <12 | 52 | 35 | |
| >12 | 26 | 17 | |
| Alcohol consumption | |||
| No | 84 | 60 | .257 |
| Yes | 13 | 5 | |
| Tea consumption | |||
| No | 91 | 62 | .669 |
| Yes | 6 | 3 | |
| B vitamin supplement | |||
| No | 86 | 60 | .446 |
| Yes | 11 | 5 | |
| MMSE | 19.55±2.29 | 28.21±3.15 | <.05 |
AD, Alzheimer's disease; MMSE, Mini‐Mental State examination.
Data are expressed as n or mean±SD as appropriate. Chi‐square test was used for enumeration variables; the Student t‐test was used for continuous variables.
3.2. Relationship among malnutrition, Hcy, and betaine
Low cholesterol and albumin levels are signs of malnutrition. As shown in Table 2, the study of MNA‐SF showed that there were 61 malnutrition patients, which accounted for 62.9% of AD patients. Cholesterol and albumin levels were much lower in the malnutrition patients than those in the non‐malnutrition patients (P<.05). In addition, an Hcy‐test was performed on every subject and the result showed that Hcy level was significantly elevated in malnutrition patients (P<.05), suggesting a strong connection between the malnutrition and Hcy.
Table 2.
Risk factors of mild senile dementia
| Factors | Malnutrition group (n=61) | Non‐malnutrition group (n=36) | P |
|---|---|---|---|
| Hemoglobin (g/L) | 121.42±9.33 | 125.15±11.84 | .089 |
| Urea nitrogen (mmol/L) | 7.12±2.75 | 6.84±2.19 | .604 |
| Cholesterol (mmol/L) | 4.41±1.36 | 5.67±1.49 | <.05 |
| Plasma albumin (g/L) | 30.38±11.03 | 42.52±9.15 | <.05 |
| Plasma Hcy (g/L) | 26.84±9.28 | 14.72±7.35 | <.05 |
Hcy, homocysteine.
Data are expressed as mean±SD. The Student t‐test was used for continuous variables.
After betaine diet intervention treatment, betaine regulated Hcy in a dose‐dependent manner, a significant difference in Hcy was identified between the untreatment group and 200 μg/kg betaine group (P<.05, Figure 1), indicating that betaine can suppress the level of Hcy.
Figure 1.
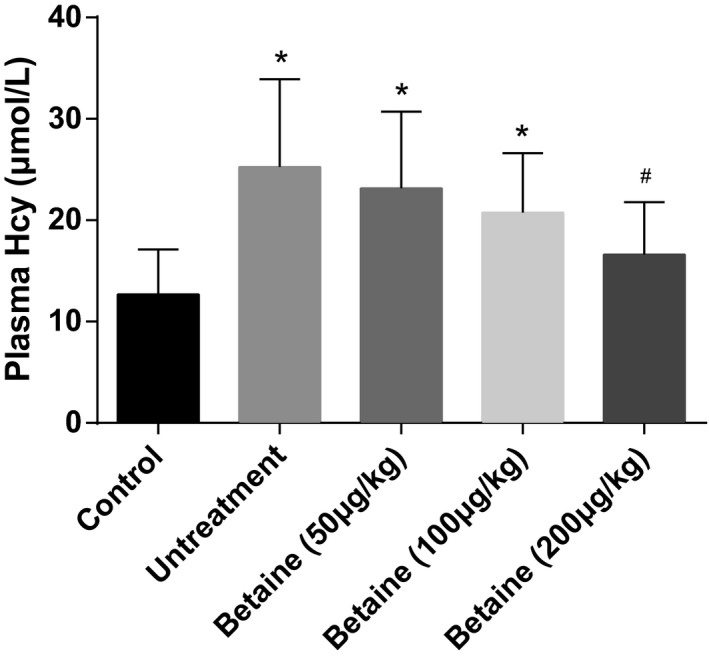
Effect of betaine on plasma Hcy. Data were expressed as mean±SD. *P<.05 vs control group; # P<.05 vs untreatment group
3.3. Betaine regulates tau phosphorylation and PP2Ac activity
Hyperphosphorylation of tau protein contributes to neuronal fiber entanglement, which is one of the mechanisms causing neurodegeneration. Western blot was used to determine the levels of phosphorylated tau proteins. We found that phosphorylated tau proteins including Thr231 (pT231), Thr205 (pT205), and Ser396 (pS396) all increased significantly in the untreatment group compared to the control group, while decreased in intervention group (treated with 200 μg/kg betaine) compared with untreatment group (P<.05, Figure 2). The results indicated that treatment of betaine was able to reduce hyperphosphorylation of tau protein.
Figure 2.
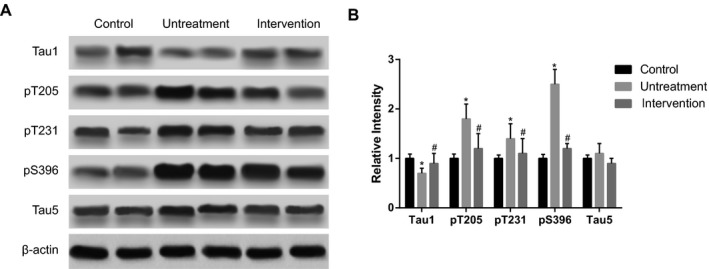
The phosphorylation levels of tau proteins. Intervention group was treated with 200 μg/kg betaine. Data were expressed as mean±SD. *P<.05 vs control group; # P<0.05 vs untreatment group
In addition, we found that intervention of betaine (200 μg/kg) increased protein phosphatase‐2Ac (PP2Ac) expression but decreased the phosphorylayion of PP2Ac, and thus regulated the activation of PP2Ac (P<.05, Figure 3).
Figure 3.
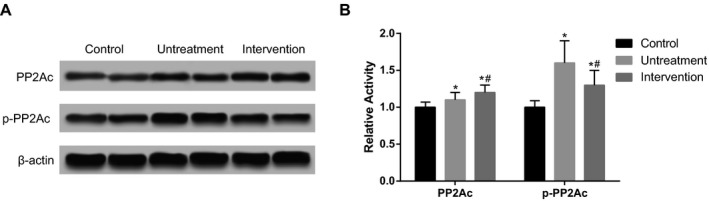
The phosphorylation level of protein phosphatase‐2Ac (PP2Ac). Intervention group was treated with 200 μg/kg betaine. Data were expressed as mean±SD. *P<.05 vs control group; # P<.05 vs untreatment group
3.4. Betaine reverses Aβ accumulation
We saw a higher level of Aβ40 and Aβ42 levels in untreatment group than control group by ELISA, and a significant decrease when betaine treatment (200 μg/kg) was given (P<.05, Figure 4). The result suggests that betaine can reverse Aβ aggregation in AD patients.
Figure 4.
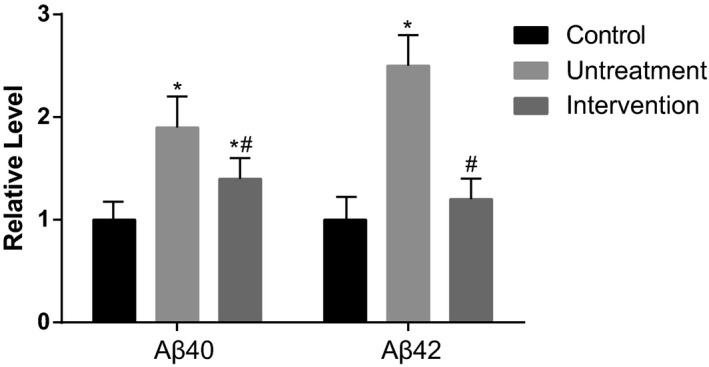
The expression levels of Aβ40 and Aβ42. Intervention group was treated with 200 μg/kg betaine. Data were expressed as mean±SD. *P<.05 vs control group; # P<.05 vs untreatment group
3.5. Analysis of inflammatory factor
The levels of IL‐lβ and TNF‐α in the untreatment group were significantly higher than those in the control group, while both reduced substantially after betaine treatment (200 μg/kg) (P<.05, Figure 5). The data show that betaine can suppress the inflammatory level and help relieve the illness.
Figure 5.
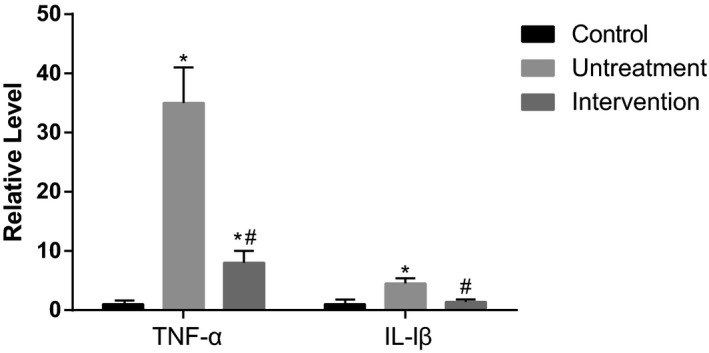
The expression levels of TNF‐α and IL‐1β. Intervention group was treated with 200 μg/kg betaine. Data were expressed as mean±SD. *P<.05 vs control group; # P<.05 vs untreatment group
3.6. Betaine stimulates memory‐related proteins (NR1, NR2A, and NR2B) levels
The Western blot results of memory‐related proteins showed that NR1 and NR2A levels were lower in untreatment group than those in the control group, whereas intervention of betaine (200 μg/kg) significantly increased the expressions of NR1 and NR2A (P<.05, Figure 6). There were no significant differences between the three groups in terms of NR2B expression.
Figure 6.
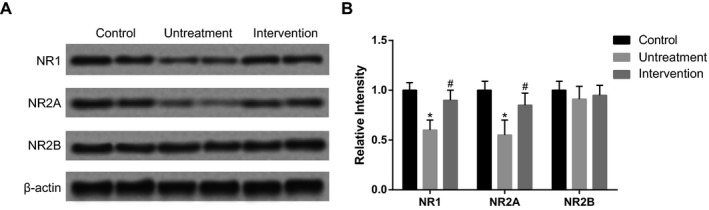
The expression levels of NR1, NR2A, and NR2B. Intervention group was treated with 200 μg/kg betaine. Data were expressed as mean±SD. *P<.05 vs control group; # P<.05 vs untreatment group
3.7. Betaine regulates synaptic protein expressions
Western blot was used to determine the levels of synaptophysin, synaptotagmin, synapsin I, and phosphorylated synapsin I (p‐synapsin I). As shown in Figure 7, the level of synaptic proteins in the untreatment group was much lower than those in the control group, while the intervention group (treated with 200 μg/kg betaine) showed an increase in synaptic protein level than untreatment group (P<.05). These results demonstrated that low level of synaptic proteins, an indicator of memory deficits in AD, could be restored to normal levels after betaine intervention.
Figure 7.
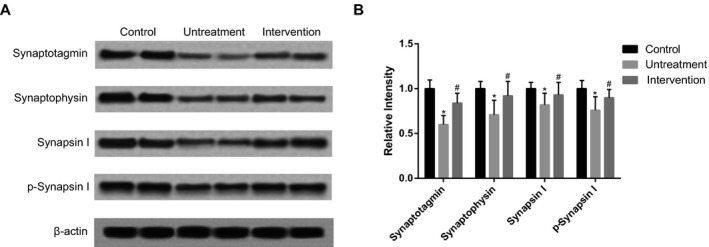
The expression levels of synaptotagmin, synaptophysin, synapsinI, and p‐synapsin I. Intervention group was treated with 200 μg/kg betaine. Data were expressed as mean±SD. * P<.05 vs control group; # P<.05 vs untreatment group
3.8. ADAS‐Cog analysis
The results of the ADAS‐Cog showed no significant difference before and after intervention (200 μg/kg betaine) in areas such as naming of items and fingers, instructions, intentional‐practice, directional ability, and verbal language skills and attention (P>.05); while significant improvements were identified in recall of words, visual‐spatial capacity, and recognition of dual words (P<.05, Table 3).
Table 3.
The results of ADAS‐Cog analysis
| Items | Before treatment | After treatment | P |
|---|---|---|---|
| Recall of words | 5.83±1.64 | 4.27±1.38 | <.05 |
| Naming of items and fingers | 0.88±1.13 | 0.96±0.86 | .591 |
| Instructions | 1.38±0.82 | 1.45±0.47 | .467 |
| Visual‐spatial capacity | 1.49±0.92 | 1.15±0.75 | <.05 |
| Intentional‐practice | 1.31±0.99 | 1.34±0.96 | .831 |
| Directional ability | 2.55±1.20 | 2.41±1.46 | .467 |
| Recognition of dual words | 5.48±1.18 | 4.25±1.15 | <.05 |
| Verbal language skills and attention | 5.20±1.16 | 5.32±1.36 | .509 |
ADAS‐Cog, Alzheimer's disease assessment scale.
Data are expressed as mean±SD. The Student t‐test was used for continuous variables.
4. Discussion
It has been reported that malnutrition is notably high in patients with dementia, and malnutrition may be presented even before classical symptoms of dementia appear.8 Furthermore, it is crucial to observe malnutrition in AD patients because malnutrition is associated not only with a faster progression of AD but also with other health problems.19 In this study, we indicated that the rate of malnutrition among AD patients was 62.9%, which was consistent with previously published studies that reported the rate of malnutrition among community‐dwelling AD patients varying between 14% and 80% in different populations.20 The results of MNA‐SF assessment and Hcy detection showed that malnutrition and Hhcy appeared in patients with dementia concurrently, which suggests that malnutrition and Hhcy are closely associated with dementia.
Increasing epidemiology and clinical researches have revealed that the Hcy level is positively related to the development of AD, and Hhcy is one of the remarkable risk factors in patients with AD.21 Javier et al. observed that Aβ level can be increased in the brain of an Hhcy mouse model with amyloidosis.22 Ho et al.23 demonstrated that Hcy impairs DNA repair in the hippocampus and made hippocampal neurons sensitive to Aβ toxity. Moreover, Zhang et al.24 indicated that increase in Hcy level was highly associated with AD‐like Aβ accumulation and augmented AD‐like tau hyperphosphorylation in brains of rats. Zhang et al.24 have also found that a concurrent supplementation of folate and vitamin B12 improved the plasma Hcy level moderately, and therefore remarkably promotes Aβ accumulation, memory damnifications, tau hyperphosphorylation, and PP2Ac deactivation. Accordingly, decreasing the plasma Hcy level could be a novel strategy to inhibit the development of AD.
In this study, we found that intervention of betaine could significantly reduce the plasma Hcy level and reverse tau protein phosphorylation, PP2Ac deactivation, as well as Aβ accumulation. By down‐regulating serum IL‐lβ and TNF‐α levels, intervention of betaine also attenuated inflammation and therefore were conducive to the mitigation of dementia. Furthermore, intervention of betaine upregulated expression levels of memory‐related proteins (NR1, NR2A) and synaptic proteins (phosphorylated synapsin I, synaptophysin, synaptotagmin), thereby relived memory ramifications. ADAS‐Cog analysis showed that brain cognition and functionality of AD patients, such as structural links, word memory and recognition, have been improved after betaine intervention. Our data were consistent with that of Chai et al.,25 which demonstrated that betaine could inhibit AD‐like pathological changes induced by Hcy.
It is well known that betaine is an endogenous catastate of bursine containing three effective methyl groups that may act as an active methyl donor for the remethylation of Hcy, and it is also related to liver function, cellular reproduction, and carnitine production.26 It has been reported that betaine contributes to a large number of diseases, such as heart and liver diseases.27, 28 Ganesan et al.29 found that betaine also plays a protective agonistic role against oxidative injury induced by stress in Wistar rats. Schiff et al.30 reported that an oral solution of anhydrous betaine were used to treat homocystinuria, indicating that betaine could down regulate the levels of Hcy and reverse the effects of Hcy, including memory impairment.
However, there were some limitations in our study, such as small sample size and the unclear molecular mechanism. We would pay attention to the effects of Hcy and malnutrition on AD in further research.
5. Conclusion
As a whole, this study showed that Hcy and malnutrition were closely related with AD, and betaine intervention can restore the plasma Hcy level as well as play protective effects in AD patients, with potential mechanisms that relate to tau hyperphosphorylation, PP2Ac activation, Aβ accumulation, inflammatory reaction, and the stimulations of memory‐related proteins. Our results provided an encouraging possibility for the treatment of senile dementia.
Acknowledgments
We would thank Dr. Tao Zhang (Department of Neurology, Shandong Provincial Qianfoshan Hospital) for his technical guidance.
References
- 1. Mi W, van Wijk N, Cansev M, Sijben JW, Kamphuis PJ. Nutritional approaches in the risk reduction and management of Alzheimer's disease. Nutrition. 2013;29:1080–1089. [DOI] [PubMed] [Google Scholar]
- 2. Nazef K, Khelil M, Chelouti H, et al. Hyperhomocysteinemia is a risk factor for Alzheimer's disease in an Algerian population. Arch Med Res. 2014;45:247–250. [DOI] [PubMed] [Google Scholar]
- 3. Wang M, Zhu Y, Shi Z, Li C, Shen Y. Meta‐analysis of the relationship of peripheral retinal nerve fiber layer thickness to Alzheimer's disease and mild cognitive impairment. Shanghai Arch Psychiatry. 2015;27:263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng Z, Wang J, Yi L, et al. Correlation between behavioural and psychological symptoms of Alzheimer type dementia and plasma homocysteine concentration. BioMed Res Int. 2014;2014:383494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin L, Latypova X, Wilson CM, Magnaudeix A, Perrin ML, Terro F. Tau protein phosphatases in Alzheimer's disease: the leading role of PP2A. Ageing Res Rev. 2013;12:39–49. [DOI] [PubMed] [Google Scholar]
- 6. Lucke‐Wold BP, Turner RC, Logsdon AF, Bailes JE, Huber JD, Rosen CL. Linking traumatic brain injury to chronic traumatic encephalopathy: identification of potential mechanisms leading to neurofibrillary tangle development. J Neurotrauma. 2014;31:1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farkas M, Keskitalo S, Smith DE, et al. Hyperhomocysteinemia in Alzheimer's disease: the hen and the egg? J Alzheimers Dis. 2013;33:1097–1104. [DOI] [PubMed] [Google Scholar]
- 8. Olde Rikkert MG, Verhey FR, Sijben JW, et al. Differences in nutritional status between very mild Alzheimer's disease patients and healthy controls. J Alzheimers Dis. 2014;41:261–271. [DOI] [PubMed] [Google Scholar]
- 9. Kamphuis PJ, Scheltens P. Can nutrients prevent or delay onset of Alzheimer's disease? J Alzheimers Dis. 2010;20:765–775. [DOI] [PubMed] [Google Scholar]
- 10. Pocernich CB, Lange ML, Sultana R, Butterfield DA. Nutritional approaches to modulate oxidative stress in Alzheimer's disease. Curr Alzheimer Res. 2011;8:452–469. [DOI] [PubMed] [Google Scholar]
- 11. Ousset PJ, Nourhashemi F, Reynish E, Vellas B. Nutritional status is associated with disease progression in very mild Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:66–71. [DOI] [PubMed] [Google Scholar]
- 12. Madsen SK, Rajagopalan P, Joshi SH, Toga AW, Thompson PM. Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer's Disease Neuroimaging Initiative. Neurobiol Aging. 2015;36(Suppl 1):S203–S210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang CW, Chang WN, Huang SH, et al. Impact of homocysteine on cortical perfusion and cognitive decline in mild Alzheimer's dementia. Eur J Neurol. 2013;20:1191–1197. [DOI] [PubMed] [Google Scholar]
- 14. Tu MC, Huang CW, Chen NC, et al. Hyperhomocysteinemia in Alzheimer dementia patients and cognitive decline after 6 months follow‐up period. Acta Neurologica Taiwanica. 2010;19:168–177. [PubMed] [Google Scholar]
- 15. Cacciapuoti F. Lowering homocysteine levels with folic acid and B‐vitamins do not reduce early atherosclerosis, but could interfere with cognitive decline and Alzheimer's disease. J Thromb Thrombolysis. 2013;36:258–262. [DOI] [PubMed] [Google Scholar]
- 16. Rajdl D, Racek J, Trefil L, Stehlik P, Dobra J, Babuska V. Effect of folic acid, betaine, vitamin B(6), and vitamin B12 on homocysteine and dimethylglycine levels in middle‐aged men drinking white wine. Nutrients. 2016;8:E34, doi: 10.3390/nu8010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhuo JM, Wang H, Pratico D. Is hyperhomocysteinemia an Alzheimer's disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol Sci. 2011;32:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Craig SA. Betaine in human nutrition. Am J Clin Nutr. 2004;80:539–549. [DOI] [PubMed] [Google Scholar]
- 19. Rullier L, Lagarde A, Bouisson J, Bergua V, Barberger‐Gateau P. Nutritional status of community‐dwelling older people with dementia: associations with individual and family caregivers’ characteristics. Int J Geriatr Psychiatry. 2013;28:580–588. [DOI] [PubMed] [Google Scholar]
- 20. Droogsma E, van Asselt DZ, Scholzel‐Dorenbos CJ, van Steijn JH, van Walderveen PE, van der Hooft CS. Nutritional status of community‐dwelling elderly with newly diagnosed Alzheimer's disease: prevalence of malnutrition and the relation of various factors to nutritional status. J Nutr Health Aging. 2013;17:606–610. [DOI] [PubMed] [Google Scholar]
- 21. Hooshmand B, Solomon A, Kareholt I, et al. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: a longitudinal study. Neurology. 2010;75:1408–1414. [DOI] [PubMed] [Google Scholar]
- 22. Pacheco‐Quinto J, Rodriguez de Turco EB, DeRosa S, et al. Hyperhomocysteinemic Alzheimer's mouse model of amyloidosis shows increased brain amyloid beta peptide levels. Neurobiol Dis. 2006;22:651–656. [DOI] [PubMed] [Google Scholar]
- 23. Ho YS, Yu MS, Yang XF, So KF, Yuen WH, Chang RC. Neuroprotective effects of polysaccharides from wolfberry, the fruits of Lycium barbarum, against homocysteine‐induced toxicity in rat cortical neurons. J Alzheimers Dis. 2010;19:813–827. [DOI] [PubMed] [Google Scholar]
- 24. Zhang CE, Tian Q, Wei W, et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging. 2008;29:1654–1665. [DOI] [PubMed] [Google Scholar]
- 25. Chai GS, Jiang X, Ni ZF, et al. Betaine attenuates Alzheimer‐like pathological changes and memory deficits induced by homocysteine. J Neurochem. 2013;124:388–396. [DOI] [PubMed] [Google Scholar]
- 26. Jin B, Gong Z, Yang N, et al. Downregulation of betaine homocysteine methyltransferase (BHMT) in hepatocellular carcinoma associates with poor prognosis. Tumour Biol. 2016;37:5911–5917. [DOI] [PubMed] [Google Scholar]
- 27. Du YP, Peng JS, Sun A, Tang ZH, Ling WH, Zhu HL. Assessment of the effect of betaine on p16 and c‐myc DNA methylation and mRNA expression in a chemical induced rat liver cancer model. BMC Cancer. 2009;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ganesan B, Anandan R. Protective effect of betaine on changes in the levels of lysosomal enzyme activities in heart tissue in isoprenaline‐induced myocardial infarction in Wistar rats. Cell Stress Chaperones. 2009;14:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ganesan B, Anandan R, Lakshmanan PT. Studies on the protective effects of betaine against oxidative damage during experimentally induced restraint stress in Wistar albino rats. Cell Stress Chaperones. 2011;16:641–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schiff M, Blom HJ. Treatment of inherited homocystinurias. Neuropediatrics. 2012;43:295–304. [DOI] [PubMed] [Google Scholar]


