Abstract
Background
The non‐invasive diagnostic approach for early detection of endometrial cancer (EC) remains limited. To date, human epididymis protein 4 (HE4) has been intensively studied but its diagnostic is controversial in EC. DJ‐1 is an oncoprotein secreted by cancer cells, recently identified as a potential diagnostic biomarker for breast cancer, melanoma, and pancreatic cancer. The aim of this study was to compare the diagnostic performances of DJ‐1 and HE4 measured in EC patients and healthy controls (HC).
Methods
Forty‐five patients (63.9±12.0 years) with EC and 29 (63.2±13.3 years) HC were enrolled. Serum concentrations of DJ‐1 and HE4 were measured using ELISA kits developed by R&D (Minneapolis, USA) and Fujirebio Diagnostic (Malvern, PA, USA), respectively. Differences between EC patients and HC were assessed by Mann‐Whitney test and associations were tested by Spearman's correlation. The diagnostic performance was assessed using receiver operating characteristics (ROC) curves analysis.
Results
Serum DJ‐1 concentrations were found to be higher in EC patients than in HC (9533.6 vs 1988.5 pg/mL; P<.0001). The area under the ROC curve (ROC‐AUC) was 0.95 (P<.0001). At the cut‐off of 3654 pg/mL, the sensitivity and specificity were 0.89 and 0.90, respectively. HE4 serum levels were higher in EC patients than in HC (75.3 vs 56.2 pmol/L; P=.019), with an AUC of 0.66 (P=.020). The AUC obtained by the combination of the two markers resulted 0.96 (P<.0001).
Conclusion
These results suggest that increased serum DJ‐1 levels are associated with EC and that this biomarker may be potentially useful for diagnosing EC.
Keywords: DJ‐1, endometrial cancer, HE4, PARK7
1. Introduction
Endometrial cancer (EC) is the fourth most common type of female cancer in developed countries, as well as the most frequent cancer of the female genital tract.1 More than 80% of new EC cases are diagnosed in post‐menopausal women, with a median age of 63 years.2
Most EC are diagnosed at early stages and the associated 5‐year overall survival approximates 80%. Nevertheless, the survival rate decreases to 57%‐46% in patients with advanced, high‐grade tumors.3
The early detection of EC patients is promoted by the presence of symptoms like abnormal vaginal bleeding, which is usually found in 93% of women diagnosed with EC. However, many other benign disorders may present with similar symptoms.4
As for many other tumors, early detection of EC is crucial to increase patient survival, so that implementation of biomarkers in early stages of the diagnostic process would be effective to improving detection of EC.
Despite several biomarkers have been studied and were found to be associated with both clinical characteristics and prognosis of EC,5, 6 none of these has been implemented in clinical practice so far.
In particular, Human Epididymis Protein 4 (HE4) has been intensively studied in gynecological tumors, used alone or in combination with other biomarkers or clinical/radiological findings,7, 8, 9, 10, 11, 12, 13, 14 but its real significance and efficacy for management of EC has not been clearly demonstrated in clinical practice, so that its diagnostic value remains controversial.15
DJ‐1, also known as Parkinson's disease‐associated protein 7 (PARK7), is a 189 amino acid protein with multiple functions. Beside its active role in promoting cell proliferation and cell cycle progression, DJ‐1 has multifunctional properties as regulatory subunit of RNA‐binding protein, redox‐regulated chaperone, cysteine protease, and transcriptional co‐activator.16 Moreover, over‐expression of DJ‐1 has been found in many cancer types including oral carcinoma and breast cancer.17, 18 A previous study also found a role of DJ‐1 as oncogene, by modulating PTEN and thus promoting cell survival.19 In accordance with this finding, Shu et al.20 showed that DJ‐1 expression in EC tissues was higher than in normal endometrial tissue and that DJ‐1 tissue levels were associated with cancer progression.
Therefore, the aim of this study was to investigate the serum concentration of both DJ‐1 in EC patients in comparison to healthy controls (HCs) and for defining its diagnostic performances in EC patients compared to HE4.
2. Materials and Methods
2.1. Patients and samples
The study population consisted of 45 women (mean age, 63.9±12.0 years) consecutively diagnosed with EC, who were scheduled to undergo radical surgery from January 2008 to December 2010 at the Obstetrics and Gynecology Clinics of the University Hospital of Verona (Italy). All patients underwent radiological imaging by pelvic ultrasonography (US), computed axial tomography (CAT) scanning, and/or magnetic resonance imaging (MRI) within 6 weeks prior to surgery, to identify the presence of endometrial mass. The histopathology data were then confirmed by surgical resection of tumors, and the cancer stage was defined according to the International Federation of Gynecology and Obstetrics (FIGO) system criteria.21
The EC patients had the following histological subtypes: serous (n=3, 6,7%), endometrioid (n=41, 91,1%), clear cell (n=1, 2,2%). Thirty‐one patients were at stage I (68,9%), seven at stage II (15,6%), six at stage III (13,3%), and one (2,2%) at stage IV (Table 1). As regard to the histological grade, nine (20%) cases were grade 1 (G1), 25 (55,6%) grade 2 (G2), and 11 (24,4%) grade 3 (G3).
Table 1.
Demographics and clinical features of EC patients and healthy controls
| Variables | EC patients (n=45) |
|---|---|
| Age, yr (±SD) | 63.9 (±12.0) |
| ≥55 yr, n (%) | 41 (91.1) |
| <55 yr, n (%) | 4 (8.9) |
| FIGO stage, n (%) | |
| I | 31 (68.9) |
| II | 7 (15.6) |
| III | 6 (13.3) |
| IV | 1 (2.2) |
| Histological grade, n (%) | |
| 1 | 9 (20.0) |
| 2 | 25 (55.6) |
| 3 | 11 (24.4) |
| Histology, n (%) | |
| Clear cells | 1 (2.2) |
| Endometrioid | 41 (91.1) |
| Serous | 3 (6.7) |
| Healthy controls (n=29) | |
| Age, yr (±SD) | 63.2 (±13.3) |
| ≥55 yr, n (%) | 4 (13.8) |
| <55 yr, n (%) | 25 (86.2) |
Twenty‐nine HC subjects (mean age, 63.2±13.3 years) were recruited from healthy hospital personnel during routine clinical and laboratory assessment. In particular, all healthy controls underwent gynecologic examinations and transvaginal sonographies in the previous 2 years. The study was carried out in accordance with the ethical standards of the revised Declaration of Helsinki, under the terms of relevant local legislation.
2.2. Laboratory methods
Blood samples were collected prior to any therapeutic procedure (i.e., surgery, chemotherapy or radiotherapy), the morning before surgery on patients who had fasted overnight and rested for 20 min. Blood was drawn in vacuum tubes containing no additives (Becton‐Dickinson, Oxford, UK). After centrifugation at 1500 g for 10 min at room temperature, serum was separated, stored in aliquots and kept frozen at −80°C until measurement.
DJ‐1 serum levels were measured using Human Park7/DJ‐1 DuoSet ELISA (R&D Systems, Inc., Minneapolis, MN, USA), according to manufacturer's instructions. The limit of detection of this method is 6.25 pg/mL, as quoted by the manufacturer. Standard curves were generated by a four parameter logistic (4‐PL) curve‐fit analyses to determine concentrations of the unknown samples.
The serum concentrations of HE4 were determined using an EIA kit developed by Fujirebio Diagnostic, Inc. (Malvern, PA, USA), and performed according to the manufacturer's specifications on Triturus Analyser (Diagnostics Grifols, Barcelona, Spain). This test is a solid‐phase, non‐competitive immunoassay based on direct sandwich technique using two mouse monoclonal antibodies, 2H5 and 3D8, against two epitopes in the C‐WFDC domain of HE4. Total imprecision, is <10%.
2.3. Statistical analysis
The results of measurement were reported as median and range. Tumor marker concentration was compared between groups using the Mann‐Whitney test. The correlation between variables was assessed with Spearman's correlation coefficient (r). The level of statistical significance was set at P<.05. The diagnostic performance for both HE4 and DJ‐1 was calculated by means of receiver operator characteristic (ROC) curves. Statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA).
3. Results
The median serum concentration of DJ‐1 and HE4 was found to be significantly higher in EC patients compared to HCs (DJ‐1: 9533.6 (2516.5‐47 938.7) vs 1988.5 (290.2‐5534.3) pg/mL, P<.0001; HE4: 75.3 (27.9‐781.8) vs 56.2 (24.4‐107.6) pmol/L, P=.019). The median serum concentration of both DJ‐1 and HE4 was not significantly higher in advanced‐stage (III‐IV) cancer than in the early stage (I‐II) (P=.86 and P=.46, respectively) (Table 2).
Table 2.
Serum levels of HE4 and DJ‐1 in different cancer stages
| Stages | HE4, pmol/L | P value | DJ‐1, pg/mL | P value |
|---|---|---|---|---|
| Stage I‐II | 73.9 (27.9‐781.8) | .46 | 9443.2 (2516.5‐47 938.7) | .86 |
| Stage III‐IV | 118.3 (30.9‐381.1) | 12 327.3 (2744.6‐44 036.9) |
The area under the curve (AUC) for identifying EC patients vs healthy controls was 0.95 (95% CI: 0.91‐0.99, P<.0001) for DJ‐1, and 0.66 (95% CI: 0.54‐0.78, P=.019) for HE4, respectively (Figure 1).
Figure 1.
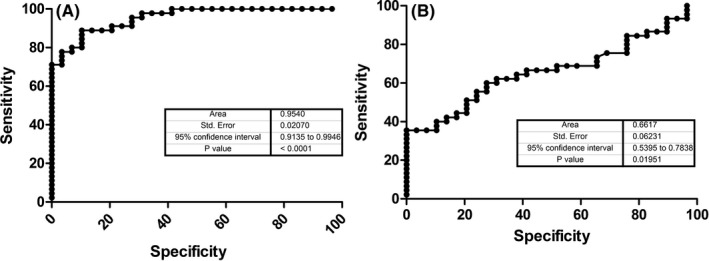
DJ‐1 (A) and HE4 (B) ROC curves performed on EC patients and healthy controls
The best ROC curve‐derived cut‐off of DJ‐1 was 3654 pg/mL, displaying 0.89 sensitivity and 0.90 specificity, respectively. The best ROC curve‐derived cut‐off of HE4 was 64 pmol/L, displaying 0.64 sensitivity and 0.62 specificity, respectively.
No significant correlation was observed between HE4 and DJ‐1 concentration (r=.25; P=.09).
The AUC obtained by the combination of the two markers resulted 0.96 (P<.0001).
4. Discussion
The worldwide incidence of EC is rapidly increasing, and this malignancy now represents the fourth most common cancer in women in developed countries.1, 22
The vast majority of EC patients present with signs or symptoms that allow a diagnosis in the early stages, so displaying an excellent prognosis (5‐year overall survival between 75%‐90%).23, 24
Despite the high frequency, no effective screening has been developed for this type of cancer so far, and no sensitive or specific biochemical markers were proven to be really clinically useful in early diagnosis or monitoring of EC.
Many studies and meta‐analysis have focused on evaluating the clinical significance of HE4 in EC in the past decade.15, 25, 26, 27, 28
According to published data, serum HE4 is seemingly helpful for distinguishing EC from healthy and benign disease, the major advantage being its higher specificity.
Despite these encouraging results, all studies concluded that the available information is still insufficient for estimating the real value of HE4 in clinical practice.
Unlike some other investigations,29, 30 we failed to find a statistically significant correlation between HE4 level and FIGO stage in our study. This finding is in agreement with two other published studies, both reporting that this biomarker may be useful for predicting the degree of myometrial involvement in EC.31, 32
These conflicting results could be attributed to the different study populations. In particular, only 15.5% of our patients were diagnosed at advanced stage. The discrepancy may also be attributable to other variables which may influence HE4 concentration, such as renal function, hormonal levels, age, later menarche, or smoke.33, 34
DJ‐1 is over‐expressed in many types of cancer tissues and is also actively released by cancer cells, so that it may be considered a potentially useful cancer biomarker.17, 18, 35
Yuen et al.36 showed that primary human malignant non‐small cell lung carcinoma and esophageal squamous cell carcinoma tumor samples were both characterized by substantial DJ‐1 over‐expression at both mRNA and protein levels compared with normal adjacent control tissue, so emphasizing that over‐expression DJ‐1 is confined to tumor cells but increases with cell transformation. Notably, DJ‐1 acts as a key negative regulator of tumor suppressor PTEN, so promoting cell proliferation and cells transformation.37
DJ‐1 has been originally identified as potential tumor antigen in the circulation of breast cancer patients,38 and in pancreatic juice of patients with pancreatic cancer,39 so supporting the hypothesis of extracellular secretion of DJ‐1.
In 2013 Shu et al.20 first observed that DJ‐1 expression in EC tissues was higher than in tumor‐adjacent tissues and normal endometrial tissues. Shortly afterward, Morelli et al.40 measured DJ‐1 concentrations with Western Blotting in serum samples of 15 patients with EC and 20 healthy women, concluding that DJ‐1 serum values were higher in EC patients than in healthy controls.
Taken together, the results of our study, which has been performed a larger population, are in accordance with these observations. Notably, our original even if preliminary findings also demonstrate for the first time that the diagnostic performance of DJ‐1 is higher than that of HE4 in patients with EC, so paving the way for additional investigations about the clinical significance of this easily measurable cancer biomarker.
Nevertheless, additional studies will be needed to assess diagnostic thresholds, to confirm the role of DJ‐1 in cancer and defining the clinical utility of this biomarker in diagnosing and monitoring EC.
Benati M, Montagnana M, Danese E, et al. The clinical significance of DJ‐1 and HE4 in patients with endometrial cancer. J Clin Lab Anal. 2018;32:e22223 10.1002/jcla.22223
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annal Oncol. 2013;24:vi33‐vi38. [DOI] [PubMed] [Google Scholar]
- 3. Yeramian A, Moreno‐Bueno G, Dolcet X, et al. Endometrial carcinoma: molecular alterations involved in tumor development and progression. Oncogene. 2013;32:403‐413. [DOI] [PubMed] [Google Scholar]
- 4. Amant F, Moerman P, Neven P, Timmerman D, Van Limbergen E, Vergote I. Endometrial cancer. Lancet. 2005;366:491‐505. [DOI] [PubMed] [Google Scholar]
- 5. Fan JT, Li MJ, Shen P, Xu H, Li DH, Yan HQ. Serum and tissue level of YKL‐40 in endometrial cancer. Eur J Gynaecol Oncol. 2014;35:304‐308. [PubMed] [Google Scholar]
- 6. Hashiguchi Y, Kasai M, Fukuda T, Ichimura T, Yasui T, Sumi T. Serum Sialyl‐Tn (STN) as a tumor marker in patients with endometrial cancer. Pathol Oncol Res. 2016;22:501‐504. [DOI] [PubMed] [Google Scholar]
- 7. Montagnana M, Lippi G, Ruzzenente O, et al. The utility of serum human epididymis protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal. 2009;23:331‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montagnana M, Lippi G, Danese E, Ruzzenente O, Franchi M, Guidi GC. Human epydidimis protein 4 (HE4): could it be useful in the diagnosis of vulvar cancer? Clin Lab. 2010;56:601‐602. [PubMed] [Google Scholar]
- 9. Capriglione S, Plotti F, Miranda A, et al. Further insight into prognostic factors in endometrial cancer: the new serum biomarker HE4. Expert Rev Anticancer Ther. 2017;17:9‐18. [DOI] [PubMed] [Google Scholar]
- 10. Angioli R, Capriglione S, Scaletta G, et al. The role of HE4 in endometrial cancer recurrence: how to choose the optimal follow‐up program. Tumour Biol. 2016;37:4973‐4978. [DOI] [PubMed] [Google Scholar]
- 11. Capriglione S, Plotti F, Miranda A, et al. Utility of tumor marker HE4 as prognostic factor in endometrial cancer: a single‐center controlled study. Tumour Biol. 2015;36:4151‐4156. [DOI] [PubMed] [Google Scholar]
- 12. Angioli R, Capriglione S, Aloisi A, et al. REM (risk of endometrial malignancy): a proposal for a new scoring system to evaluate risk of endometrial malignancy. Clin Cancer Res. 2013;19:5733‐5739. [DOI] [PubMed] [Google Scholar]
- 13. Angioli R, Plotti F, Capriglione S, et al. The role of novel biomarker HE4 in endometrial cancer: a case control prospective study. Tumour Biol. 2013;34:571‐576. [DOI] [PubMed] [Google Scholar]
- 14. Angioli R, Plotti F, Capriglione S, et al. Preoperative local staging of endometrial cancer: the challenge of imaging techniques and serum biomarkers. Arch Gynecol Obstet. 2016;294:1291‐1298. [DOI] [PubMed] [Google Scholar]
- 15. Hu L, Du S, Guo W, Chen D, Li Y. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in endometrial cancer: a meta‐analysis. Int J Gynecol Cancer. 2016;26:331‐340. [DOI] [PubMed] [Google Scholar]
- 16. van der Merwe C, Jalali Sefid Dashti Z, Christoffels A, Loos B, Bardien S. Evidence for a common biological pathway linking three Parkinson's disease‐causing genes: parkin, PINK1 and DJ‐1. Eur J Neurosci. 2015;41:1113‐1125. [DOI] [PubMed] [Google Scholar]
- 17. Xu S, Ma D, Zhuang R, et al. DJ‐1 is upregulated in oral squamous cell carcinoma and promotes oral cancer cell proliferation and invasion. J Cancer. 2016;7:1020‐1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kawate T, Iwaya K, Koshikawa K, et al. High levels of DJ‐1 protein and isoelectric point 6.3 isoform in sera of breast cancer patients. Cancer Sci. 2015;106:938‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lin JP, Pan BC, Li B, Li Y, Tian XY, Li Z. DJ‐1 is activated in medulloblastoma and is associated with cell proliferation and differentiation. World J Surg Oncol. 2014;12:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shu K, Xiao Z, Long S, et al. Expression of DJ‐1 in endometrial cancer: close correlation with clinicopathological features and apoptosis. Int J Gynecol Cancer. 2013;23:1029‐1035. [DOI] [PubMed] [Google Scholar]
- 21. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103‐104. [DOI] [PubMed] [Google Scholar]
- 22. McAlpine JN, Temkin SM, Mackay HJ. Endometrial cancer: not your grandmother's cancer. Cancer. 2016;122:2787‐2798. [DOI] [PubMed] [Google Scholar]
- 23. Braun MM, Overbeek‐Wager EA, Grumbo RJ. Diagnosis and management of endometrial cancer. Am Fam Physician. 2016;93:468‐474. [PubMed] [Google Scholar]
- 24. Morice P, Leary A, Creutzberg C, Abu‐Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094‐1108. [DOI] [PubMed] [Google Scholar]
- 25. Li X, Gao Y, Tan M, et al. Expression of HE4 in endometrial cancer and its clinical significance. Biomed Res Int. 2015;2015:437468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zanotti L, Bignotti E, Calza S, et al. Human epididymis protein 4 as a serum marker for diagnosis of endometrial carcinoma and prediction of clinical outcome. Clin Chem Lab Med. 2012;50:2189‐2198. [DOI] [PubMed] [Google Scholar]
- 27. Chen Y, Ren YL, Li N, Yi XF, Wang HY. Serum human epididymis protein 4 vs. carbohydrate antigen 125 and their combination for endometrial cancer diagnosis: a meta‐analysis. Eur Rev Med Pharmacol Sci. 2016;20:1974‐1985. [PubMed] [Google Scholar]
- 28. Bie Y, Zhang Z. Diagnostic value of serum HE4 in endometrial cancer: a meta‐analysis. World J Surg Oncol. 2014;12:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saarelainen SK, Peltonen N, Lehtimäki T, Perheentupa A, Vuento MH, Mäenpää JU. Predictive value of serum human epididymis protein 4 and cancer antigen 125 concentrations in endometrialcarcinoma. Am J Obstet Gynecol. 2013;209:142. [DOI] [PubMed] [Google Scholar]
- 30. Angioli R, Miranda A, Aloisi A, et al. A critical review on HE4 performance in endometrial cancer: where are we now? Tumour Biol. 2014;35:881‐887. [DOI] [PubMed] [Google Scholar]
- 31. Moore RG, Miller CM, Brown AK, Robison K, Steinhoff M, Lambert‐Messerlian G. Utility of tumor marker HE4 to predict depth of myometrial invasion in endometrioid adenocarcinoma of the uterus. Int J Gynecol Cancer. 2011;21:1185‐1190. [DOI] [PubMed] [Google Scholar]
- 32. Mutz‐Dehbalaie I, Egle D, Fessler S, et al. HE4 is an independent prognostic marker in endometrial cancer patients. Gynecol Oncol. 2012;126:186‐191. [DOI] [PubMed] [Google Scholar]
- 33. Qu W, Li J, Duan P, et al. Physiopathological factors affecting the diagnostic value of serum HE4‐test for gynecologic malignancies. Expert Rev Mol Diagn. 2016;16:1271‐1282. [DOI] [PubMed] [Google Scholar]
- 34. Lowe KA, Shah C, Wallace E, et al. Effects of personal characteristics on serum CA125, mesothelin, and HE4 levels in healthy postmenopausal women at high‐risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2480‐2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan J, Yu H, Lv Y, Yin L. Diagnostic and prognostic value of serum thioredoxin and DJ‐1 in non‐small cell lung carcinoma patients. Tumour Biol. 2016;37:1949‐1958. [DOI] [PubMed] [Google Scholar]
- 36. Yuen HF, Chan YP, Law S, et al. DJ‐1 could predict worse prognosis in esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2008;17:3593‐3602. [DOI] [PubMed] [Google Scholar]
- 37. Kim RH, Peters M, Jang Y, et al. DJ‐1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7:263‐273. [DOI] [PubMed] [Google Scholar]
- 38. Le Naour F, Misek DE, Krause MC, et al. Proteomics‐based identification of RS/DJ‐1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res. 2001;7:3328‐3335. [PubMed] [Google Scholar]
- 39. Tian M, Cui YZ, Song GH, et al. Proteomic analysis identifies MMP‐9, DJ‐1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 2008;8:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morelli M, Scumaci D, Di Cello A, et al. DJ‐1 in endometrial cancer: a possible biomarker to improve differential diagnosis between subtypes. Int J Gynecol Cancer. 2014;24:649‐658. [DOI] [PubMed] [Google Scholar]


