Abstract
Background
Hypercoagulability induced by the imbalance between von Willebrand factor (VWF) secretion and its cleaving protease (ADAMTS‐13) has been correlated with cancer metastasis. The aim of this study was to explore the prognostic significance of the VWF/ADAMTS‐13 ratio in advanced non‐small‐cell lung cancer (NSCLC).
Methods
Pre‐treatment sera/plasma levels of VWF, ADAMTS‐13, VWF/ADAMTS‐13 ratio, factor (F) VIII, and other clinical/laboratory parameters were measured in 119 patients with advanced NSCLC and 102 healthy controls. All patients were followed up to determine the predictive value of these parameters for prognosis of advanced NSCLC.
Results
Elevated VWF, VWF/ADAMTS‐13 ratio, and reduced ADAMTS‐13 were significantly correlated with the stage and grade of advanced NSCLC and the final status of disease (P<.05). VWF levels and the VWF/ADAMTS‐13 ratio were also associated with response to chemotherapy (P<.05). Multivariate analysis identified the VWF/ADAMTS‐13 ratio and D‐dimer as significant independent predictors of patient mortality. The area under the curve showed that the VWF/ADAMTS‐13 ratio was more useful than VWF, ADAMTS‐13, and D‐dimer to predict mortality. Kaplan‐Meier analysis showed that a low VWF/ADAMTS‐13 ratio was significantly predictive of improved survival (P=.004).
Conclusion
These results suggest that the imbalance between VWF secretion and ADAMTS‐13 may play a critical role in the hypercoagulability state in advanced NSCLC. Moreover, elevation of the plasma VWF/ADAMTS‐13 ratio may serve as an independent predictive factor for mortality in patients with advanced NSCLC.
Keywords: ADAMTS‐13, non‐small‐cell lung cancer, prognosis, Von Willebrand factor
1. Introduction
Von Willebrand factor (VWF) is a multimeric glycoprotein synthesized by endothelial cells and megakaryocytes/platelets that circulates in the plasma, and plays an essential role in primary hemostasis, mediating the adhesion of platelets to thrombogenic surfaces, and acting as a carrier protein for coagulation factor (F) VIII.1, 2 In the development of cancer metastasis, tumor cells interact with platelets and the vessel wall to extravasate from the circulation; thus, VWF presents a potential candidate to mediate platelet‐tumor cell interactions.3 A number of potential receptors for VWF, including glycoprotein Ib and the integrins αIIbβ3 and αVβ3, have been identified on the surface of tumor cells, while direct interactions between VWF and tumor cells have also been reported.4, 5
The distinctive different molecular sizes of VWF influence its ability to regulate adhesive interactions with platelets.6 “Unusually large” VWF multimers (UL‐VWFMs) are stored in Weibel‐Palade bodies and released into the plasma, where they are rapidly degraded into smaller VWF multimers by the plasma VWF‐cleaving protease ADAMTS‐13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). ADAMTS‐13 specifically cleaves UL‐VWFMs between Tyr1605 and Met1606 within the VWF A2 domain.7 Smaller VWF multimers, such as VWF‐pp, are more rapidly cleared from the circulation than VWF. A deficiency in ADAMTS‐13 increases plasma VWF levels, thereby disrupting the balance between levels of VWF and ADAMTS‐13, resulting in microcirculatory disturbances.8, 9 It is known that severe ADAMTS‐13 deficiency, due to either mutations in the ADAMTS‐13 gene or the presence of inhibitory antibodies,10, 11 is strongly associated with thrombotic thrombocytopenic purpura.
In fact, impaired VWF‐cleaving activity of ADAMTS‐13 was recently demonstrated in patients with metastasizing and malignant tumors. Oleksowicz et al.12 first reported that deficient ADAMTS‐13 activity could result in elevated levels of highly polymeric VWF in patients with disseminated malignancies and Koo et al.13 reported mild ADAMTS‐13 deficiency in patients with stage IV colon cancer and various advanced‐stage malignant tumors. Mannucci et al.14 also found lower mean levels of ADAMTS‐13 activity in patients with disseminated tumors, as compared to those with localized tumors. However, few reports have focused on the contribution of VWF and ADAMTS‐13 to the development of advanced non‐small‐cell lung cancer (NSCLC). Therefore, the purpose of this study was to investigate the balance of VWF secretion and plasma ADAMTS‐13 level in patients with advanced NSCLC, and to explore the association of VWF/ADAMTS‐13 ratio with the progression or prognosis of advanced NSCLC.
2. Materials and Methods
2.1. Patients
A total of 119 patients with histologically confirmed advanced NSCLC who visited the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou City, Zhejiang Province, China), between May 10, 2014, and July 6, 2015, were enrolled in this study. Pre‐treatment evaluation included a complete medical history, physical examination, complete blood cell count, and serum chemistry analysis. Pathological diagnoses were determined by biopsies or cytological specimens collected at bronchoscopy, transthoracic needle aspiration, or from surgical specimens. All patients were classified according to the revised World Health Organization classification of lung tumors and staged according to the revised TNM staging system for lung cancer.15, 16 Additionally, 102 age‐ and sex‐matched healthy controls were recruited from those who attended the First Affiliated Hospital of Zhejiang University for routine health examinations.
All subjects enrolled in this study had normal liver, renal, and cardiac function with no history of cardio‐, cerebro‐, or peripheral‐vascular atherosclerotic disease. Individuals with diabetes mellitus or thromboembolic diseases, including pulmonary embolism, those who underwent surgery within 3 months before enrollment, had acute inflammatory disease or a second primary tumor, received anticoagulant therapy or medical contraception, or any prior history of alcohol or drug abuse were excluded. This study was performed in accordance with the principles embodied in the Declaration of Helsinki and approved by the Institutional Research Ethics Committee of the First Affiliated Hospital of Zhejiang University. Written informed consent was obtained from all subjects prior to participation.
2.2. Response to treatment and follow‐up
All patients underwent chemotherapy or chemoradiotherapy. Cisplatin‐based chemotherapy included etoposide, irinotecan, gemcitabine, docetaxel, or pemetrexed. The tumor response to chemotherapy was evaluated using computed tomography of the chest and defined according to the revised Response Evaluation Criteria in Solid Tumours (version 1.1).17 In short, a complete response (CR) was defined as the disappearance of all target lesions; a partial response (PR) was defined as at least a 30% decrease in the sum of diameters of target lesions; progressive disease (PD) was defined as at least a 20% increase in the sum of diameters of target lesions and that the sum must demonstrate an absolute increase of at least 5 mm; and stable disease (SD) was defined as neither sufficient shrinkage to qualify as a PR nor sufficient increase to qualify as a PD.
The follow‐up period for each patient began at the time of blood sampling and ended for overall survival at the occurrence of death, self‐withdrawal from the study, or on February 25, 2016, whichever came first. During follow‐up, clinical, laboratory, and radiological reassessments were performed at three‐ to four‐week intervals. Additionally, a telephone interview with the patient or patient's family was established to determine the status of the patients who abandoned the follow‐up regimen.
2.3. Sample collection and coagulation assays
Venous blood samples were collected from both the healthy controls and patients in the early morning after overnight fasting into three tubes at the time of clinical diagnosis, and prior to any treatment. One tube coated with ethylenediaminetetraacetic acid (2.4 mg per 2 mL venous blood) was used for the immediate measurement of hemoglobin. One tube with 0.109 mol/L trisodium citrate (9:1 v/v) was used for the measurement of D‐dimer, fibrinogen, VWF, ADAMTS‐13, and F VIII levels. Plasma was obtained by immediately centrifuging the blood in a refrigerated centrifuge at 1500 g for 10 minutes. Blood was also collected in one tube without anticoagulant and allowed to clot for the isolation of serum after centrifugation. All plasma and serum samples were aliquoted and stored at −80°C until assayed. Serum levels of lactate dehydrogenase (LDH) were determined using a Hitachi 7600 analyzer (Hitachi Ltd., Tokyo, Japan) with dedicated reagents (Roche Diagnostics, Mannheim, Germany). Plasma levels of fibrinogen (Clauss method), D‐dimer (immunoturbidometric method), and F VIII (coagulation method) were measured using the Sysmex CA7000 coagulation analyzer (Sysmex Corporation, Kobe, Japan) with commercial kits (Siemens AG, Marburg, Germany). Plasma levels of VWF antigen and ADAMTS‐13 activity were measured using commercial enzyme‐linked immunosorbent assay kits (R&D Systems Inc., Minneapolis, MN, USA) according to the manufacturer's instructions. The intra‐ and inter‐assay coefficients of variation for VWF and ADAMTS‐13 were <9% and <11%, respectively, according to the manufacturer.
2.4. Statistical analysis
Continuous variables are expressed as the mean and standard deviation. The mean values of each item between two groups were compared using the Student's ttest and Mann‐Whitney U test, whereas differences among the three groups were analyzed using one‐way analysis of variance and the Kruskal‐Wallis H test, as appropriate for continuous variables. Nonparametric chi‐square tests were used to compare categorical variables. Spearman's rank correlation was used to evaluate the relationship between parameters. Univariate analysis of risk factors for their predictive value of patient death was performed using Cox proportional hazard regression analyses. A multivariable stepwise logistic regression test was used to evaluate independent clinical parameters predicting mortality. Receiver‐operating characteristic (ROC) curves of the parameters were further obtained, and the differences in the area under the curve (AUC) were tested using the z test. Survival curves were determined using the Kaplan‐Meier method. The log‐rank test was used to evaluate differences between survival curves. All statistical analyses were performed using SPSS version 16.0 J for Windows software (IBM‐SPSS, Inc., Chicago, IL, USA). A P‐value <.05 was considered statistically significant.
3. Results
3.1. Comparison of clinical/laboratory parameters between patients and healthy controls
Clinical and laboratory characteristics in advanced NSCLC patients prior to commencing treatment and in healthy controls are shown in Table 1. Compared to the healthy controls, levels of LDH, D‐dimer, fibrinogen, VWF, and F VIII, as well as VWF/ADAMTS‐13 ratio were significantly increased, whereas body mass index (BMI) and levels of hemoglobin and ADAMTS‐13 were significantly decreased, in the sera or plasma of advanced NSCLC patients. However, there were no significant differences in sex, age, blood group distribution, or smoking status between the two groups.
Table 1.
Clinical and laboratory characteristics of the study participants
| Variables | NSCLC (n=119) | Healthy control (n=102) | P value |
|---|---|---|---|
| Sex (male/female) | 78/41 | 64/38 | .665 |
| Age (y) | 56.6±8.6 | 54.5±8.5 | .098 |
| BMI (kg/m2) | 22.3±3.1 | 23.1±2.9 | .030 |
| Smoking status (yes/no) | 30/89 | 19/83 | .240 |
| Blood group (O/non‐O) | 38/81 | 40/62 | .259 |
| Histology | |||
| Squamous | 36 (30.3%) | ||
| Adeno | 68 (57.1%) | ||
| Other (unclassified) | 15 (12.6%) | ||
| Tumor grade | |||
| Well/moderately differentiated | 87 (73.1%) | ||
| Poorly differentiated | 32 (26.9%) | ||
| TNM stage | |||
| Stage IIIB | 55 (46.2%) | ||
| Stage IV | 64 (53.7%) | ||
| Treatment | |||
| Chemotherapy | |||
| Cisplatin+etoposide or irinotecan | 37 (31.1%) | ||
| Cisplatin+gemcitabine or paclitaxel | 54 (45.4%) | ||
| Cisplatin+pemetrexed | 28 (23.5%) | ||
| Radiotherapy (thoracic) | |||
| Radical | 26 (21.8%) | ||
| Palliative | 36 (30.3%) | ||
| Hemoglobin (g/L) | 122.2±20.6 | 146.9±15.4 | <.001 |
| LDH (U/L) | 205.8±79.3 | 130.0±20.7 | <.001 |
| D‐dimer (μg/L FEU) | 1249.9±1583.5 | 154.5±135.7 | <.001 |
| Fibrinogen (g/L) | 3.8±1.1 | 2.4±0.5 | <.001 |
| VWF (U/L) | 1583.5±787.7 | 1019.9±789.4 | <.001 |
| ADAMTS‐13 (U/L) | 1392.6±562.1 | 1699.9±907.5 | .016 |
| VWF/ADAMTS‐13 ratio | 1.37±0.99 | 0.59±0.26 | <.001 |
| F VIII (%) | 142.9±41.2 | 103.8±23.1 | <.001 |
BMI, body mass index; LDH, lactate dehydrogenase; VWF, von Willebrand factor; ADAMTS‐13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; F, factor.
3.2. Relationship of VWF/ADAMTS‐13 ratio with clinical/pathological parameters
The relationships between plasma levels of VWF, ADAMTS‐13, F VIII, and VWF/ADAMTS‐13 ratio of advanced NSCLC patients and clinical/pathological parameters were analyzed. As shown in Table 2 and Figure 1, the levels of VWF, ADAMTS‐13, VWF/ADAMTS‐13 ratio, and F VIII were found to be significantly correlated with stage and grade of advanced NSCLC. Patients with stage IV or poorly differentiated NSCLC exhibited higher levels of VWF, VWF/ADAMTS‐13 ratio, and F VIII, but lower levels of ADAMTS‐13 (all P<.05). In addition, there was a significant correlation between chemotherapy response and levels of VWF, VWF/ADAMTS‐13 ratio, and F VIII (P=.042, .011, and .049, respectively), but not ADAMTS‐13. Besides, plasma levels of VWF, ADAMTS‐13, and VWF/ADAMTS‐13 ratio were also associated with the final status of disease. Nonsurvivors had higher levels of VWF and VWF/ADAMTS‐13 ratio, but lower levels of ADAMTS‐13, as compared to survivors (P=.027, .005, and .040, respectively).
Table 2.
Results of comparisons between VWF, ADAMTS‐13, VWF/ADAMTS‐13 ratio, F VIII, and clinical/pathological parameters in patients with advanced NSCLC
| Variable | Number (119) | VWF (U/L) | ADAMTS‐13 (U/L) | VWF/ADAMTS‐13 ratio | F VIII (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean±SD | P value | Mean±SD | P value | Mean±SD | P value | Mean±SD | P value | ||
| Sex | |||||||||
| Male | 78 | 1648.4±754.6 | .593 | 1452.5±587.6 | .158 | 1.37±0.88 | .730 | 156.4±41.3 | .259 |
| Female | 41 | 1555.5±847.1 | 1278.1±567.3 | 1.54±1.13 | 165.5±40.7 | ||||
| Age (y) | |||||||||
| ≤55 | 51 | 1424.5±820.6 | .069 | 1230.8±590.0 | .108 | 1.58±1.30 | .841 | 160.7±47.9 | .786 |
| >55 | 68 | 1732.3±752.5 | 1480.2±561.6 | 1.35±0.72 | 159.7±36.4 | ||||
| BMI (kg/m2) | |||||||||
| ≤25 | 92 | 1660.7±783.8 | .165 | 1356.1±574.4 | .488 | 1.53±1.02 | .070 | 165.0±42.1 | .039 |
| >25 | 27 | 1359.2±800.9 | 1513.3±627.0 | 1.00±0.67 | 136.8±24.6 | ||||
| Histology | |||||||||
| Squamous | 36 | 1502.5±759.0 | .208 | 1473.6±673.9 | .753 | 1.27±0.79 | .667 | 150.4±29.9 | .261 |
| Adeno | 68 | 1573.6±735.2 | 1328.3±593.5 | 1.52±1.12 | 163.8±44.5 | ||||
| Other (unclassified) | 15 | 2033.2±102.7 | 1417.4±169.0 | 1.46±0.72 | 165.2±46.5 | ||||
| Tumor grade | |||||||||
| Well/moderately differentiated | 87 | 1488.2±762.0 | .010 | 1472.3±514.1 | .019 | 1.14±0.66 | .002 | 148.6±36.8 | .004 |
| Poorly differentiated | 32 | 1954.3±765.3 | 1162.0±685.8 | 2.30±1.23 | 187.9±39.5 | ||||
| TNM stage | |||||||||
| Stage IIIB | 55 | 1448.3±850.1 | .018 | 1526.3±550.6 | .035 | 1.12±0.87 | .004 | 147.1±37.8 | .011 |
| Stage IV | 64 | 1812.3±675.5 | 1226.3±581.9 | 1.80±0.98 | 174.0±40.2 | ||||
| Response to chemotherapy | |||||||||
| Responsive (CR+PR) | 75 | 1477.2±764.3 | .042 | 1434.6±546.4 | .151 | 1.18±0.76 | .011 | 153.0±35.3 | .049 |
| Nonresponsive (SD+PD) | 44 | 1859.7±779.3 | 1234.3±641.8 | 1.96±1.19 | 173.8±49.8 | ||||
| Final status | |||||||||
| Alive | 92 | 1546.8±832.3 | .027 | 1464.9±565.0 | .040 | 1.22±0.76 | .005 | 155.2±39.5 | .073 |
| Dead | 27 | 1905.1±525.5 | 1080.4±558.7 | 2.17±1.14 | 177.8±42.9 | ||||
BMI, body mass index; VWF, von Willebrand factor; ADAMTS‐13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; SD, standard deviation; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; F, factor.
Figure 1.
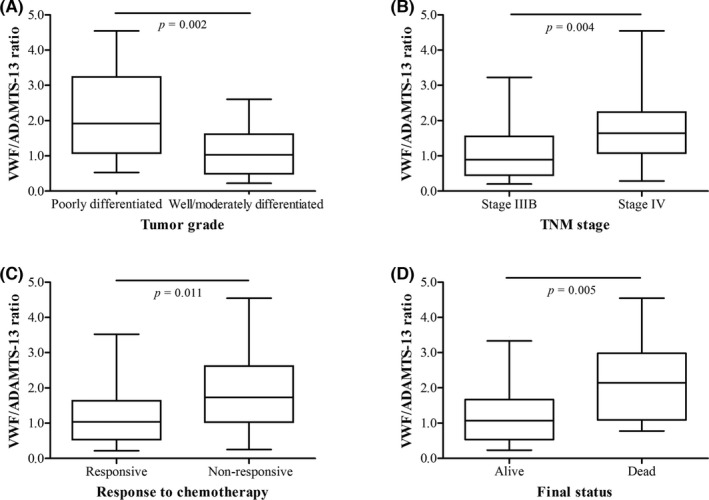
Comparison of the VWF/ADAMTS‐13 ratio in NSCLC patients according to tumor grade (A), TNM stage (B), response to chemotherapy (C), and final status (D). Data are expressed as box plots, in which the horizontal lines illustrate the 25th, 50th, and 75th percentiles of the VWF/ADAMTS‐13 ratio. The vertical lines represent the 2.5th and 97.5th percentiles
The relationship between the VWF/ADAMTS‐13 ratio and clinical variables was further analyzed (Table 3). The VWF/ADAMTS‐13 ratio correlated positively with LDH, D‐dimer, fibrinogen, VWF, and F VIII and correlated negatively with BMI, hemoglobin, and ADAMTS‐13.
Table 3.
Correlation coefficients between the VWF/ADAMTS‐13 ratio and clinical variables in patients with advanced NSCLC
| Variables | Correlation coefficients | P value |
|---|---|---|
| Age (y) | .078 | .321 |
| BMI (kg/m2) | −.170 | .032 |
| Hemoglobin (g/L) | −.393 | <.001 |
| LDH (U/L) | .382 | <.001 |
| D‐dimer (μg/L FEU) | .492 | <.001 |
| Fibrinogen (g/L) | .277 | <.001 |
| VWF (U/L) | .750 | <.001 |
| ADAMTS‐13 (U/L) | −.318 | <.001 |
| F VIII (%) | .376 | <.001 |
BMI, body mass index; LDH, lactate dehydrogenase; VWF, von Willebrand factor; ADAMTS‐13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; F, factor.
Spearman's rank correlation coefficients between the VWF/ADAMTS‐13 ratio and the variables.
3.3. Correlation of VWF/ADAMTS‐13 ratio with prognosis of NSCLC
Cox regression analysis was performed to identify correlations between patient prognosis and clinical/laboratory factors. Among these factors, age (>55 vs ≤55 years), sex (female vs male), BMI (>25 vs ≤25 kg/m2), smoking status (yes vs no), histology (adeno vs non‐adeno), tumor grade (poorly differentiated vs well/moderately differentiated), TNM stage (IV vs IIIB), and response to chemotherapy (nonresponsive vs responsive) were available, while the median values in advanced NSCLC patients were used to dichotomize the levels of hemoglobin, LDH, D‐dimer, fibrinogen, and F VIII, as well as VWF/ADAMTS‐13 ratio to identify the prognostic relevance of these markers by univariate and multivariate analyses (Table 4). Univariate analyses showed that a higher VWF/ADAMTS‐13 ratio, higher plasma D‐dimer levels, poorly differentiated grade, and stage IV were significant risk factors for prediction of mortality in advanced NSCLC patients (all P<.05). Based on multivariate analysis, furthermore, TNM stage, an increase in VWF/ADAMTS‐13 ratio, and higher plasma D‐dimer levels were still significant independent prognostic factors for prediction of mortality (P=.024, .013, and .030, respectively).
Table 4.
Cox regression analyses of risk factors for prediction of patient mortality
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (>55 vs ≤55 y) | 0.72 (0.26‐2.00) | .535 | 0.99 (0.93‐1.06) | .853 |
| Sex (female vs male) | 1.94 (0.72‐5.19) | .187 | 2.84 (0.66‐12.13) | .160 |
| BMI (>25 vs ≤25 kg/m2) | 0.74 (0.21‐2.59) | .637 | 1.12 (0.23‐5.42) | .815 |
| Smoking status (yes vs no) | 1.71 (0.66‐4.45) | .270 | 2.36 (0.86‐6.48) | .097 |
| Histology (adeno vs non‐adeno) | 1.65 (0.62‐4.37) | .318 | 3.21 (0.63‐16.44) | .162 |
| Tumor grade (poorly vs well/moderately) | 3.28 (1.21‐8.95) | .020 | 2.25 (0.76‐6.67) | .140 |
| TNM stage (IV vs IIIB) | 3.91 (1.41‐10.82) | .009 | 4.76 (1.23‐18.41) | .024 |
| Response to chemotherapy (nonresponsive vs responsive) | 2.43 (0.89‐6.62) | .083 | 0.98 (0.23‐4.11) | .958 |
| Hemoglobin (>122 vs ≤122 g/L) | 0.51 (0.19‐1.34) | .169 | 0.86 (0.16‐4.59) | .820 |
| LDH (>193 vs ≤193 U/L) | 2.77 (0.89‐8.60) | .078 | 0.570 (0.13‐2.51) | .458 |
| D‐dimer (>996 vs ≤996 μg/L FEU) | 4.07 (1.33‐12.53) | .014 | 3.49 (1.13‐10.80) | .030 |
| Fibrinogen (>3.7 vs ≤3.7 g/L) | 2.39 (0.84‐6.80) | .104 | 2.15 (0.62‐7.52) | .231 |
| VWF/ADAMTS‐13 ratio (>1.31 vs ≤1.31) | 5.71 (1.63‐19.99) | .006 | 4.89 (1.39‐17.21) | .013 |
| F VIII (>159 vs ≤159%) | 2.33 (0.76‐7.19) | .140 | 1.76 (0.43‐7.19) | .432 |
HR: hazard ratio; CI: confidence interval; TNM, tumor node metastasis; BMI, body mass index; vs, versus; LDH, lactate dehydrogenase; F, factor; VWF, von Willebrand factor; ADAMTS‐13, a disintegrin and metalloproteinase with thrombospondin type‐1 motif, member 13.
The AUCs for the VWF/ADAMTS‐13 ratio, VWF, ADAMTS‐13, and D‐dimer were, respectively, 0.790 (95% confidence interval [CI]=0.677‐0.906), 0.673 (95% CI=0.558‐0.794), 0.651 (95% CI=0.538‐0.775), and 0.708 (95% CI=0.601‐0.824) for prediction of mortality of advanced NSCLC patients (Figure 2). The AUC for the VWF/ADAMTS‐13 ratio tended to be highest for the prediction of mortality. However, these differences were not significant among the VWF/ADAMTS‐13 ratio, VWF, ADAMTS‐13, and D‐dimer for prediction of mortality among advanced NSCLC patients.
Figure 2.
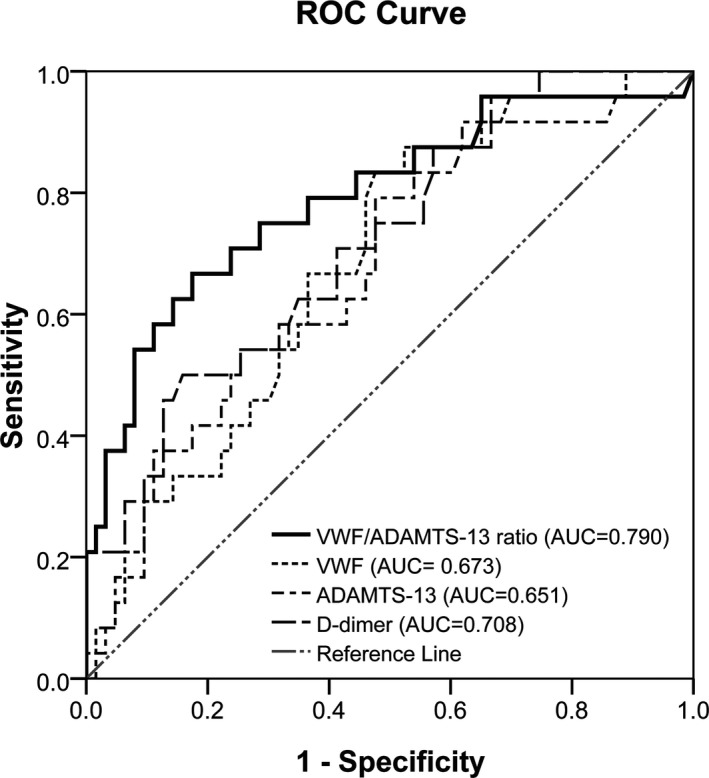
ROC curves of the VWF/ADAMTS‐13 ratio and levels of VWF, ADAMTS‐13, and D‐dimer for the prediction of mortality in advanced NSCLC
The cumulative survival rate of 119 NSCLC patients according to the VWF/ADAMTS‐13 ratio is shown in Figure 3. The median length of survival time was 336 days (95% CI=306‐376 days) and 270 days (95% CI=229‐310 days), respectively, for patients with low VWF/ADAMTS‐13 ratios (≤1.31) and high VWF/ADAMTS‐13 ratios (>1.31). As determined by the Kaplan‐Meier curves, the probability of survival of patients with a lower VWF/ADAMTS‐13 ratio was more favorable than for patients with a higher VWF/ADAMTS‐13 ratio (P=.004).
Figure 3.
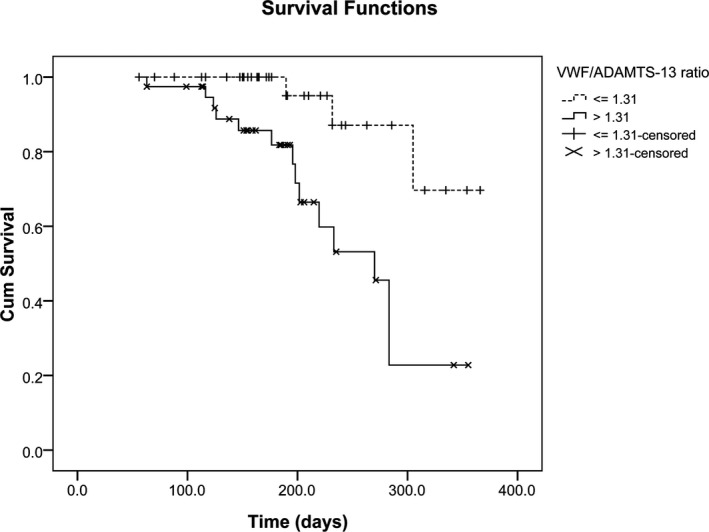
Kaplan‐Meier analysis survival curves of 119 advanced NSCLC patients according to the VWF/ADAMTS‐13 ratio. Differences between groups were evaluated using the log‐rank test (P=.004)
4. Discussion
It is well established that clotting activation is frequently encountered in cancer and that the hypercoagulability state is directly involved in cancer progression.18 Although the biological significance of hemostatic abnormalities in cancer remains unclear, some evidence suggests that the capacity of neoplastic cells to activate the coagulation and fibrinolytic system facilitates their invasive and metastatic behaviors.19 NSCLC patients are also suspected to be more prone to a hypercoagulable state. In NSCLC, host macrophages release procoagulant factors, which could activate the coagulation and fibrinolytic system.20 Previous studies showed that prolonged prothrombin time, international normalized ratio, high D‐dimer level, and low antithrombin III level were associated with worse survival in lung cancer patients.21, 22 Additionally, a low ratio of plasmin‐α2‐plasmin inhibitor complex to thrombin‐antithrombin complex and low protein S levels were reported as significant independent negative prognostic factors for survival in patients with advanced NSCLC.23 The plasma VWF level, currently regarded as a marker of endothelial cell activation, is increased in colorectal, breast, and ovarian cancer. It has been suggested that the plasma VWF level may be associated with tumor stage and has prognostic significance in cancer patients.24, 25, 26 Furthermore, it is known that a deficiency in ADAMTS‐13, which is also known as von Willebrand factor‐cleaving protease, resulting in the presence of UL‐VWFM and increased levels of VWF antigen in plasma, was associated with the metastatic process.13 However, little is known about the association of VWF, ADAMTS‐13, and VWF/ADAMTS‐13 ratio with histopathological types, pretherapeutic tumor stage and grade, and prognosis in advanced NSCLC.
In the present study, we demonstrated significantly increased levels of VWF and the VWF/ADAMTS‐13 ratio, as well as significantly decreased level of ADAMTS‐13, in the plasma of advanced NSCLC patients. Furthermore, plasma levels of VWF, ADAMTS‐13, and VWF/ADAMTS‐13 ratio were significantly associated with tumor grade, disease stage, and final status of advanced NSCLC patients. Meanwhile, we found increased levels of VWF and VWF/ADAMTS‐13 ratio in advanced NSCLC patients with no response to chemotherapy. The results of the present study support the idea that plasma levels of VWF, as well as its cleaving protease ADAMTS‐13, possibly play an important role in tumor spread and might be valuable to predict the outcomes of advanced NSCLC patients. These results are in agreement with the findings of Wang et al.27 and Zietek et al.28 who reported increased levels of plasma VWF in colorectal and urinary bladder carcinoma and may be correlated with clinical staging and poor prognosis. Nevertheless, the significant difference found in the present study is inconsistent with the results obtained by Martini et al.29 who found a similar distribution of VWF between NSCLC patients and control subjects. As the control population consisted of age‐ and sex‐matched healthy subjects in both studies, different prevalence of metastatic disease might explain the discrepancies observed in both studies. Additionally, the imbalance in the distribution of blood types between patients with NSCLC and healthy controls might explain the lack of differences between the two groups in a study by Martini et al.29 as previous studies demonstrated that the proteolysis of VWF by ADAMTS‐13 seems to occur more rapidly in the O blood group, as compared to non‐O blood groups,30, 31 thus explaining the higher VWF plasma levels observed in subjects with non‐O blood groups. In the present study, we found no significant difference in the distribution of blood groups between advanced NSCLC patients and healthy controls.
The results of the present study also confirmed those of previous studies in that a deficiency in ADAMTS‐13 activity was consistently observed in patients with malignancies, especially in advanced or metastatic diseases.13, 32 However, the severity of ADAMTS‐13 deficiency differed among these previous studies, as well as the present study. The different methodology used for measuring plasma ADAMTS‐13 levels might account for the variant results among these studies. Another explanation for the variant results may be a consequence of the heterogeneous study population and cancer types. F VIII plays a crucial role in the propagation phase of coagulation activation. The present study also showed higher levels of F VIII activity in patients with a lower BMI, poorly differentiated grade, stage IV, and no response to chemotherapy. In addition, a marginal significant increase in F VIII activity was observed in nonsurvivors, partly in accordance with a previous study which demonstrated that elevated F VIII was associated with an increased risk of thrombosis and a decrease in survival in patients with lung adenocarcinoma. However, despite this association, F VIII activity did not appear to be a significant risk factor for thrombosis and mortality in the multivariate analysis.33 The effects of disease on F VIII plasma levels may be mainly mediated by VWF, which is well known to carry F VIII and indirectly prevent its plasmatic clearance.2
The VWF/ADAMTS‐13 ratio was reported to be more useful than VWF alone for the diagnosis of hypercoagulability induced by an imbalance between VWF secretion and ADAMTS‐13 in patients with organ failure.34 In the present study, a marked increase in the VWF/ADAMTS‐13 ratio was positively correlated with D‐dimer, fibrinogen, and F VIII in advanced NSCLC patients. These results suggest that an imbalance between VWF secretion and ADAMTS‐13 level induced by endothelial activation or dysfunction may cause highly prothrombotic states that lead to thrombosis in patients with advanced NSCLC. Moreover, the results of the present study established for the first time a possible correlation between a high VWF/ADAMTS‐13 ratio and poor prognosis of advanced NSCLC. These results also suggest that the VWF/ADAMTS‐13 ratio may be a better marker of poor prognosis than VWF, ADAMTS‐13, and D‐dimer in the early phase of advanced NSCLC. However, it is necessary to determine if an imbalance between VWF secretion and ADAMTS‐13 would directly favor metastasis and tumor progression or if some of the thrombogenic factors, including VWF and F VIII, could create a hypercoagulability environment that would result in thrombosis and a subsequent reduction in survival.33
von Willebrand factor is secreted from endothelial cells in response to thrombin, vasoactive amines, and a variety of cytokines by either a constitutive or regulated pathway, and is an adhesive protein capable of adhering tumor cells and platelets, which may lead to the formation of micro‐thrombi, resulting in prolonged survival of tumor cells by protecting them from the immune system and the disruptive effects of turbulence and sheer stress.3 Formation of platelet‐tumor cell aggregates also facilitates the metastatic process by allowing tumor cells to more easily adhere to and migrate through the vessel wall.35 The VWF receptors expressed on the membrane of tumor cells possibly augment GPIb‐mediated VWF attachment, especially for UL‐VWFMs,36, 37 which could arise due to a deficiency in ADAMTS‐13 activity and interactions with more than one ligand at one time. However, it remains unclear whether these tumor‐platelet aggregates further augment secretion of VWF from endothelial Weibel‐Palade bodies. Nonetheless, we speculate that the reduced ADAMTS‐13 activity, which regulates the size and, hence, affects the clearance of VWF from the circulation, might result in an increase in plasma VWF. Although the mechanism that drives the reduction in plasma ADAMTS‐13 in cancer patients is not fully understood, various oncogenes have been found to regulate expression of extracellular proteinases, including the matrix‐degrading metalloproteinases, which can directly result in perturbations in ADAMTS‐13 activity.38 Additionally, the functional properties of ADAMTS‐13 could be impacted by a network of oncogene‐mediated kinases via protein phosphorylation, which not only alters the activity of ADAMTS‐13 itself, but influence the activities of associated regulatory molecules, cofactors, and docking‐type receptors.12, 39
Interestingly, several studies have reported that VWF induces tumor cells to enter apoptosis instead of influencing tumor cell proliferation or invasion.40, 41 Terraube et al.4 reported that VWF plays a protective role against tumor cell dissemination in a VWF‐deficient mouse model. They found that VWF induced the death of tumor cells in a period of hours following their arrest in the target organ, but VWF did not affect their subsequent growth. More recently, Mochizuki et al.40 reported that aggressive cancer cells produced high levels of disintegrin and metalloproteinase 28, which bind and degrade VWF in carcinoma cells, thus avoiding VWF‐induced apoptosis at micrometastatic sites. These results suggested that inactivation of VWF is required in the initial establishment of metastatic foci. However, because the functional absence of glycoprotein Ib alpha results in reduced experimental metastasis,42 the mechanism by which VWF inhibits metastasis may be independent of its role in hemostasis.3 Furthermore, a previous study in mice reported that antibody‐mediated inhibition of VWF led to an inhibition of metastasis.43 Thus, opposite results were found in VWF‐null mice compared to an antibody‐mediated inhibition study and the present study. Because previous studies indicated that tumor‐associated procoagulant activity correlated with increased metastatic potential,42, 44 the protective role of VWF in metastasis formation is unique. It is noteworthy that mouse experimental metastasis occurs within weeks, whereas human metastases develop over years.45 Moreover, the extensive intravascular proliferation of metastatic cancer cells in the mouse model differs from the pathogenesis of human metastasis.46 Further studies are therefore required to confirm and to increase our knowledge of the precise functional mechanism of VWF in tumorigenesis and tumor progression.
There were a few limitations to this study that should be addressed. First, this was a single‐center study with a relatively small sample size, which was not large enough to establish the usefulness of the measurement of the preoperative plasma VWF/ADAMTS‐13 ratio. Therefore, the current findings must be confirmed in larger, multicenter, prospective studies. Second, measurement of the VWF antigen was insufficient to arrive at specific information regarding circulating VWF molecular species, such as VWF propeptide and VWF multimers. A deeper analysis of VWF propeptides, VWF multimers, and VWF activity might be more relevant to reveal the imbalance between VWF secretion and ADAMTS‐13 level in cancer patients, and to clarify the mechanisms behind our findings.
5. Conclusion
In summary, our results demonstrated that an imbalance between VWF secretion and ADAMTS‐13 may play a critical role in the hypercoagulability state in patients with advanced NSCLC. A high VWF/ADAMTS‐13 ratio was associated with tumor grade, tumor metastasis, poor response to chemotherapy, and mortality in advanced NSCLC patients. Our study showed that measurement of the plasma VWF/ADAMTS‐13 ratio can provide additional data for evaluating the prognosis of advanced NSCLC patients.
Acknowledgments
This work was financially supported by grants from the Health Bureau of Zhejiang Province (2016KYA074).
Guo R, Yang J, Liu X, Wu J, Chen Y. Increased von Willebrand factor over decreased ADAMTS‐13 activity is associated with poor prognosis in patients with advanced non‐small‐cell lung cancer. J Clin Lab Anal. 2018;32:e22219 10.1002/jcla.22219
References
- 1. Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185‐190. [DOI] [PubMed] [Google Scholar]
- 2. Sadler JE. Biochemistry and genetics of von Willebrand factor. Annu Rev Biochem. 1998;67:395‐424. [DOI] [PubMed] [Google Scholar]
- 3. Terraube V, Marx I, Denis CV. Role of von Willebrand factor in tumor metastasis. Thromb Res. 2007;120(suppl 2):S64‐S70. [DOI] [PubMed] [Google Scholar]
- 4. Terraube V, Pendu R, Baruch D, et al. Increased metastatic potential of tumor cells in von Willebrand factor‐deficient mice. J Thromb Haemost. 2006;4:519‐526. [DOI] [PubMed] [Google Scholar]
- 5. Pilch J, Habermann R, Felding‐Habermann B. Unique ability of integrin alpha(v)beta 3 to support tumor cell arrest under dynamic flow conditions. J Biol Chem. 2002;277:21930‐21938. [DOI] [PubMed] [Google Scholar]
- 6. Ruggeri ZM, Ware J. The structure and function of von Willebrand factor. Thromb Haemost. 1992;67:594‐599. [PubMed] [Google Scholar]
- 7. Furlan M, Robles R, Galbusera M, et al. Von Willebrand factor‐cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic‐uremic syndrome. N Engl J Med. 1998;339:1578‐1584. [DOI] [PubMed] [Google Scholar]
- 8. Uemura M, Fujimura Y, Ko S, Matsumoto M, Nakajima Y, Fukui H. Determination of ADAMTS13 and its clinical significance for ADAMTS13 supplementation therapy to improve the survival of patients with decompensated liver cirrhosis. Int J Hepatol. 2011;2011:759047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takaya H, Uemura M, Fujimura Y, et al. ADAMTS13 activity may predict the cumulative survival of patients with liver cirrhosis in comparison with the Child‐Turcotte‐Pugh score and the Model for End‐Stage Liver Disease score. Hepatol Res. 2012;42:459‐472. [DOI] [PubMed] [Google Scholar]
- 10. Tsai HM, Lian EC. Antibodies to von Willebrand factor‐cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med. 1998;339:1585‐1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lotta LA, Garagiola I, Palla R, Cairo A, Peyvandi F. ADAMTS13 mutations and polymorphisms in congenital thrombotic thrombocytopenic purpura. Hum Mutat. 2010;31:11‐19. [DOI] [PubMed] [Google Scholar]
- 12. Oleksowicz L, Bhagwati N, DeLeon‐Fernandez M. Deficient activity of von Willebrand's factor cleaving protease in patients with disseminated malignancies. Cancer Res. 1999;59:2244‐2250. [PubMed] [Google Scholar]
- 13. Koo BH, Oh D, Chung SY, et al. Deficiency of von Willebrand factor‐cleaving protease activity in the plasma of malignant patients. Thromb Res. 2002;105:471‐476. [DOI] [PubMed] [Google Scholar]
- 14. Mannucci PM, Karimi M, Mosalaei A, Canciani MT, Peyvandi F. Patients with localized and disseminated tumors have reduced but measurable levels of ADAMTS‐13 (von Willebrand factor cleaving protease). Haematologica. 2003;88:454‐458. [PubMed] [Google Scholar]
- 15. Brambilla E, Travis WD, Colby TV, Corrin B, Shimosato Y. The new World Health Organization classification of lung tumors. Eur Respir J. 2001;18:1059‐1068. [DOI] [PubMed] [Google Scholar]
- 16. Detterbeck FC, Boffa DJ, Tanoue LT. The new lung cancer staging system. Chest. 2009;136:260‐271. [DOI] [PubMed] [Google Scholar]
- 17. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 18. Goad KE, Gralnick HR. Coagulation disorders in cancer. Hematol Oncol Clin North Am. 1996;10:457‐484. [DOI] [PubMed] [Google Scholar]
- 19. Gabazza EC, Taguchi O, Yamakami T, Machishi M, Ibata H, Suzuki S. Evaluating the prethrombotic state in lung cancer using molecular markers. Chest. 1993;103:196‐200. [DOI] [PubMed] [Google Scholar]
- 20. Pavey SJ, Hawson G, Marsch NA. Alterations to the fibrinolytic enzyme system in patients with non small cell lung carcinoma. Blood Coagul Fibrinolysis. 1999;10:261‐267. [DOI] [PubMed] [Google Scholar]
- 21. Unsal E, Atalay F, Atikcan S, Yilmaz A. Prognostic significance of hemostatic parameters in patients with lung cancer. Respir Med. 2004;98:93‐98. [DOI] [PubMed] [Google Scholar]
- 22. Tas F, Kilic L, Serilmez M, Keskin S, Sen F, Duranyildiz D. Clinical and prognostic significance of coagulation assays in lung cancer. Respir Med. 2013;107:451‐457. [DOI] [PubMed] [Google Scholar]
- 23. Masago K, Fujita S, Mio T, et al. Clinical significance of the ratio between the alpha 2 plasmin inhibitor‐plasmin complex and the thrombin‐antithrombin complex in advanced non‐small cell lung cancer. Med Oncol. 2011;28:351‐356. [DOI] [PubMed] [Google Scholar]
- 24. Röhsig LM, Damin DC, Stefani SD, Castro CG Jr, Roisenberg I, Schwartsmann G. Von Willebrand factor antigen levels in plasma of patients with malignant breast disease. Braz J Med Biol Res. 2001;34:1125‐1129. [DOI] [PubMed] [Google Scholar]
- 25. Damin DC, Rosito MA, Gus P, Roisemberg I, Bandinelli E, Schwartsmann G. Von Willebrand factor in colorectal cancer. Int J Colorectal Dis. 2002;17:42‐45. [DOI] [PubMed] [Google Scholar]
- 26. Gadducci A, Baicchi U, Marrai R, Del Bravo B, Fosella PV, Facchini V. Pretreatment plasma levels of fibrinopeptideA (FPA), D‐dimer (DD), and von Willebrand factor (vWF) in patients with ovarian carcinoma. Gynecol Oncol. 1994;53:352‐356. [DOI] [PubMed] [Google Scholar]
- 27. Wang WS, Lin JK, Lin TC, et al. Plasma von Willebrand factor level as a prognostic indicator of patients with metastatic colorectal carcinoma. World J Gastroenterol. 2005;11:2166‐2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zietek Z, Iwan‐Zietek I, Paczulski R, Kotschy M, Wolski Z. Von Willebrand factor antigen in blood plasma of patients with urinary bladder carcinoma. Thromb Res. 1996;83:399‐402. [DOI] [PubMed] [Google Scholar]
- 29. Martini F, Ferroni P, Guadagni F, et al. Plasma von Willebrand factor antigen levels in non‐small cell lung cancer patients. Anticancer Res. 2005;25:403‐407. [PubMed] [Google Scholar]
- 30. Paiva SG, Sabino AP, Carvalho MG, et al. Polymorphisms in exons 6 and 7 of the ABO locus and their association with venous thrombosis in young Brazilian patients. Blood Coagul Fibrinolysis. 2009;20:122‐128. [DOI] [PubMed] [Google Scholar]
- 31. O'Donnell J, Boulton FE, Manning RA, Laffan MA. Amount of H antigen expressed on circulating von Willebrand factor is modified by ABO blood group genotype and is a major determinant of plasma von Willebrand factor antigen levels. Arterioscler Thromb Vasc Biol. 2002;22:335‐341. [DOI] [PubMed] [Google Scholar]
- 32. Fontana S, Gerritsen HE, Kremer Hovinga J, Furlan M, Lammle B. Microangiopathic haemolytic anaemia in metastasizing malignant tumours is not associated with a severe deficiency of the von Willebrand factor‐cleaving protease. Br J Haematol. 2001;113:100‐102. [DOI] [PubMed] [Google Scholar]
- 33. de Meis E, Pinheiro VR, Zamboni MM, et al. Clotting, immune system, and venous thrombosis in lung adenocarcinoma patients: a prospective study. Cancer Invest. 2009;27:989‐997. [DOI] [PubMed] [Google Scholar]
- 34. Claus RA, Bockmeyer CL, Budde U, et al. Variations in the ratio between von Willebrand factor and its cleaving protease during systemic inflammation and association with severity and prognosis of organ failure. Thromb Haemost. 2009;101:239‐247. [PubMed] [Google Scholar]
- 35. Suter CM, Hogg PJ, Price JT, Chong BH, Ward RL. Identification and characterisation of a platelet GPIb/V/IX‐like complex on human breast cancers: implications for the metastatic process. Jpn J Cancer Res. 2001;92:1082‐1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pépin M, Kleinjan A, Hajage D, et al. ADAMTS‐13 and von Willebrand factor predict venous thromboembolism in patients with cancer. J Thromb Haemost. 2016;14:306‐315. [DOI] [PubMed] [Google Scholar]
- 37. Blot E, Decaudin D, Veyradier A, Bardier A, Zagame OL, Pouillart P. Cancer‐related thrombotic microangiopathy secondary to Von Willebrand factor‐cleaving protease deficiency. Thromb Res. 2002;106:127‐130. [DOI] [PubMed] [Google Scholar]
- 38. Rocks N, Paulissen G, Quesada Calvo F, et al. Expression of a disintegrin and metalloprotease (ADAM and ADAMTS) enzymes in human non‐small‐cell lung carcinomas (NSCLC). Br J Cancer. 2006;94:724‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zheng XL. Structure‐function and regulation of ADAMTS‐13 protease. J Thromb Haemost. 2013;11(suppl 1):11‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mochizuki S, Soejima K, Shimoda M, et al. Effect of ADAM28 on carcinoma cell metastasis by cleavage of von Willebrand factor. J Natl Cancer Inst. 2012;104:906‐922. [DOI] [PubMed] [Google Scholar]
- 41. Franchini M, Frattini F, Crestani S, Bonfanti C, Lippi G. von Willebrand factor and cancer: a renewed interest. Thromb Res. 2013;131:290‐292. [DOI] [PubMed] [Google Scholar]
- 42. Jain S, Zuka M, Liu J, et al. Platelet glycoprotein Ib alpha supports experimental lung metastasis. Proc Natl Acad Sci USA. 2007;104:9024‐9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012‐1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease‐activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397‐401. [DOI] [PubMed] [Google Scholar]
- 45. Zucker S, Cao J. New wrinkle between cancer and blood coagulation, metastasis and cleavage of von Willebrand factor by ADAM28. J Natl Cancer Inst. 2012;104:887‐888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wong CW, Song C, Grimes MM, et al. Intravascular location of breast cancer cells after spontaneous metastasis to the lung. Am J Pathol. 2002;161:749‐753. [DOI] [PMC free article] [PubMed] [Google Scholar]


