Abstract
Background
To establish maternal thyroid‐stimulating hormone (TSH) reference ranges for first trimester screening from 11 + 0 to 13 + 6 weeks of gestation.
Methods
A total of 10 592 singleton and 201 twin consecutive Caucasian pregnant women who underwent simultaneously prenatal first trimester Down's syndrome screening and thyroid function screening from January 2010 to November 2017 were included in the study. Women with positive antithyroid peroxidase antibody (TPOAb) and positive personal history of thyroid disease were previously excluded. TSH was measured by immunochemiluminescent assay on ci 16200 Abbott Architect analyzer. Nonparametric percentile method (also known as CLSI C28.A3) was used for the determination of reference ranges.
Results
We established reference ranges of TSH for the period of gestation from 11 + 0 to 13 + 6 weeks of pregnancy as 0.16‐3.43 mU/L for singleton Caucasian pregnancies and 0.02‐2.95 mU/L for twin Caucasian pregnancies. The median (IQR) of TSH for singleton pregnancies was higher than that for twin pregnancies (1.25 mU/L (0.83‐1.81) vs 0.84 (0.37‐1.47), respectively; P < .0001).
Conclusions
Each first trimester screening center should be aware of which type of immunoassay their laboratory uses. TSH reference ranges in women during the first trimester of pregnancy are lower than those for general population. Twin pregnancies have lower TSH than singleton pregnancies.
Keywords: gestation, immunoassay, pregnancy, reference interval, thyroid disease
1. INTRODUCTION
The production of thyroid hormones is controlled by thyroid‐stimulating hormone (TSH) produced by anterior pituitary gland. Human chorionic gonadotropin (HCG) produced by placenta also directly stimulates thyroid hormones production. TSH production is regulated by negative feedback of free thyroid hormones.
Pregnancy has a significant impact on the thyroid gland function. Production of the thyroid hormones, thyroxine (T4), and triiodothyronine (T3) increases by nearly 50%, in conjunction with a separate 50% increase in the daily iodine requirement.1
Thyroid hormones have a key role in embryo and fetus development. The lack of maternal thyroid hormones in pregnancy is associated with serious maternal, fetal, and newborn complications: spontaneous abortions, preterm birth, preeclampsia, gestational diabetes, induction, cesarean section, intensive care unit admission, placental abruption, and breech presentation. Screening for thyroid dysfunction should be applied in all high‐risk women—and, according to some authorities—in all women in early pregnancy.2
Today, 70% of objective information on patients is based on laboratory test results. Valid reference ranges and/or cut‐off points of any laboratory test results are needed for further clinical decision making.3
Manufacturers usually provide only reference ranges for general population but these reference ranges cannot be applied in early pregnancy because high maternal plasma HCG levels cause increase in plasma level of thyroid hormones and subsequently, due to negative feedback, decrease in TSH production.
Moreover, different immunoassays yield different results. It is visible in external quality proficiency testing results. Certainly, no two methods are exactly the same; the issue is whether the difference is important relative to the clinical question.4
That is why we decided to establish reference ranges of TSH in the period of first trimester Down's syndrome screening, that is from 11 to 13 + 6 weeks of gestation.
2. MATERIALS AND METHODS
2.1. Subjects
Reference ranges were established from 12 278 consecutive patients who underwent prenatal first trimester Down's syndrome screening together with thyroid function screening at the Department of obstetrics and gynecology of Tomas Bata Hospital in Zlín from January 2010 to November 2017. The screening is performed from 11 + 0 to 13 + 6 weeks of gestation. Patients with personal history of thyroid dysfunction were not included in thyroid function screening.
A total of 1391 patients with positive TPOAb (11.3% of screened population), 52 patients with Asian ethnicity, 3 patients with Afro‐American ethnicity, 2 patients with triple pregnancy, 3 patients with undetectable TSH and elevated free thyroxine, and 34 patients with TSH over the upper reference range for general population were excluded from the study.
In the end, a total of 10 592 singleton and 201 twin Caucasian pregnancies were analyzed. The age median (interquartile range) was 29 (26‐33) years in singleton pregnancies and 31 (27‐34) years in twin pregnancies.
Adequate iodine intake was assumed because iodized salt has been distributed in the Czech Republic since 1947.5 The study was approved by the hospital Ethics committee in Zlín, Czech Republic and performed according to Declaration of Helsinki.
2.2. Methods
Fasting venous blood samples were obtained in the morning. VACUETTE® red top 6 mL tubes (Greiner Bio‐One GmbH, Kremsmünster, Austria) with clot activator and without gel separator were used for venous blood collection. The separation of cells from serum was performed within 45 minutes.
TSH, TPOAb, freeT4 were measured by immunochemiluminescent assay on ci 16200 Abbott Architect analyzer (Abbott Laboratories,100 Abbott Park Road, Abbott Park, Illinois 60064‐3500, USA). Manufacturer‐recommended reference range for general population is 0.35‐4.94 mU/L.
2.3. Statistical analysis
MedCalc statistical software version 17.4 (MedCalc Software bvba, Ostend, Belgium) was used for statistical analysis. D'Agostino‐Pearson test was used for normal distribution testing.
We used International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) recommended nonparametric percentile method (also known as CLSI C28.A3) for the determination of reference ranges.6
Lower reference limit is defined as 2.5th percentile and upper reference limit is defined as 97.5th percentile. Calculation of 90% confidence interval (CI) is recommended for both limits. Mann‐Whitney test for independent samples was used for comparison of TSH medians between singleton and twin pregnancies.
3. RESULTS
The median (IQR) of gestation age of singleton pregnancies was 12 weeks + 4 days (12 + 2‐13 + 0). The median (IQR) of gestation age of twin pregnancies was 12 weeks +5 days (12 + 3‐13 + 1). The results of TSH in both types of pregnancies did not have normal distribution (P < .0001). Both skewness and kurtosis were present. For singleton pregnancies, coefficient of skewness was 1.02 (P < .0001) and coefficient of kurtosis 1.39 (P < .0001). For twin pregnancies, coefficient of skewness was 1.30 (P < .0001) and coefficient of kurtosis 2.44 (P = .0001).
Figures 1 and 2 show the positively skewed distribution of TSH values for both singleton and twin pregnancies. The reference ranges (90% CI) for singleton and twin pregnancies are presented in Table 1. The median (IQR) of TSH for singleton pregnancies was higher than that for twin pregnancies (1.25 mU/L (0.83‐1.81) vs 0.84 (0.37‐1.47), respectively; P < .0001).
Figure 1.
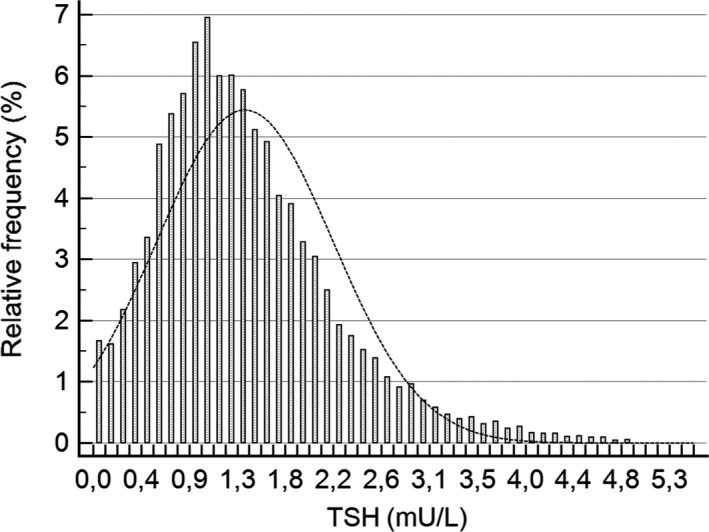
Distribution of TSH results for singleton pregnancies (10 592 cases)
Figure 2.
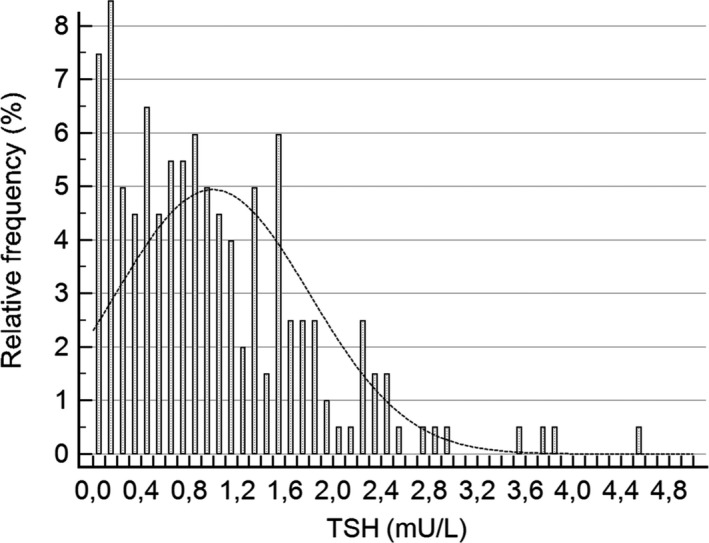
Distribution of TSH results for twin pregnancies (201 cases)
Table 1.
Reference ranges of singleton and twin pregnancies from 11 to 13 + 6 wk of gestation
| Lower Reference Limit (90% CI) mU/L | Upper reference limit (90% CI) mU/L | |
|---|---|---|
| Singleton pregnancy (n = 10592) | 0.16 (0.15‐0.18) | 3.43 (3.36‐3.49) |
| Twin pregnancy (n = 201) | 0.02 (0.01‐0.05) | 2.95 (2.41‐3.88) |
CI, confidence interval.
4. DISCUSSION
We determined reference ranges for TSH for singleton and twin pregnancies in Caucasian ethnic group. We performed TSH test simultaneously with first trimester Down's syndrome screening from 11 to 13 + 6 weeks of gestation.
It is well known that different immunochemical tests give different results even after standardization with traceability of measurement. Each first trimester center should know their immunoassays of all included tests.
Reference ranges of TSH in early pregnancy on seven different analytical systems were compared in a study by Springer et al. The Abbott Architect platform was also included with established reference ranges of 0.22‐3.27 mU/L. Their serum samples were collected from pregnant women with median gestation age of 10 + 4 weeks. Their study included only singleton pregnancies.7 It is consistent with our results.
The study by Gilbert et al included 1817 pregnant women (9‐13 weeks of gestation) attending a private pathology practice for first trimester screening. Like in our study, they too used Abbott Architect platform for TSH measurement. The reference group for deriving reference intervals comprised the 1817 antibody‐negative women (maternal age range, 14.3‐45.8 years; mean, 30.9 years). For the whole reference group, the first trimester‐specific reference intervals were as follows: TSH 0.02‐2.15 mU/L, fT4 10.4‐17.8 pmol/L, and fT3 3.3‐5.7 pmol/L. The TSH reference range is substantially lower than the established nonpregnant TSH reference range of 0.4‐4.0 mU/L.8 The reference ranges of TSH in Gilbert's study were lower than those in this study. It may be explained by the fact that Gilbert did not separate twin and multiple pregnancies. They also did not have medical records of possible hormone replacement therapy.
Rosario et al established TSH reference values in the first trimester of gestation. The 2.5th and 97.5th percentiles of the values obtained were 0.04 and 2.68 mIU/L, respectively. TSH was measured with a chemiluminescent assay (Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA, USA).9 They used different analytical system, so it is difficult to compare results. Ashoor et al demonstrated that TSH in a twin pregnancy is lower than in a singleton pregnancy.10 It confirms our results.
Establishment of 97.5th TSH percentile has implications for clinical practice. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum provide recommendation 1: When possible, population‐based trimester‐specific reference ranges for serum TSH should be defined through the assessment of local population data representative of a healthcare provider's practice. Reference range determinations should only include pregnant women with no known thyroid disease, optimal iodine intake, and negative TPOAb status.
Its recommendation 29 states that L‐thyroxine therapy is recommended for TPOAb‐positive women with TSH greater than the pregnancy‐specific reference range.1 We created reference ranges according to this approach.
First trimester thyroid function testing may impact prediction of Down's syndrome risk. When screening simultaneously for maternal thyroid disease and Down's syndrome, thyroid marker levels should be used in the calculation of Down's syndrome risk. The benefit is modest but can be achieved with no additional cost.11 The major limitations of this study are that we did not perform ultrasonography of the thyroid gland, and we had low number of other ethnic group patients for establishment of reference ranges.
In summary, each first trimester screening center should be aware of which type of immunoassay their laboratory uses. TSH reference ranges in women during the first trimester of pregnancy are lower than those for general population. We established reference ranges of TSH for the period of gestation from 11 + 0 to 13 + 6 weeks of pregnancy as 0.16‐3.43 mU/L for singleton Caucasian pregnancies and 0.02‐2.95 mU/L for twin Caucasian pregnancies. Twin pregnancies have TSH levels lower than singleton pregnancies.
Šálek T, Dhaifalah I, Langova D, Havalová J. Maternal thyroid‐stimulating hormone reference ranges for first trimester screening from 11 to 14 weeks of gestation. J Clin Lab Anal. 2018;32:e22405 10.1002/jcla.22405
REFERENCES
- 1. Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid. 2017;27:315‐389. [DOI] [PubMed] [Google Scholar]
- 2. Springer D, Jiskra J, Limanova Z, Zima T, Potlukova E. Thyroid in pregnancy: from physiology to screening. Crit Rev Clin Lab Sci. 2017;54:102‐116. [DOI] [PubMed] [Google Scholar]
- 3. Šálek T, Franeková J, Jabor A, et al. Postanalytical phase and interpretation of laboratory tests. Klin Biochem Metab. 2016;24:82‐87. [Google Scholar]
- 4. Jones GRD. The role of EQA in harmonization in laboratory medicine – a global effort. Biochem Med (Zagreb). 2017;27:23‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zamrazil V, Bilek R, Cerovska J, Delange F. The elimination of iodine deficiency in the Czech Republic: the steps toward success. Thyroid. 2004;14:49‐56. [DOI] [PubMed] [Google Scholar]
- 6. Guidi GC, Salvagno GL. Reference intervals as a tool for total quality management. Biochem Med (Zagreb). 2010;20:165‐172. [Google Scholar]
- 7. Springer D, Bartos V, Zima T. Reference intervals for thyroid markers in early pregnancy determined by 7 different analytical systems. Scand J Clin Lab Invest. 2014;74:95‐101. [DOI] [PubMed] [Google Scholar]
- 8. Gilbert RM, Hadlow NC, Walsh JP, et al. Assessment of thyroid function during pregnancy: first‐trimester (weeks 9‐13) reference intervals derived from Western Australian women. Med J Aust. 2008;189:250‐253. [DOI] [PubMed] [Google Scholar]
- 9. Rosario PW, Carvalho M, Calsolari MR. TSH reference values in the first trimester of gestation and correlation between maternal TSH and obstetric and neonatal outcomes: a prospective Brazilian study. Arch Endocrinol Metab. 2016;60:314‐318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashoor G, Muto O, Poon LCY, Muhaisen M, Nicolaides KH. Maternal thyroid function at gestational weeks 11–13 in twin pregnancies. Thyroid. 2013;23:1165‐1171. [DOI] [PubMed] [Google Scholar]
- 11. Dhaifalah I, Salek T, Langova D, Cuckle H. Incorporating thyroid markers in Down's syndrome screening protocols. Prenat Diagn. 2017;37:510‐514. [DOI] [PubMed] [Google Scholar]


