Abstract
Background
Avidity of antiphospholipid antibodies may be clinically useful as a valuable additional characteristic. The aim of this study was to compare several ELISA modifications with different chaotropic agents and calculation of avidity indices for the determination of anticardiolipin antibody (aCL) avidity.
Methods
We examined 28 serum samples with positive IgG aCL by adapted ELISA using various concentrations of urea and sodium chloride as chaotropic agents and different dilution of sera. We tested these conditions of ELISA—a single diluted serum sample with fixed concentration of a chaotrope and a serially diluted serum in the constant concentration of a chaotropic agent.
Results
We demonstrated that ELISA method for avidity determination based on a single dilution of serum in the presence of fixed concentration of chaotrope is convenient for determination of IgG aCL antibody avidity. Concentrations 6 and 8 mol/L of urea or 1 and 2 mol/L of NaCl were suitable for sufficient dissociation of immune complexes during ELISA procedure.
Conclusion
This way was in good agreement with more demanding procedures. Both urea and sodium chloride may be used as chaotropic agents. Reference values of avidity indices essential for interpretation of patients’ results must be established individually for distinct assay conditions.
Keywords: anticardiolipin antibodies, antiphospholipid antibodies, avidity, chaotropic reagents, ELISA, serum, sodium chloride, urea
1. Introduction
Antiphospholipid antibodies (aPLs) are a heterogeneous group of autoantibodies targeted at different phospholipids and/or their complexes with protein cofactors.1, 2 Laboratory testing of aPL includes predominantly assays for anticardiolipin antibodies (aCL), antibodies against β2‐glycoprotein I (anti‐β2GPI), and lupus anticoagulant.3, 4 Together with the occurrence of recurrent vascular thrombosis and/or pregnancy morbidity, the presence of aPL is required for the diagnosis of antiphospholipid syndrome (APS) known more than 30 years. The antiphospholipid syndrome is also recognized as a major cause of stroke, migraine, and heart attack.5 The aPLs are even considered to be a non‐traditional risk factor in atherosclerosis in coronary artery diseases, ischemic stroke, or peripheral artery diseases 6 and may occur in other conditions in high frequency.7
With a view to a large spectrum of clinical conditions associated with the elevated aPL, it is very desirable to improve continuously the actual approaches for aPL determination. It is known that pathogenic potential of autoantibodies depends not only on their levels but also on the qualitative characteristics such as affinity and avidity, which may contribute to the severity of appropriate disease.8, 9 It seems that avidity of aPL may be clinically useful as a valuable additional characteristic of aPL.10 Cucnik et al.11 assume that avidity of anti‐β2GPI may be a more reliable laboratory feature than aPL titer for the evaluation of long‐term thrombotic risk. One of the recent multicenter studies clarified a clear association between high‐avidity anti‐β2‐GPI and obstetric complications.12 Previously, avidity of aCL had been also studied in patients with primary biliary cirrhosis, primary sclerosing cholangitis, and type 1 autoimmune hepatitis.13, 14
Various methods for avidity determination have been described.8, 15, 16 The common techniques in clinical laboratories utilize the solid‐phase immune assays (predominantly enzyme‐linked immunosorbent assay—ELISA) in the presence of chaotropic agents. The immune complexes formed during the antibody binding in the course of ELISA are temporarily exposed to the action of chaotropic agents during the extra step.16, 17, 18, 19, 20 The interactions of low‐avidity antibodies with antigens are easily broken by chaotropic agents, while high‐avidity antibodies remain bound to antigens. The low‐avidity antibodies released after incubation with chaotropic reagents are removed from the appropriate wells. The antibodies with higher avidity bound in the immune complexes on the surface on the wells are quantified and equated to the amount of antibodies detected in the wells without chaotrope.
Several modifications for avidity ELISAs differ in a dilution of analyzed serum or concentration of chaotropic agents.16, 18, 21 The simplest method uses a single diluted serum with fixed concentration of chaotrope. Another approach is based on the determination of avidity on condition of serially diluted serum in the constant concentration of chaotropic agent.13, 21, 22 Alternatively, a single diluted serum is exposed to an increasing concentration of chaotrope.23 The expression of avidity values varies according to applied ELISA modification.
The intensity in interrupting of the antibody‐antigen bindings seems to be highly dependent on the kind of examined antigen and its specific antibody with respect to a distinct type and a number of bounds and interactions participated in the formation of immune complexes.16, 19 On this account, various chaotropes interrupt immune complexes formed by a certain antigen and a corresponding antibody differently.18 It is advisable to test the use of suitable chaotropes and their concentration individually for antibodies with various specificities. In addition, the results of avidity determination are influenced by experimental conditions.19
The aim of this study was to test various possibilities for aCL avidity determination by ELISA procedure in the presence of chaotropes and to choose the optimal modification which would be suitable for routine aCL avidity examination. Therefore, we (a) compared two different chaotropic agents (urea and sodium chloride), which had been already used for determination of aPL avidity,12, 20, 24 (b) compared several approaches for the ELISA modification using various concentrations of chaotropes and various dilution of serum samples, and (c) compared corresponding avidity index (AI) calculation.
2. Materials and Methods
2.1. Participants
We analyzed 28 serum samples from patients carried out at the Immunological Department of the Institute of Medical Biochemistry and Laboratory Diagnostics in Prague (Czech Republic) (age: 40±17 years, mean±SD; sex: female 22, male 6). IgG aCL levels (ELISA Anti‐cardiolipin antibodies; Orgentec, Mainz, Germany) had been determined in the sera as a part of the immunological follow‐up of patients. IgG aCL levels were higher than 10 GPL in the patients. The predominant diagnoses of patients enrolled in the study were following: systemic lupus erythematosus (n=9), glomerulonephritis and nephritis (n=5), immunodeficiency (n=3), infertility (n=2), and coagulation disorders (n=2).
All subjects gave written informed consent regarding study participation. The Ethics Committee of the General University Hospital, Prague, approved the study.
2.2. Methods
We used ELISA according to a protocol25 for the IgG aCL determination; 10% adult bovine serum (ABS) in phosphate buffered saline (PBS) was used as a blocking solution and for a dilution of patient samples. This ELISA procedure provides sufficient β2‐GPI for a valid test and meets the necessities recommended by international consensus guidelines on anticardiolipin and anti‐β2‐glycoprotein I testing.26
2.2.1. IgG aCL avidity assay
The procedure described by Vlachoyiannopoulos et al.20 in our modification was used for the avidity of IgG aCL examination. The detailed description of the ELISA procedure is shown in Fialova et al.24
We examined sera serially diluted with 10% ABS in PBS (pH 7.2) 1:50, 1:100, 1:200, and 1:400. In the extra step of ELISA, the avidity of antibodies in each appropriated diluted serum was tested using increasing concentrations of urea solutions (2.0, 4.0, 6.0, and 8.0 mol/L) or sodium chloride (0.25, 0.5, 1.0, and 2.0 mol/L).
With regard to an insufficient amount of serum in some patients, all ELISA modifications were not done in every serum.
2.2.2. Expression of avidity results
We used several modifications for avidity determination and expression of avidity results.
2.2.3. Method 1: a single dilution of serum, a single concentration of chaotrope
Avidity determination by this method is based on the analysis of a single diluted serum exposed a single concentration of a chaotrope. The avidity index used for the expression of avidity values represents the ratio (or percentage) of the residual antibodies bound in the wells in the presence of the chaotrope solution to the total antibodies bound in the absence of a chaotrope.
AI is calculated by dividing absorbance (A) values obtained from the wells with chaotrope addition (urea 6 or 8 mol/L; NaCl 1 or 2 mol/L) by A values obtained from the wells without chaotropes (the standard ELISA method).
2.2.4. Methods 2: a multiple diluted serum, a single concentration of chaotrope
In contrast to the previous approach, this modification for avidity determination uses a multiple diluted serum sample exposed a single concentration of a chaotrope.13, 22 Sera were diluted 1:50, 1:100, 1:200, and 1:400 in our experiments. The AI was expressed as the ratio (or percentage) of the serum dilution (antibody titer) value after chaotrope treatment accordant to the certain cutoff absorbance value to the value of the serum dilution without chaotrope treatment corresponding to the same cutoff absorbance value (Figure 1A).
Figure 1.
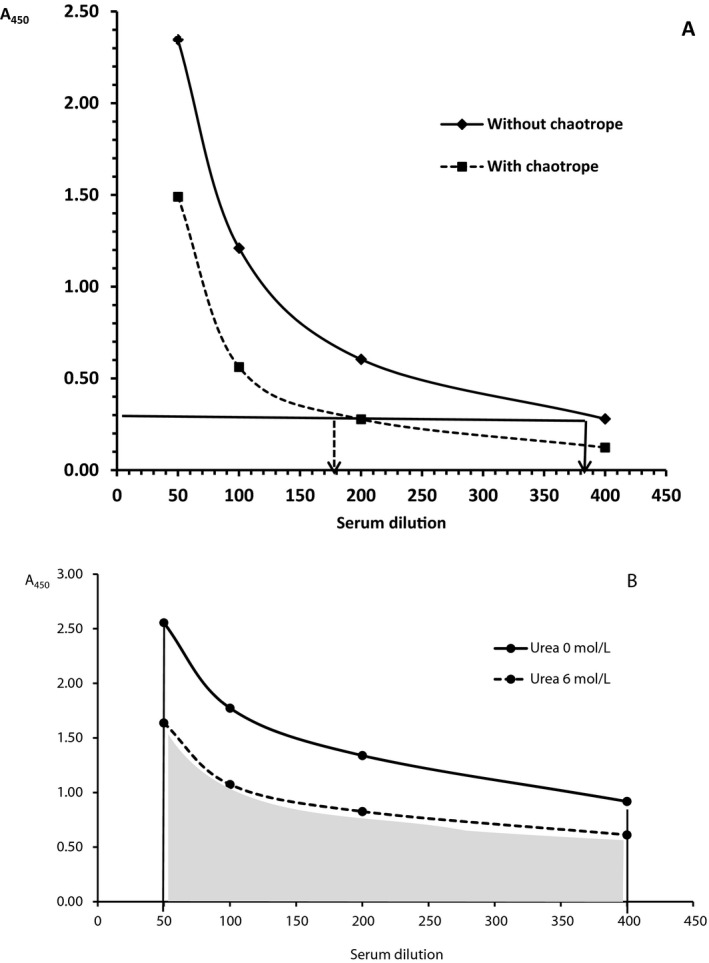
(A) Determination of avidity index by ELISA using a multiple diluted serum exposed a single concentration of chaotropic agents. The arrow of the dashed line in the x‐axis shows dilution of serum exposed to a chaotropic agent accordant to the certain cutoff absorbance; the arrow of the solid line in the x‐axis shows dilution of serum without chaotrope exposition accordant to the certain cutoff absorbance. (B) Determination of avidity index based on the ratio of areas derived from the antibody titration curve. The solid line borders the area without chaotrope and the dashed line borders the area with chaotrope
A more precise manner of AI calculation is based on the ratio of the areas derived from the antibody titration curve value obtained with and without treatment by a chaotrope reagent 21 (Figure 1B).
We applied a simplified formula proposed by Perciani et al.21 who assessed the ratio between areas of curves without using integral functions. A relative simple mathematic formula may be used for the calculation of both areas. AI can be calculated as the ratio of two times the summation of the absorbances obtained in the ELISA plot under the chaotrope condition minus absorbances of the first and last serum dilution data divided by two times the summation of the absorbances obtained in the ELISA plot without chaotrope minus the absorbances of the first and last serum dilution data.21
n=number of dilutions
We modified this method using only two optimal serum dilutions 1:50 and 1:100 with the aim to labor‐save the ELISA procedure.
2.3. Statistics
The statistical analyses were performed by parametric tests after evaluation of normal distribution using Kolmogorov‐Smirnov test. The relationship between AI calculated by different ways was evaluated by Pearson's correlation coefficient. The paired t test was used for the statistical analysis of differences in the paired measurements of aCL avidity in serum or various expression of avidity indices. The significance level for all tests was P<.05. The comparison of two different measurement techniques was performed by Bland‐Altman plots.27 Statistical analysis was performed using Statistica 12 (StatSoft, Prague, CR) and MedCalc (Ostend, Belgium).
3. Results
3.1. Method 1: a single dilution of serum, a single concentration of chaotropes
The individual concentrations of urea 2 and 4 mol/L or NaCl 0.25 and 0.5 mol/L did not dissociate the immune complexes effectively. They were less suitable for differentiation between high‐ and low‐avidity antibodies if chaotrope solution was used in a single concentration.
For the evaluation of AI calculation by this approach, we compared the values obtained: (a) at different concentration of chaotropes during ELISA (urea 6 and 8 mol/L; NaCl 1 and 2 mol/L) and (b) at distinct dilution of sera (1:50 and 1:100).
A high correlation was found between AI based on various concentrations of urea or NaCl independently on dilution serum (serum dilution 1:50: urea 6 vs 8 mol/L, r=0.94; NaCl 1 vs 2 mol/L r=0.89; serum dilution 1:100: urea 6 vs 8 mol/L r=0.88; NaCl 1 vs 2 mol/L r=0.88; P<.0001 for each comparison). AI was significantly lower in the higher concentrations of urea (urea 6 vs 8 mol/L—P<.001 for dilution of sera 1:50 and P=.0008 for dilution of sera 1:100), while no significant decrease of AI values determined in the presence of NaCl 2 mol/L was observed in comparison with NaCl 1 mol/L.
Dilutions of serum samples (1:50 and 1:100) influenced AI more than concentration of chaotropes, but the correlation was still high for urea in both concentration (urea 6 mol/L: serum 1:50 vs 1:100 r=0.85; urea 8 mol/L: serum 1:50 vs 1:100 r=0.87; P<.0001 for each comparison) and lesser for NaCl, but still statistically significant (NaCl 1 mol/L: serum 1:50 vs 1:100 r=0.55, P=.01; NaCl 2 mol/L: serum 1:50 vs 1:100 r=0.65, P=.002). The action of urea was more effective in more diluted sera. AI for sera diluted 1:100 was significantly lower than those for sera diluted 1:50 (serum diluted 1:50 vs serum diluted 1:100, P<.05 for each urea concentration). The effect of NaCl to immune complex dissociation was similar in sera diluted both 1:100 and 1:50 and AI did not differ (serum diluted 1:50 vs serum diluted 1:100 n.s. for each concentration of NaCl).
3.2. Methods 2: a multiple diluted serum, a single concentration of chaotropes
We compared this ELISA modification in the condition of different chaotrope concentrations. Significant correlations between AI determined to the same cutoff absorbance value 0.3 using concentration 6 or 8 mol/L for urea and 1 or 2 mol/L for NaCl were observed (urea 6 vs 8 mol/L r=0.96, P<.0001; NaCl 1 vs 2 mol/L r=0.85, P=.002). Similarly as in the previous method, the AI was significantly lower when urea 8 mol/L was used for the dissociation of immune complexes in comparison with urea 6 mol/L (P=.047). No difference was seen in the presence of various concentrations of NaCl as chaotropic agent. Unfortunately, without an additional dilution of serum, it was not possible to assess the AI in the samples with too high levels of aCL, which provided too high absorbance exceeding the cutoff value.
Moreover, we also analyzed the AI based on the ratio of the areas of the plot under chaotrope action and those without chaotrope calculated by the formula proposed by Perciani et al.21 and in our modification (see Result). The correlation between AI calculated by original formula and by our modification is high especially when urea is used for disruption of immune complexes (Table 1). This statement was also confirmed by Bland‐Altman plots (Figure 2A). In contrast to use of AI based on the cutoff absorbance, this variation enabled to determine the AI independently on the value of absorbance.
Table 1.
Comparison between avidity indices determined by ELISA using a multiple diluted serum with a single concentration of a chaotrope calculated according formula of Perciani et al. 21 (area 6 mol/L or area 8 mol/L) and our modified method included only serum dilution 1:50 and 1:100 (area 6 mol/L modified or area 8 mol/L modified)
| Comparison urea as chaotrope | n | r | P | Comparison NaCl as chaotrope | n | r | P |
|---|---|---|---|---|---|---|---|
| Area 6 mol/L vs area 8 mol/L | 18 | .95 | <.0001 | Area 1 mol/L vs area 2 mol/L | 20 | .93 | <.0001 |
| Area 6 mol/L vs area 6 mol/L modified | 18 | .96 | <.0001 | Area 1 mol/L vs area 1 mol/L modified | 20 | .92 | <.0001 |
| Area 8 mol/L vs area 8 mol/L modified | 18 | .98 | <.0001 | Area 2 mol/L vs area 2 mol/L modified | 20 | .92 | <.0001 |
| Area 6 mol/L modified vs area 8 mol/L modified | 24 | .95 | <.0001 | Area 1 mol/L modified vs area 2 mol/L modified | 20 | .89 | <.0001 |
n, number of samples; r, Pearson's correlation coefficient; P, level of significance; vs, versus.
Figure 2.
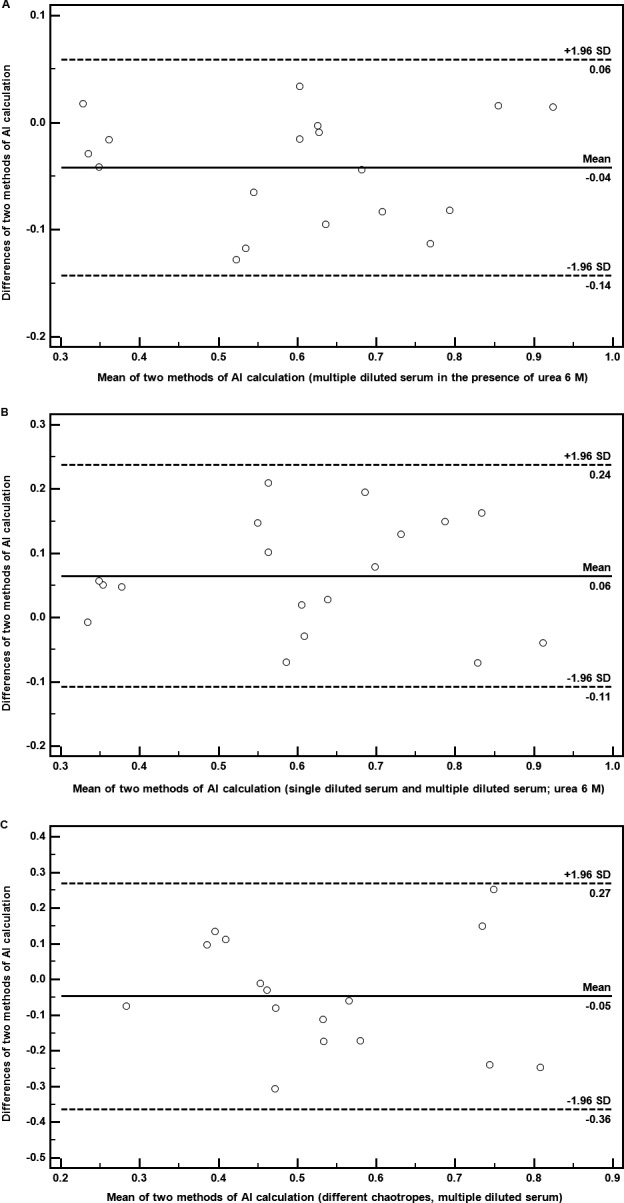
(A) Representative Bland‐Altman plot comparing avidity indices determined by ELISA using a multiple diluted serum in the presence of urea 6 mol/L calculated according formula of Perciani et al. 21 and our modified method. (B) Representative Bland‐Altman plot comparing avidity indices based on ELISA using a multiple diluted serum and a single diluted serum (1:50) in the presence of urea 6 mol/L. (C) Representative Bland‐Altman plot comparing avidity indices based on ELISA using different chaotropes (area calculation: urea 6 mol/L and NaCl 1 mol/L)
The comparison between tested modifications is shown in Table 2. The correlation coefficients and Bland‐Altman plots (Figure 2B) also showed good agreement between AI determination using a multiple diluted serum at single concentration of chaotrope and that using a single diluted serum.
Table 2.
Comparison between avidity indices determined by ELISA using a single diluted serum (1:50 or 1:100) with a fixed concentration of chaotrope (urea or NaCl) and by ELISA using a multiple diluted serum
| Urea concentration | Comparisons | n | r | P | NaCl concentration | Comparisons | n | r | P |
|---|---|---|---|---|---|---|---|---|---|
| Urea 6 mol/L | Single diluted serum 1:50 vs multiple diluted serum | 18 | .88 | <.0001 | NaCl 1 mol/L | Single diluted serum 1:50 vs multiple diluted serum | 20 | .74 | .0002 |
| Single diluted serum 1:50 vs multiple diluted serum modified | 18 | .97 | <.0001 | Single diluted serum 1:50 vs multiple diluted serum modified | 20 | .94 | <.0001 | ||
| Single diluted serum 1:100 vs multiple diluted serum | 18 | .97 | <.0001 | Single diluted serum 1:100 vs multiple diluted serum | 20 | .91 | <.0001 | ||
| Single diluted serum 1:100 vs multiple diluted serum modified | 24 | .94 | <.0001 | Single diluted serum 1:100 vs multiple diluted serum modified | 20 | .80 | <.0001 | ||
| Urea 8 mol/L | Single diluted serum 1:50 vs multiple diluted serum | 18 | .91 | <.0001 | NaCl 2 mol/L | Single diluted serum 1:50 vs multiple diluted serum | 20 | .81 | <.0001 |
| Single diluted serum 1:50 vs multiple diluted serum modified | 24 | .97 | <.0001 | Single diluted serum 1:50 vs multiple diluted serum modified | 20 | .95 | <.0001 | ||
| Single diluted serum 1:100 vs multiple diluted serum | 18 | .97 | <.0001 | Single diluted serum 1:100 vs multiple diluted serum | 20 | .87 | <.0001 | ||
| Single diluted serum 1:100 vs multiple diluted serum modified | 24 | .95 | <.0001 | Single diluted serum 1:100 vs multiple diluted serum modified | 20 | .84 | <.0001 |
n, number of samples; r, Pearson's correlation coefficient; P, level of significance; vs, versus.
3.3. Relationship between AI based on using urea or NaCl
AI calculated from the absorbances obtained in the presence of NaCl did not correlate with those in the presence of urea using sera diluted 1:50. When the sera were more diluted (1:100), the correlation between ELISA performed with different chaotropes became evident (Table 3). However, the agreement was lesser expressed in comparison to the various procedures for AI determination using the same chaotropic agent. Similar correlations were seen by comparison of methods described by Perciani et al.21 including our modification (Table 4). There was a good correlation between methods using four serum dilutions and limited between those using only two serum dilutions for urea and NaCl. Bland‐Altman plots also presented an agreement between AI determined via various chaotropic agents (Figure 2C).
Table 3.
Comparison between avidity indices determined by ELISA using a single diluted serum (1:50 or 1:100) in the presence of various chaotropes (urea concentration 6 or 8 mol/L; NaCl 1 or 2 mol/L)
| Serum dilution | Comparisons | n | r | P |
|---|---|---|---|---|
| 1:50 | Urea: 6 mol/L vs NaCl 1 mol/L | 16 | .32 | n.s. |
| Urea: 6 mol/L vs NaCl 2 mol/L | 16 | .25 | n.s. | |
| Urea: 8 mol/L vs NaCl 1 mol/L | 16 | .34 | n.s. | |
| Urea: 8 mol/L vs NaCl 2 mol/L | 16 | .35 | n.s. | |
| 1:100 | Urea: 6 mol/L vs NaCl 1 mol/L | 16 | .67 | .004 |
| Urea 6 mol/L vs NaCl 2 mol/L | 16 | .62 | .01 | |
| Urea: 8 mol/L vs NaCl 1 mol/L | 16 | .81 | .0001 | |
| Urea: 8 mol/L vs NaCl 2 mol/L | 16 | .57 | .02 |
n, number of samples; r, Pearson's correlation coefficient; P, level of significance; vs, versus.
Table 4.
Comparison between avidity indices determined by ELISA using a multiple diluted serum with fixed concentration of various chaotropes (urea vs NaCl)
| Comparisons | r | P | |
|---|---|---|---|
| Area urea 6 mol/L vs area NaCl 1 mol/L | 16 | .57 | .022 |
| Area urea 6 mol/L vs area NaCl 2 mol/L | 16 | .56 | .025 |
| Area urea 8 mol/L vs area NaCl 1 mol/L | 16 | .68 | .004 |
| Area urea 8 mol/L vs area NaCl 2 mol/L | 16 | .64 | .008 |
| Area urea 6 mol/L modified vs area NaCl 1 mol/L modified | 16 | .49 | .053 |
| Area urea 6 mol/L modified vs area NaCl 2 mol/L modified | 16 | .45 | .081 |
| Area urea 8 mol/L modified vs area NaCl 1 mol/L modified | 16 | .54 | .032 |
| Area urea 8 mol/L modified vs area NaCl 2 mol/L modified | 16 | .48 | .057 |
r, Pearson's correlation coefficient; P, level of significance; vs, versus.
3.4. Avidity of IgG aCL in patients with their high levels
Four patients in our patient's group had levels of aCL exceeded 100 GPL (a unit for IgG antiphospholipid antibody concentration). None of the avidity values determined at a single serum dilution in concentration of urea 6 or 8 mol/L and NaCl 1 or 2 mol/L was higher than appropriate values of mean+SD (standard deviation). The representative values of AI in various concentration of urea are shown in Figure 3.
Figure 3.
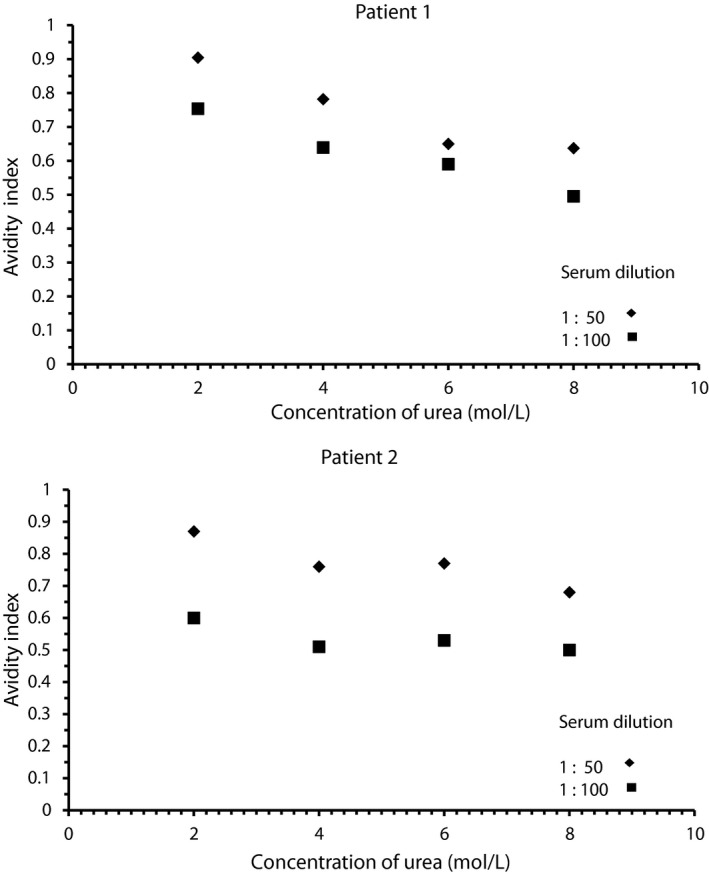
Avidity indices in two representative patients with high levels of anticardiolipin antibodies (≥100GPL) determined by ELISA in the presence of various concentrations of urea
4. Discussion
We compared two chaotropic agents urea and sodium chloride described in the literature for avidity determination of aPL and tested the several possibilities for calculation of avidity indices. The investigated concentrations of chaotropes—2‐8 mol/L for urea and 0.25‐2 mol/L—were chosen with respect to the experience presented in previous studies addressing to aPL avidity.12, 20, 24
From the chemical principle, it is obvious that urea and NaCl will not dissociate the immune complexes formed during ELISA by the same manner. While urea disturbs hydrogen bonding and Van der Waals forces,16 NaCl as an ionic compound may also dissociate electrostatic interactions. Therefore, the results obtained by methods utilized various chaotropes differing in their chemical properties cannot be equal. Nevertheless, it seems that both tested chaotropes may be used, and if the appropriate concentration of chaotropes and serum dilution are chosen, the avidity values may be comparable each other. A good relationship between AI determined via various chaotropic agents under suitable conditions was demonstrated by correlation coefficients and by Bland‐Altman plots. A limited comparability between AI in the presence of various chaotropes was observed in the method using a single dilution of sera 1:50 at fixed concentration of chaotrope, while AI determination based on higher serum dilution 1:100 or multiple dilutions of sera showed a better agreement (Tables 3 and 4).
Because a reliable estimation of antibody avidity requires a 50% decrease in the binding of antibodies,28 the lower tested concentrations of urea (2 and 4 mol/L) and NaCl (0.25 and 0.5 mol/L) were not convenient for the methods using a fixed concentration of dissociation agents. Concentrations of 6 and 8 mol/L of urea and 1 and 2 mol/L of NaCl were suitable for sufficient dissociation of immune complexes at dilution of sera 1:50 and 1:100. Both dilutions of sera and concentrations of chaotropes influenced the intensity of dissociation of immune complexes during an extra step of ELISA. The higher concentration of urea and more diluted sera yielded in more effective disruption of immune complexes and in significant decrease of AI.
The simplest way for avidity determination is the analysis of a single dilution of serum exposed only a single concentration of chaotropic agents. The calculation of AI expressed as the ratio of absorbance values obtained in the presence of chaotropic agent to those without chaotropic agents is also uncomplicated. The shortcoming of this modification might be the error caused by the initial levels of specific antibodies.29, 30 The opinion on the impact of high concentration of antibodies to the avidity estimation by ELISA is not unique.16, 19 Dimitrov et al. 16 mentioned that the assay for avidity determination is not enough sensitive to moderate decreases in the binding at high concentrations of IgG. On the basis of our results, it seems that determination of IgG aCL avidity is not influenced by their levels similarly as it was described for IgG antibody avidity against pertussis toxin and filamentous hemagglutinin.19 Our avidity method for aCL with a single dilution of serum and that used a serially diluted serum which is not affected by the antibody levels exhibited a good correlation. Regarding the AI values in our patients with very high levels of IgG aCL did not exceed values of mean+standard deviation corresponding to the total tested group of patients, we can anticipate that used concentrations of chaotropes were sufficient even for higher levels of aCL. However, the levels of antibodies may be important when the values of expected antibody titer in individual samples varied in large range (several order of values).19
Determination of AI using serially diluted patient serum (end‐point titration of antibodies) is considered to be the gold standard method for avidity determination by ELISA.29 The results of avidity are not influenced by the concentration of tested specific antibodies.16 A main disadvantage of this methodological approach consists in the necessity for four or more dilution series of a specimen, which sometimes have to be repeated due to insufficient serial dilution.30 This procedure increases the requirements for time and reagent consumption. With aim to abolish shortage, we modified this method by the reduction of serum dilutions only for two ones which showed to be suitable within testing the method with single diluted serum. A high correlation of results obtained by our modified method with that based on multiple dilutions suggests that the performance of whole titration curve need not be necessary. In past, the serially diluted sera were applied for the determination of aCL and anti‐β2‐glycoprotien‐I avidity using urea as a denaturing agent in monitoring of patients with various diseases.13, 20 The AI was denoted as “residual activity.”
In conclusion, we demonstrated that simplest method for AI determination based on ELISA using a single dilution of serum in the presence of fixed concentration of chaotrope is convenient for determination of IgG aCL antibody avidity. This way was in good agreement with more exacting procedures. Both urea and sodium chloride may be used as chaotropic agents. Concentrations 6 and 8 mol/L of urea and 1 and 2 mol/L of NaCl were suitable for sufficient dissociation of immune complexes during extra step of ELISA procedure. Considering the AI values differed in dependence of serum dilution and concentration of chaotropes especially urea, the reference values of avidity indices essential for interpretation of patients’ results must be established individually for distinct assay conditions.
Acknowledgments
We thank Dr. Karin Malíčková for the selection of the serum samples for our avidity analyses.
This work was supported by the project of Ministry of Health, Czech Republic, for conceptual development of research organization RVO 64165 (General University Hospital in Prague, Czech Republic) and by research project of Charles University in Prague PRVOUK P25/LF1/2.
Fialová L, Petráčková M, and Kuchař O. Comparison of different enzyme‐linked immunosorbent assay methods for avidity determination of antiphospholipid antibodies. J Clin Lab Anal. 2017;31:e22121 10.1002/jcla.22121
References
- 1. Ke K, Strango ZI, Harper PE, Zhao M. Influence of phosphatidylethanolamine concentration and composition on the detection of antiphosphatidylethanolamine antibodies by ELISA. J Clin Lab Anal. 2016;30:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krilis SA, Giannakopoulos B. Laboratory methods to detect antiphospholipid antibodies. Hematology Am Soc Hematol Educ Program. 2014;2014:321–328. [DOI] [PubMed] [Google Scholar]
- 3. Pengo V, Denas G, Banzato A, et al. Interpretation of laboratory data and need for reference laboratories. Lupus. 2012;21:732–733. [DOI] [PubMed] [Google Scholar]
- 4. Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. 2006;4:295–306. [DOI] [PubMed] [Google Scholar]
- 5. Hughes GR, Shoenfeld Y. Antiphospholipid antibody testing ‐ slow progress? Int J Clin Pract. 2012;66:533–535. [DOI] [PubMed] [Google Scholar]
- 6. Artenjak A, Lakota K, Frank M, et al. Antiphospholipid antibodies as non‐traditional risk factors in atherosclerosis based cardiovascular diseases without overt autoimmunity. A critical updated review. Autoimmun Rev. 2012;11:873–882. [DOI] [PubMed] [Google Scholar]
- 7. Mankai A, Manoubi W, Ghozzi M, Melayah S, Sakly W, Ghedira I. High frequency of antiphospholipid antibodies in primary biliary cirrhosis. J Clin Lab Anal. 2015;29:32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Božič B, Čučnik S, Kveder T, Rozman B. Affinity and avidity of autoantibodies In: Shoenfeld Y, Gershwin ME, Meroni PL, eds. Autoantibodies. Amsterdam: Elsevier; 2007: 21–28. [Google Scholar]
- 9. Fialova L. Avidity of antiphospholipid antibodies ‐ our current knowledge. Epidemiol Mikrobiol Imunol. 2014;63:221–225. [PubMed] [Google Scholar]
- 10. Bozic B, Cucnik S, Kveder T, Rozman B. Avidity of anti‐beta‐2‐glycoprotein I antibodies. Autoimmun Rev. 2005;4:303–308. [DOI] [PubMed] [Google Scholar]
- 11. Cucnik S, Bozic B, Kveder T, Tomsic M, Rozman B. Avidity of anti‐beta2‐glycoprotein I and thrombosis or pregnancy loss in patients with antiphospholipid syndrome. Ann N Y Acad Sci. 2005;1051:141–147. [DOI] [PubMed] [Google Scholar]
- 12. Cucnik S, Kveder T, Artenjak A, et al. Avidity of anti‐beta2‐glycoprotein I antibodies in patients with antiphospholipid syndrome. Lupus. 2012;21:764–765. [DOI] [PubMed] [Google Scholar]
- 13. Zachou K, Liaskos C, Rigopoulou E, et al. Presence of high avidity anticardiolipin antibodies in patients with autoimmune cholestatic liver diseases. Clin Immunol. 2006;119:203–212. [DOI] [PubMed] [Google Scholar]
- 14. Liaskos C, Rigopoulou E, Zachou K, et al. Prevalence and clinical significance of anticardiolipin antibodies in patients with type 1 autoimmune hepatitis. J Autoimmun. 2005;24:251–260. [DOI] [PubMed] [Google Scholar]
- 15. Lynch HE, Stewart SM, Kepler TB, Sempowski GD, Alam SM. Surface plasmon resonance measurements of plasma antibody avidity during primary and secondary responses to anthrax protective antigen. J Immunol Methods. 2014;404:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dimitrov JD, Lacroix‐Desmazes S, Kaveri SV. Important parameters for evaluation of antibody avidity by immunosorbent assay. Anal Biochem. 2011;418:149–151. [DOI] [PubMed] [Google Scholar]
- 17. Cucnik S, Kveder T, Ulcova‐Gallova Z, et al. The avidity of anti‐beta2‐glycoprotein I antibodies in patients with or without antiphospholipid syndrome: a collaborative study in the frame of the European forum on antiphospholipid antibodies. Lupus. 2011;20:1166–1171. [DOI] [PubMed] [Google Scholar]
- 18. Dauner JG, Pan Y, Hildesheim A, Kemp TJ, Porras C, Pinto LA. Development and application of a GuHCl‐modified ELISA to measure the avidity of anti‐HPV L1 VLP antibodies in vaccinated individuals. Mol Cell Probes. 2012;26:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Almanzar G, Ottensmeier B, Liese J, Prelog M. Assessment of IgG avidity against pertussis toxin and filamentous hemagglutinin via an adapted enzyme‐linked immunosorbent assay (ELISA) using ammonium thiocyanate. J Immunol Methods. 2013;387:36–42. [DOI] [PubMed] [Google Scholar]
- 20. Vlachoyiannopoulos PG, Petrovas C, Tektonidou M, Krilis S, Moutsopoulos HM. Antibodies to beta 2‐glycoprotein‐I: urea resistance, binding specificity, and association with thrombosis. J Clin Immunol. 1998;18:380–391. [DOI] [PubMed] [Google Scholar]
- 21. Perciani CT, Peixoto PS, Dias WO, Kubrusly FS, Tanizaki MM. Improved method to calculate the antibody avidity index. J Clin Lab Anal. 2007;21:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jenum PA, Stray‐Pedersen B, Gundersen AG. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J Clin Microbiol. 1997;35:1972–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Polanec J, Seppälä I, Rousseau S, Hedman K. Evaluation of protein‐denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J Clin Lab Anal. 1994;8:16–21. [DOI] [PubMed] [Google Scholar]
- 24. Fialova L, Malbohan I, Malickova K. Avidity of anticardiolipin antibodies‐A factor that could be important for their detection by ELISA methods. J Appl Biomed. 2014;12:277–284. [Google Scholar]
- 25. Pierangeli SS, Harris EN. A protocol for determination of anticardiolipin antibodies by ELISA. Nat Protoc. 2008;3:840–848. [DOI] [PubMed] [Google Scholar]
- 26. Lakos G, Favaloro EJ, Harris EN, et al. International consensus guidelines on anticardiolipin and anti‐beta2‐glycoprotein I testing: report from the 13th International Congress on Antiphospholipid Antibodies. Arthritis Rheum. 2012;64:1–10. [DOI] [PubMed] [Google Scholar]
- 27. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. [DOI] [PubMed] [Google Scholar]
- 28. Pullen GR, Fitzgerald MG, Hosking CS. Antibody avidity determination by ELISA using thiocyanate elution. J Immunol Methods. 1986;86:83–87. [DOI] [PubMed] [Google Scholar]
- 29. Hedman K, Lappalainen M, Seppaia I, Makela O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J Infect Dis. 1989;159:736–740. [DOI] [PubMed] [Google Scholar]
- 30. Prince HE, Wilson M. Simplified assay for measuring Toxoplasma gondii immunoglobulin G avidity. Clin Diagn Lab Immunol. 2001;8:904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]


