Abstract
Background
There are very few biomarkers available to diagnose cases with premature ovarian failure. Some complete blood count parameters have been introduced to be diagnostic biomarkers for several disorders associated with inflammatory process. Due to the evidence that indicated chronic inflammatory process to be underlying pathophysiology in premature ovarian insufficiency (POI), we aimed to assess the predictive value of complete blood count parameters for POI diagnosis.
Method
A total of 96 women diagnosed to have premature ovarian failure were compared with 110 otherwise healthy women in terms of some basal hormone levels and complete blood count parameters.
Results
Mean age was similar between groups. Neutrophil/lymphocyte and mean platelet volume/lymphocyte ratios were significantly higher in group with POI (P < .001, P < .003, respectively). In group with POI, there were significant correlations between anti‐Mullerian hormone and follicle stimulating hormone (r = −.30, P <.05), anti‐Mullerian hormone and white blood cell count (r = .23, P < .05). Mean platelet volume/lymphocyte ratio significantly predicted cases with POI (AUC = 0.607, %95 CI: 0.529‐0.684; P < .001).
Conclusions
Neutrophil/lymphocyte and mean platelet volume/lymphocyte ratios are elevated in POI. There have been some controversies about the value of neutrophil/lymphocyte in POI diagnosis. We suggest mean platelet volume/lymphocyte ratio as a new biomarker in early POI because it is cheap and easily accessible compared to anti‐Mullerian hormone.
Keywords: anti‐Mullerian hormone, inflammation markers, premature ovarian insufficiency
1. INTRODUCTION
Although the prevalence of premature ovarian insufficiency (POI) varies between different populations, it is encountered in %1‐2 of the cases <40 years of age, its prevalence decreases to 0.1% in women <30 years and 0.01% in women younger than 20 years of age.1
Among all women with POI, some may have cyclic normal ovarian function after diagnosis and 5‐10% of the cases were reported to conceive following certain diagnosis.2, 3
Although the exact mechanism of POI has not been clarified, accelerated follicle losses and dysfunction are most commonly proposed etiologies in these cases.4
Several etiological mechanisms have been introduced up to date, including genetic causes, autoimmune disorders, infections, toxins and iatrogenic causes (pelvic surgery, chemotherapy, radiotherapy).5, 6 Additionally, estrogen depletion was shown to result in pro‐inflammatory process secondary to the increased pro‐inflammatory active metabolites.7
It is well known that POI is associated with the decreased estrogen levels, and as we mentioned above, decreased estrogen levels lead to increase pro‐inflammatory markers; additionally, oral estrogen replacement therapy was shown to result in decreased concentrations of all inflammatory marker. 8, 9, 10
Generally POI is diagnosed in women <40 years of age with elevated FSH levels >25 mIU/mL and accompanying 4‐6 months of amenorrhea or oligomenorrhea periods.11
Currently, serum FSH and anti‐Mullerian hormone (AMH) levels are utilized to diagnose POI cases. AMH levels were shown to decrease secondary to the decreased antral follicle numbers.12 Neutrophil/lymphocyte (NLR), platelet/lymphocyte (PLR), RDW/platelet (RPR), and mean platelet volume/lymphocyte ratios have been introduced to be markers for systemic inflammation. These markers were shown to have significant predictive values for the diagnosis and prognosis of some cancers such as papillary thyroid, lung, gastrointestinal cancers, and additionally, most commonly encountered changes secondary to systemic inflammation were shown to be thrombocytopenia and neutrophilia.13
In this study, we aimed to assess the utility of some complete blood count parameters to predict POI.
2. MATERIAL AND METHODS
In this retrospective study, women <40 years of age with elevated FSH levels > 25 mIU/mL and oligomenorrhea who admitted to outpatient clinic of Zeynep Kamil Women and Children's Health Training and Research Hospital between January 2013 and February 2017 were included in this study. Study protocol was approved by institutional ethics committee. Exclusion criteria were women with PCOS, chronic systemic disorders, hypothalamic amenorrhea, intensive exercise, dietary abnormalities, hyperprolactinemia, hyperthyroidism, hypothalamic or pituitary disorders, chemo‐radiotherapy, previous ovarian surgery, and other systemic disorders. Blood samples were obtained to laboratory tubes with EDTA and analyzed by automated hematology analyzer (CELL‐DYN 3700, Abbott diagnostics, Abbott park, IL) within 60 minutes following venopuncture. Ratios were calculated by dividing mean platelet volume by number of lymphocyte for mean platelet volume/lymphocyte ratios (MPLRs), whereas neutrophil/lymphocyte was calculated by dividing number of neutrophils by number of lymphocyte, and finally, platelet/lymphocyte ratio was obtained by dividing number of platelets by the number of lymphocytes.
2.1. Statistical analysis
While the findings obtained in the study were evaluated, the IBM SPSS Statistics 15 program was used for statistical analysis. Student t test was used for the comparison of two groups of normal distribution parameters in comparison of descriptive statistical methods (mean, standard deviation), and Mann‐Whitney U test was used for comparison of two groups of parameters without normal distribution. Pearson correlation analysis and Spearman's rho correlation analysis were used to examine the relationships between parameters with non‐normal distribution. Significance was assessed at P < .05 level.
3. RESULTS
The basic characteristics and hormone levels of the groups are shown in Table 1.
Table 1.
Comparison of some demographic and clinical characteristics of groups with and without POI
| Control | POI | P | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| Age (Years) | 31.52 + 4.64 | 32.83 + 5.22 | .059a |
| FSH (mIU/mL) | 5.68 + 1.37 | 61.56 + 22.56 | .001a , d |
| LH (mIU/mL) | 4.72 + 4.19 (4.2) | 32.15 + 67 (14.92) | .001b , d |
| AMH (ng/mL) | 2.53 + 1.43 (2.09) | 0.38 ± 0.36 (0.48) | .001b , d |
| PRL (ng/mL) | 15.88 ± 6.23 | 13.93 ± 10.09 | .099b |
| TSH (mIU/L) | 1.9 + 1.04 (1.7) | 2.06 + 93 (1.59) | .673b |
| Glucose (mg/dL) | 90.56 + 6.53 | 88.07 + 13.62 | .136a |
| Progesterone (ng/mL) | 0.28 + 0.19 (0.3) | 0.33 + 96 (0.41) | .801b |
| Estrogen (pg/mL) | 36.12 + 12.42 (36) | 24.4 + 89 (19.65) | .001b , d |
| WBC (103) | 6.89 + 1.92 | 7.51 + 1.53 | .010a , c |
| NEU (103) | 3.93 + 1.46 | 4.29 + 1.32 | .040a , c |
| LYMP (103) | 2.45 ± 0.71 | 2.25 ± 0.67 | .047a , c |
| RDW | 15.44 + 2.04 | 15.27 + 2.44 | .603a |
| PLT (103) | 273.79 + 66.29 | 273.23 + 69.84 | .953a |
| MPV (fL) | 7.95 ± 0.83 | 8.28 + 1.07 | .018a , c |
| NEU/LYMP | 1.73 ± 0.80 | 2.08 ± 0.89 | .001a , d |
| RDW/PLT | 0.06 + 0.06 (0.06) | 0.06 + 96 (0.02) | .828b |
| PLT/LYMP | 119.87 ± 41.52 | 128.64 ± 39.33 | .123a |
| MPV/LYMP | 3.52 ± 1.06 | 4.01 ± 1.30 | .003a , d |
| Hemoglobin (gr/dL) | 12.53 + 1.27 | 12.88 + 1.12 | .035a , c |
| Hematocrit (%) | 38.06 + 3.67 | 38.61 + 3.58 | .277a |
Student's t test.
Mann‐Whitney U test.
P < .05.
P < .01.
The age of the patients ranged from 20 to 39 years, and the mean age was 31.78 ± 4.96 years. There was no statistically significant difference between the groups in terms of age (P > .05).
Prolactin, TSH, glucose, progesterone, red cell distribution width (RDW), number of platelets (PLT), mean platelet volume (MPV), RDW/PLT, PLT/number of lymphocytes (LYMP), and hematocrit levels (P > .05, for all) were not statistically different between two groups. However, the FSH levels were significantly higher and the AMH levels were lower in the POI group (P < .001, for all) (Table 1). There was statistically significant correlation between FSH and LH in the same direction (positive) 54.1% (P < .01), but there was no significant correlation between FSH and other parameters (P > .05). There was statistically significant correlation between AMH and FSH in the reverse direction (negative) 30% (P < .01), and statistically significant correlation with white blood cell count (WBC) 23% (P < .05) in the same direction (positive). There was no statistically significant correlation between AMH and other parameters (P > .05).
WBC, number of neutrophil (NEU) and MPV levels between groups were different, the differences were statistically significant (P < .05, for all). Moreover, the hemoglobin level of POI group was statistically significantly higher (P < .05); LYMP level was statistically significantly lower in POI (P < .05).
The POI group had a mean NLR of 2.08 ± 0.89, an RPR of 0.02, a PLR of 128.64 ± 39.33, and an MPLR of 4.01 ± 1.30. The NLR of the POI group was statistically significantly higher (P < .01). Similarly, the MPLR of patients in the POI group was statistically significantly higher than the mean MPLR (P < .01).
We also performed ROC curve analysis for MPLR in the POI group. The area under ROC curve for MPLR in POI was 0.60 (Figure 1), with a threshold value 3.5 with a sensitivity = 61% and specificity = 53%. (95% CI: 0.529‐0.684; P < .001).
Figure 1.
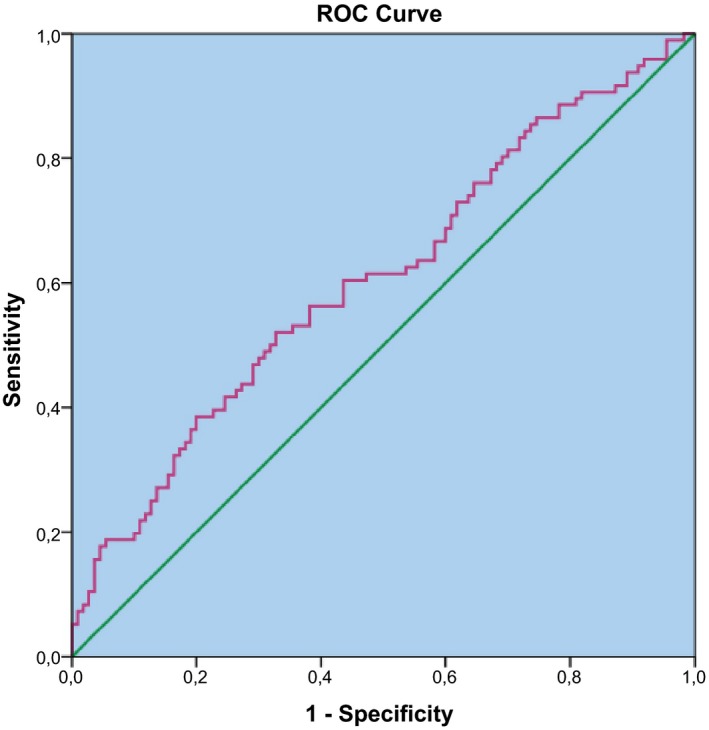
ROC curve of MPLR to predict cases with POI
4. DISCUSSION
The precise etiology of POI is not fully known in 90% of cases. There are genetic, autoimmune, iatrogenic, and idiopathic causes leading to this disorder. The most common autoimmune diseases associated with POI are hypothyroidism, type 1 diabetes mellitus, hyperparathyroidism, Addison's disease, myasthenia gravis, thymus aplasia, systemic lupus erythematosus, rheumatoid arthritis, Crohn's disease, vitiligo, pernicious anemia, and type I and type II autoimmune polyglandular insufficiency syndromes. In all of the cases mentioned above, premature follicular atresia and follicular dysfunction are present.14, 15 The mechanisms that cause or induce ovarian autoimmunity and inflammation in POI etiology are not fully known.16 It is well known that, during pathogenesis, the biological effects of inflammation are increased, and the ability to adapt to cellular proliferation, inhibition of apoptosis, angiogenesis, oxidative stress is reduced. Platelets were proposed to play an important metabolic role in the background of inflammation due to their angiogenic, metastatic, and proteolytic activities.17, 18 MPV is also an inflammatory marker that reflects platelet activity. Metabolic and enzymatic activities of the platelets were shown to be related with its volume.19
The anti‐inflammatory and pro‐inflammatory effects of estrogens depend on estradiol concentration. During the pathogenesis of POI, the pro‐inflammatory pathway becomes active as the body's estrogen concentration decreases, resulting in B‐cell activation, TNF, IFNγ, and IL‐1β activation in dendritic cells, and also the NK‐cell activation. B‐cell activation is a harmful factor in B cell‐mediated diseases such as SLE. On the other hand, as the estrogen concentration increases, the anti‐inflammatory pathway becomes active.7 To date, the relationship between many types of cancer and MPV has been investigated.20, 21, 22, 23, 24, 25, 26 All authors reported that MPV could be used as a cheap, non‐invasive marker for cancer diagnosis and prognosis in these publications. MPV has also been used as a diagnostic and prognostic marker in active lupus disease as an autoimmune disease. Guillermo et al. reported that MPV was reduced in active lupus adult patients and MPV correlated with other biomarkers of disease activity and serum albumin level in the same direction.23
NLR and MPLR are simple, inexpensive biomarkers of inflammation that can be accessed fairly easily. Tsai and colleagues reported that increased pre‐treatment NLR in patients with colorectal cancer patients was associated with poor prognosis in these patients; furthermore, NLR was an independent prognostic factor in patients with colorectal cancer.27 Ilhan and colleagues used NLR, PLR, and RPR as diagnostic markers in POI cases and found that only NLR was statistically significant to diagnose POI. As a result of their studies, NLR was proposed as a new marker in POI as an easily accessible marker that is cheaper than AMH.28 In a study conducted by Yildirim and colleagues, the relationship between POI and NLR, CRP, serum amyloid A protein (SAA) was investigated. Yıldırım and colleagues found no significant difference between groups in terms of CRP and SAA; on the other hand, NLR was significantly lower in the POI group. Authors concluded that NLR might be used as an inflammatory marker in the diagnosis of POI.18 In our study, we found that MPLR was significantly higher in the POI group. However, this statistically significant difference between the two groups was not the case for PLR and RPR. ROC analysis showed that MPLR may be a marker for the diagnosis of POI. We used NLR as well as MPLR as markers for POI diagnosis; our study has a higher number of cases than the number in previous publications.
Many cancer types have an alternative biomarker to reveal the pro‐inflammatory process. We investigated the efficacy of MPLR as an adjunct diagnostic marker for POI. To the best of our knowledge, MPLR is the first hematological marker to be studied in POI.
Today, we use ovarian‐reserve markers such as FSH, antral follicle count, inhibin B, and AMH for POI diagnosis. Among them, the predictive value of FSH in POI and menopausal diagnosis is well supported by many authors in the literature. When compared with other markers, AMH was shown to best reflect the steady decline in lifelong follicles and oocyte pool.29 As a result of this study, we found that the AMH is a better marker of POI. However, for a more reliable prediction, both tests can confirm each other. However, AMH is still a diagnostic marker that has a certain cost and is harder to achieve than a full blood count.
In conclusion, our findings show that NLR and MPLR are effective as diagnostic biomarkers in premature ovarian failure patients. Our results showed increased MPLR's ability to work by supporting NLR results; even more importantly because it is inexpensive and easily accessible compared to AMH, we are proposing a new biomarker for POI. With this biomarker, POI can be diagnosed early and infertility that is concerned about future child possession can be done on time.
Sanverdi I, Kilicci C, Cogendez E, Abide Yayla C, Ozkaya E. Utility of complete blood count parameters to detect premature ovarian insufficiency in cases with oligomenorrhea/amenorrhea. J Clin Lab Anal. 2018;32:e22372 10.1002/jcla.22372
REFERENCES
- 1. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67(4):604‐606. [PubMed] [Google Scholar]
- 2. Van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483‐492. [DOI] [PubMed] [Google Scholar]
- 3. Luborsky JL, Meyer P, Sowers MF, Gold EB, Santoro N. Premature menopause in a multi‐ethnic population study of the menopause transition. Hum Reprod. 2003;18:199‐206. [DOI] [PubMed] [Google Scholar]
- 4. Nelson LM, Bakalov VK. Mechanisms of follicular dysfunction in 46 XX spontaneous premature ovarian failure. Endocrinol Metab Clin North Am. 2003;32:613‐637. [DOI] [PubMed] [Google Scholar]
- 5. Laml T, Preyer O, Umek W, Hengstschlager M, Hanzal H. Genetic disorders in premature ovarian failure. Hum Reprod Update. 2002;8:483‐491. [DOI] [PubMed] [Google Scholar]
- 6. Meskhi A, Seif MW. Premature ovarian failure. Curr Opin Obstet Gynecol. 2006;18:418‐426. [DOI] [PubMed] [Google Scholar]
- 7. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28(5):521‐574. [DOI] [PubMed] [Google Scholar]
- 8. Frohlich M, Muhlberger N, Hanke H, et al. Markers of inflammation in women on different hormone replacement therapies. Ann Med. 2003;35:353‐361. [DOI] [PubMed] [Google Scholar]
- 9. Lacut K, Oger E, Le Gal G, et al. Differential effects of oral and transdermal postmenopausal estrogen replacement therapies on C‐reactive protein. Thromb Haemost. 2003;90:124‐131. [PubMed] [Google Scholar]
- 10. Silvestri A, Gebara O, Vitale C, et al. Increased levels of C‐reactive protein after oral hormone replacement therapy may not be related to an increased inflammatory response. Circulation. 2003;107:3165‐3169. [DOI] [PubMed] [Google Scholar]
- 11. Webber L, Davies M, Anderson Bartlett J, et al. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926‐937. [DOI] [PubMed] [Google Scholar]
- 12. Themmen AP. Anti‐Müllerian hormone: its role in follicular growth initiation and survival and as an ovarian reserve marker. J Natl Cancer Inst Monogr. 2005;34:18‐21. [DOI] [PubMed] [Google Scholar]
- 13. Ueno H, Hawrylowicz CM, Banchereau J. Immunological intervention in human diseases. J Transl Med. 2007;5:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107‐134. [DOI] [PubMed] [Google Scholar]
- 15. La Marca A, Marzotti S, Brozzetti A, et al. Italian Addison Network. Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with a preserved pool of functioning follicles. J Clin Endocrinol Metab. 2009;94:3816‐3823. [DOI] [PubMed] [Google Scholar]
- 16. Yıldırım G, Tokmak A, Kokanalı MK, et al. Association between some inflammatory markers and primary ovarian insufficiency. Menopause. 2015;22(9):1000‐1005. [DOI] [PubMed] [Google Scholar]
- 17. Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology. 2002;16:217‐226. [PubMed] [Google Scholar]
- 18. Kisucka J, Butterfield CE, Duda DG, et al. Platelets and platelet adhesion support angiogenesis while preventing excessive hemorrhage. Proc Natl Acad Sci. 2006;103:855‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mangalpally KK, Siqueiros‐Garcia A, Vaduganathan M, Dong JF, Kleiman NS, Guthikonda S. Platelet activation patterns in platelet size subpopulations: differential responses to aspirin in vitro. J Thrombosis Thrombolysis. 2010;30:251‐262. [DOI] [PubMed] [Google Scholar]
- 20. Yılmaz B, Şengül E, Şereflican M, et al. Prognostic value of mean platelet volume and platelet to lymphocyte ratio in laryngeal carcinoma. JCEI. 2016;7(2):134‐138. [Google Scholar]
- 21. Baldane S, Ipekci SH, Sozen M, Kebapcilar L. Mean platelet volume could be a possible biomarker for papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2015;16(7):2671‐2674. [DOI] [PubMed] [Google Scholar]
- 22. Shen XM, Xia YY, Lian L, et al. Mean platelet volume provides beneficial diagnostic and prognostic information for patients with resectable gastric cancer. Oncol Lett. 2016;12(4):2501‐2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Delgado‐García G, Galarza‐Delgado DÁ, Colunga‐Pedraza I, et al. Mean platelet volume is decreased in adults with active lupus disease. Rev Bras Reumatol Engl Ed. 2016;56(6):504‐508. [DOI] [PubMed] [Google Scholar]
- 24. Cho SY, Yang JJ, You E, et al. Mean platelet volume/platelet count ratio in hepatocellular carcinoma. Platelets. 2013;24:375‐377. [DOI] [PubMed] [Google Scholar]
- 25. Kemal Y, Demirağ G, Ekiz K, Yücel I. Mean platelet volume could be a useful biomarker for monitoring epithelial ovarian cancer. J Obstet Gynaecol. 2014;34:515‐518. [DOI] [PubMed] [Google Scholar]
- 26. Kılıncalp S, Ekiz F, Başar O, et al. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25:592‐594. [DOI] [PubMed] [Google Scholar]
- 27. Tsai PL, Su WJ, Leung WH, Lai CT, Liu CK. Neutrophil‐lymphocyte ratio and CEA level as prognostic and predictive factors in colorectal cancer: a systematic review and meta‐analysis. J Cancer Res Ther. 2016;12(2):582‐589. [DOI] [PubMed] [Google Scholar]
- 28. Ilhan G, Atmaca FFV, Altan E, et al. Evaluation of neutrophil‐lymphocyte ratio, platelet‐lymphocyte ratio and red blood cell distribution width‐platelet ratio for diagnosis of premature ovarian insufficiency. J Family Reprod Health. 2016;10(4):211‐216. [PMC free article] [PubMed] [Google Scholar]
- 29. Silberstein T, MacLaughlin DT, Shai I, et al. Mullerian inhibiting substance levels at the time of HCG administration in IVF cycles predict both ovarian reserve and embryo morphology. Hum Reprod. 2006;21:159‐163. [DOI] [PubMed] [Google Scholar]


