Abstract
Introduction
Infections represent a major complication of hematological malignancies. C‐reactive protein (CRP) and procalcitonin (PCT) have been used as diagnostic biomarkers of infections, but do not produce definitive findings. Recently, a new biomarker, presepsin, has been used as a diagnostic tool for detecting infections in the fields of emergency and neonatal medicine. However, the usefulness of presepsin for identifying infections in patients with hematological malignancies, including those who develop febrile neutropenia, remains unclear.
Methods
In this study, we retrospectively analyzed the utility of PCT, presepsin, and CRP as biomarkers of infections during 49 febrile episodes that occurred in 28 patients with hematological malignancies.
Results
The levels of PCT, but not those of CRP or presepsin, were significantly higher in the infection group than in the uninfected group (P<.03), indicating that PCT might be a more sensitive biomarker of infections. No differences in presepsin levels were detected between the patients with and without neutropenia, or between the infected and uninfected patients with neutropenia, indicating that presepsin might have less diagnostic value in patients with neutropenia.
Conclusions
We conclude that PCT might provide additional information and could be used in combination with other biomarkers to detect infections in patients with hematological malignancies.
Keywords: C‐reactive protein, hematological malignancy, neutropenia, presepsin, procalcitonin
1. Introduction
Fever is frequently encountered as a complication in patients with hematological malignancies who undergo intensive chemotherapy or hematopoietic stem cell transplantation (HSCT).1, 2 The most common cause of fever is infection. Patients with neutropenia are particularly at risk of severe infections. However, some patients experience fever without any evidence of infection. Identifying the origin of a fever is very important for enabling the rapid initiation of suitable treatment in cases of severe infection.
Biomarkers of infection play important roles in identifying the causes of fever in patients with hematological malignancies.3
C‐reactive protein (CRP) is probably the gold‐standard biomarker of inflammatory status, but it does not seem to be a very specific or diagnostically definitive marker of infection.4, 5
Procalcitonin (PCT) is the 116‐amino acid precursor molecule of calcitonin and is rapidly produced by the C cells of the thyroid gland as well as several other cell types and organs in response to pro‐inflammatory stimulation.6 High levels of PCT are seen during systemic bacterial infections, and less elevated PCT levels are observed during localized bacterial infections. To date, the diagnostic and predictive value of PCT has been investigated in several types of patients and diseases, including those that develop neutropenia after chemotherapy or an HSCT.7, 8
Recently, a new biomarker, soluble CD14‐subtype (presepsin), was identified.9 Presepsin is a 13‐kD molecule comprising the soluble fraction of CD14, a surface marker of monocytes/macrophages. At the infection, CD14 was phagocytized with pathogens and cleaved. After this process, soluble CD14 subtype (presepsin) was generated and released.10 Elevated presepsin levels in the peripheral blood have widely been used as a biomarker of sepsis, especially in the field of critical care medicine.11 It was reported that presepsin levels are related to the severity of sepsis and can be used to predict the prognosis of such patients.12 However, the usefulness of presepsin in patients with hematological malignancies, including those that develop febrile neutropenia, remains unclear.
In this study, we retrospectively analyzed the utility of PCT, presepsin, and CRP as biomarkers of infection in patients with hematological malignancies who developed febrile episodes.
2. Patients and Methods
This retrospective single‐center analysis was performed at the International Medical Center, Saitama Medical University. Approval for this study was provided by the ethics committee of the International Medical Center, Saitama Medical University, according to the Declaration of Helsinki (#16‐075). The need for informed consent was waived in view of the retrospective and anonymous nature of the study.
2.1. Study design
Between March 2014 and March 2016, 28 patients who fulfilled the inclusion criteria were enrolled in the study. All data and clinical diagnosis were drawn from patient individual charts. The enrolled patients were classified according to criteria (mentioned below) when we reviewed the clinical, radiological, and microbiological data retrospectively. We did not take account of the levels of biomarkers at the classification. The inclusion criteria were as follows: (1) The patient was suffering from a hematological malignancy and was admitted to our hospital to receive chemotherapy and/or HSCT against hematological malignancy at the Department of Hematology/Oncology; (2) the patient's PCT, presepsin (soluble CD14‐subtype), and CRP levels were measured simultaneously within 72 hours after each febrile episode; and (3) the patients exhibited estimated glomerular filtration rate: >60 mL/min/m2, which is the cutoff level the renal dysfunction affected the presepsin levels,13, 14 at the time of the measurement of PCT, presepsin, and CRP.
Each definition used in this study was shown as followed. Neutropenia was defined as a neutrophil count of <0.5×109/L or a neutrophil count of <1.0×109/L in patients whose neutrophil counts were expected to decline to <0.5×109/L. Fever was defined as an axillary temperature of ≥37.5°C based on a single measurement as previously reported.12, 15
In some of analyses, we classified the febrile episodes by the absence or presence of infections or neutropenia (see text).
2.2. Measurements of biomarkers
Serum and plasma samples were collected during each febrile episode (within 72 hours of the onset of fever). Almost patients were free from antimicrobial therapy when three biomarkers were measured. The plasma concentrations of presepsin were measured based on a chemiluminescent enzyme immunoassay using the PATHFAST Presepsin kit (LSI Medience Corporation, Tokyo, Japan), performed on the PATHFAST point‐of‐care analyzer (Mitsubishi Chemical Medicine Corporation, Tokyo, Japan). The measurement range is 20‐20 000 pg/dL. The serum concentrations of PCT were measured by Elecsys BRAHMA PCT assay electrochemiluminescence immunoassay (Roche Diagnostics, Tokyo, Japan) using a cobas 8000 (Roche Diagnostics). The measurement range is 0.02‐100 ng/mL. The serum concentrations of CRP were evaluated by latex‐enhanced turbidimetric immunoassay using CRP‐LATEX (II) X2 “SEIKEN” (DENKA SEIKEN CO., LTD., Tokyo, Japan), performed by the TBA‐c16000 chemistry analyzer (TOSHIBA Medical System Corporation, Tokyo, Japan). The limit of detection level was 0.010 mg/dL. A result was considered to be positive if it met the following criteria: 314 pg/mL for presepsin, 0.05 ng/mL for PCT, and 0.26 mg/dL for CRP, according to manufacturer's instructions, respectively.
2.3. Statistical analysis
Statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).16 Continuous variables were expressed as median (range). Categorical variables were compared using the Mann‐Whitney test (two groups), or the Kruskal‐Wallis test (more than two groups). During comparisons among three or four groups, the Kruskal‐Wallis test was performed, and then the Mann‐Whitney test with Bonferroni correction was employed. Correlations were evaluated using Spearman's rank test. The diagnostic properties of biomarkers were evaluated by receiver‐operating characteristic (ROC) analysis. This statistical method provided index of diagnostic accuracy of a test as area under the ROC curve (AUC).
Statistical significance was estimated when P‐value <.05.
3. Results
A total of 49 febrile episodes that occurred in 28 patients with hematological malignancies were analyzed in this study. The patients’ profiles were shown in Table 1.
Table 1.
Patients’ characteristics
| Patients, n | 28 |
| Male/female, n | 19/9 |
| Median age, y (range) | 50 (21‐85) |
| Diagnosis | |
| Leukemia [n (%)] | 19 (68) |
| de novo AML, n | 12 |
| ALL, n | 4 |
| Myeloid/NK cell leukemia, n | 1 |
| Mixed lineage leukemia, n | 1 |
| CLL, n | 1 |
| Lymphoma [n (%)] | 5 (18) |
| MDS [n (%)] | 4 (14) |
| RCMD, n | 1 |
| Advanced, n | 3 |
| Treatment | |
| Chemotherapy, n | 21 |
| Allogeneic HSCT, n | 7 |
| Samples, n | 49 |
| Post‐HSCT, n | 9 |
| Neutropenia, n | 26 |
AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; RCMD, refractory cytopenia with multilineage dysplasia; Advanced, patients with MDS‐related secondary AML; NK, natural killer; HSCT, hematopoietic stem cell transplantation.
3.1. Analysis of the levels of PCT, presepsin, and CRP in the patients with hematological malignancies
We first compared the patients’ PCT, presepsin, and CRP levels according to the presence or absence of infection (Table 2). The median PCT, presepsin, and CRP levels of the infection group were 0.38 ng/mL (range: 0.08‐83.1), 415 pg/mL (155‐2680), and 7.317 mg/dL (0.467‐26.075), respectively, and those of the uninfected group were 0.16 (0.04‐5.14), 381.5 (114‐1480), and 7.28 (0.055‐31.493), respectively. The infection group exhibited higher presepsin levels than the uninfected group, but the difference was not significant. The infection group displayed significantly higher PCT levels than the uninfected group (P=.027, Table 2). These results indicated that PCT might be a more sensitive biomarker of infections.
Table 2.
Comparison of the biomarker levels between the infection and uninfected groups
| Group: median (range) | P‐value | ||
|---|---|---|---|
| Infection group (n=19) | Uninfected group (n=30) | ||
| PCT (ng/mL) | 0.38 (0.08‐83.1) | 0.16 (0.04‐5.14) | .035 |
| Presepsin (pg/mL) | 415 (155‐2680) | 381.5 (114‐1480) | .272 |
| CRP (mg/dL) | 7.317 (0.467‐26.075) | 7.28 (0.055‐31.493) | .511 |
Next, we classified the patients into three groups according to the causes of their fevers; (1) the infection group (n=19), (2) the primary disease group (n=6), and (3) the uninfected group (n=24). Each criterion of three groups was as followed: (1) the infection group: patients who exhibited clinical or microbiological signs of infection in addition to fever; (2) the primary disease group: patients who did not exhibit any signs of infection except fever and whose febrile episodes were thought to be caused by primary malignant disease; and (3) the uninfected group: patients who demonstrated negative culture results and were confirmed to be free from infection by a physician. As shown in Figure 1, the patients’ median PCT, presepsin, and CRP levels were 0.38 ng/mL (range: 0.08‐83.1), 415 pg/mL (155‐2680), and 7.317 mg/dL (0.467‐26.075) in the infected group; 0.15 (0.07‐5.14), 416 (154‐549), and 7.666 (0.574‐21.584) in the primary disease group; and 0.16 (0.04‐0.79), 358.5 (114‐1480), and 7.28 (0.055‐31.493) in the uninfected group, respectively. The presepsin levels of the infection group were higher than those of the uninfected group, but the difference was not significant. Significant differences in PCT levels were only detected between the infection and uninfected groups (P<.01). These results indicated that PCT, but not presepsin, might have diagnostic value for discriminating between infected and uninfected patients.
Figure 1.
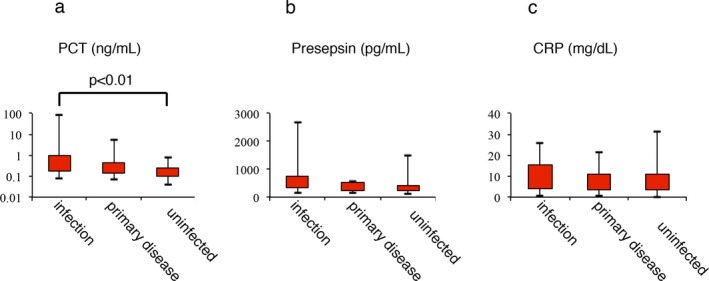
Levels of procalcitonin (PCT; A), presepsin (PRES; B), and CRP (C‐reactive protein; C) in patients in the infection, primary disease, or uninfected groups
Nine febrile episodes occurred in seven patients who had treated with allogeneic HSCT. The PCT, presepsin, and CRP levels of the patients that had undergone HSCT were higher than those of the patients that had not undergone HSCT, but the differences were not significant (data not shown). Seven of the nine episodes occurred in the presence of a cytokine storm or during the recovery phase after myeloablative treatment at HSCT.
3.2. Analysis of the levels of PCT, presepsin, and CRP in the patients with neutropenia
When we divided the patients according to whether their neutrophil counts were above or below 0.5×109/L, we found that the patients with neutropenia exhibited significantly higher levels of PCT and CRP than the patients without neutropenia; however, the presepsin levels of these two groups did not differ (Table 3), indicating that neutropenia itself might influence the levels of some biomarkers.
Table 3.
Comparison of the biomarker levels between the neutropenia and non‐neutropenia groups
| Group: median (range) | P‐value | ||
|---|---|---|---|
| Neutropenia group (n=26) | Non‐neutropenia group (n=23) | ||
| PCT (ng/mL) | 0.38 (0.05‐83.1) | 0.14 (0.04‐5.14) | .008 |
| Presepsin (pg/mL) | 397.5 (114‐2680) | 389 (180‐1200) | .764 |
| CRP (mg/dL) | 9.881 (3.097‐31.493) | 3.94 (0.055‐21.584) | .001 |
Next, we examined the biomarker levels in neutropenic patients according to the presence or absence of infection. As shown in Table 4, the patients’ median PCT, presepsin, and CRP levels were 0.43 ng/mL (range: 0.14‐83.1), 358 pg/mL (155‐2680), and 11.4 mg/dL (4.229‐26.075) in the infected group, and 0.19 (0.05‐0.64), 398 (114‐1480), and 8.358 (3.044‐31.493) in the uninfected group, respectively. Only PCT showed the significant difference between the infection and uninfected groups (P<.03). These results indicated that PCT, but not presepsin, might have diagnostic value for discriminating between infected and uninfected patients in neutropenic status.
Table 4.
Comparison of biomarker levels between infection and uninfected groups in neutropenic patients
| Group: median (range) | P value | ||
|---|---|---|---|
| Infection group (n=13) | Uninfected group (n=13) | ||
| PCT (ng/mL) | 0.43 (0.14‐83.1) | 0.19 (0.05‐0.64) | .033 |
| Presepsin (pg/mL) | 358 (155‐2680) | 398 (114‐1480) | .663 |
| CRP (mg/dL) | 11.4 (4.229‐26.075) | 8.358 (3.044‐31.493) | .626 |
We divided the patients with neutropenia into (1) the bacteremia/sepsis group (n=6), (2) the local infection group (n=7), and (3) the uninfected group (n=13). Each classification was below: (1) the bacteremia/sepsis group: patients who displayed positive blood culture results or were diagnosed with sepsis; (2) the local infection group: patients who demonstrated negative blood culture results and were diagnosed with an infection by a physician (all of these patients were diagnosed with pneumonia); and (3) the uninfected group: patients who displayed negative culture results and were confirmed to be free from infection by a physician. The discrimination of severity about infection was very important in the patients with neutropenia when we treated these patients. The patients’ median PCT, presepsin, and CRP levels were as follows: 1.035 ng/mL (range: 0.38‐83.1), 471.5 pg/mL (309‐1200), and 13.207 mg/dL (5.923‐18.153) in the bacteremia/sepsis group; 0.37 (0.14‐3.04), 326 (115‐2680), and 13.207 (5.923‐18.153) in the local infection group; and 0.19 (0.05‐0.64), 398 (114‐1480), and 8.358 (3.044‐31.493) in the uninfected group, respectively. Figure 2 shows the PCT, presepsin, and CRP levels of the three groups. No significant differences in the levels of presepsin or CRP were detected between any of the groups. As for the patients’ PCT levels, the bacteremia/sepsis group exhibited significantly higher PCT levels than the uninfected group, but no other intergroup differences were detected (P<.02). These results indicated that these biomarkers could not be used to determine a patient's infection status and were not of diagnostic value (at least when used alone) in patients with neutropenia.
Figure 2.
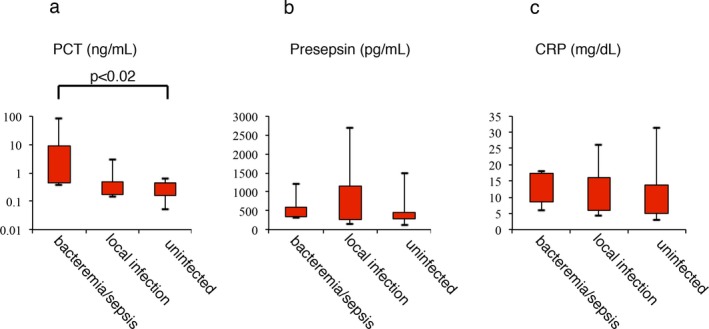
Levels of procalcitonin (PCT; A), presepsin (PRES; B), and CRP (C‐reactive protein; C) in neutropenic patients with bacteremia, localized infections, or no infection
3.3. Accuracy of infection diagnosis
We performed the ROC analysis of three biomarkers for the accuracy of infection diagnosis. The ROC curves in infection and the uninfected group of all febrile episodes (n=49) were shown in Figure 3A,B. The area under the curve (AUC) of PCT was calculated from ROC curve was 0.753 [95% confidence interval (CI): 0.6151‐0.89]. This value of PCT was higher than the AUC of presepsin (0.622 [95%CI: 0.4573‐0.7858]) or CRP 0.453 [95% CI: 0.2856‐0.6213]). When we analyzed the ROC curves of febrile episodes (n=26) in infection and the uninfected group from neutropenic patients were shown in Figure 3C,D. The area under the curve (AUC) of PCT was calculated from ROC curve was 0.746 [95% confidence interval (CI): 0.5528‐0.9383]. This value of PCT was higher than the AUC of presepsin (0.45 [95%CI: 0.2152‐0.6842]) or CRP 0.556 [95% CI: 0.3213‐0.7911]). These results indicated that PCT, but not presepsin or CRP might have diagnostic value to some extent for discriminating between infected and uninfected patients, and this tendency was seen in the patients with neutropenia.
Figure 3.
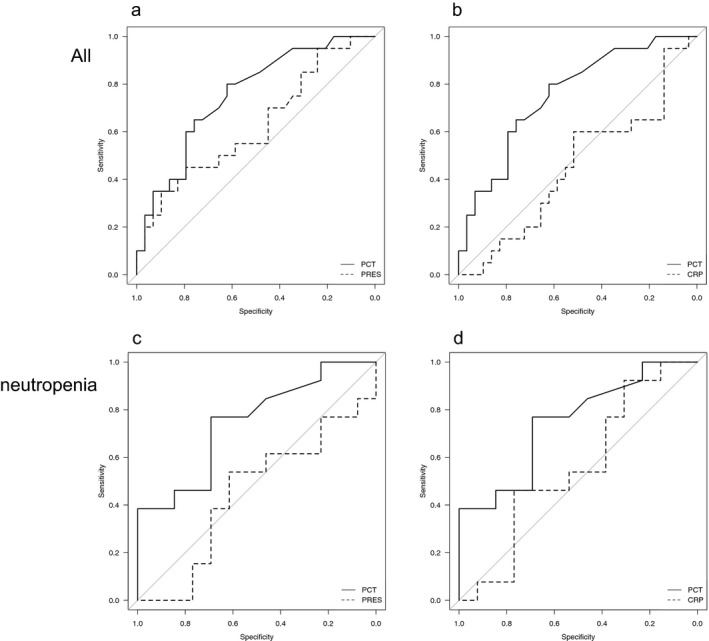
Receiver‐operating characteristic curves of biomarkers. (A) all patients (procalcitonin: PCT and presepsin: PRES), (B) all patients (PCT and C‐reactive protein: CRP). (C) patients with neutropenia (PCT and PRES), (D) patients with neutropenia (PCT and CRP)
3.4. Correlations between each biomarker
We finally examined the correlations between the levels of each pair of biomarkers in patients with hematological malignancies. As shown in Figure 4, significant correlations were detected between each combination.
Figure 4.
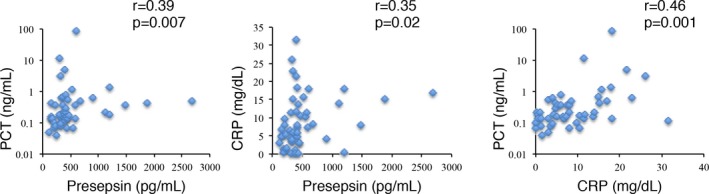
Correlations between the levels of each pair of biomarkers
4. Discussion
In this study, we investigated the levels of PCT, presepsin, and CRP during 49 febrile episodes that occurred in 28 patients with hematological malignancies. We found that the patients’ levels of PCT, presepsin, and CRP were significantly correlated with each other (Figure 4). However, our results indicated that while PCT is somewhat useful for discriminating between infected and uninfected patients, it could not be used to definitively diagnose infections. On the other hand, presepsin and CRP were even less useful biomarkers for identifying infections in such patients.
C‐reactive protein was reported to be useful for aiding treatment‐related decision‐making,17 and it was suggested that high CRP levels are associated with sepsis and microbiologically documented infections in patients with febrile neutropenia.3 However, it was subsequently reported that CRP is not very specific and hence is a poor diagnostic marker of infection in patients with febrile neutropenia.4, 5 In our study, patients with infections tended to exhibit higher CRP levels, indicating that CRP has insufficient diagnostic value for detecting infections.
It was reported that PCT worked well as a diagnostic biomarker in patients without acute kidney injuries,18 while Urbonas et al.8 showed that PCT had additional diagnostic value for detecting bacteremia/sepsis in pediatric patients with febrile neutropenia. However, Aimoto et al.19 reported that neither PCT nor CRP levels were useful for determining the cause of a fever. In our study, the PCT levels of the patients with infections were significantly higher than those of the patients without infections (Table 2), and so PCT could be of diagnostic value for discriminating between patients that have and have not suffered infections (Table 2). Moreover, PCT had highest AUC in three biomarkers (Figure 3). The neutropenic patients exhibited significantly higher PCT levels than the non‐neutropenic patients, but PCT was not found to be of diagnostic value in terms of its ability to distinguish between systemic and local infection group, or between local infection and uninfected group (Figure 2). Thus, PCT might provide additional information for determining the presence/absence of infection when used in combination with other biomarkers, as reported previously.20
In the fields of emergency medicine and neonatology, it was suggested that presepsin has diagnostic value for detecting infections.11, 21 Few studies have evaluated the presepsin levels of patients with hematological malignancies, even though they frequently suffer severe infections or sepsis during the clinical courses of their diseases.8, 12 Among the patients with hematological malignancies examined in the current study, the patients with infections tended to exhibit higher presepsin levels, but the difference was not significant. And presepsin showed lower AUC than PCT (Figure 4). The dynamics of presepsin levels in neutropenic patients are still undefined. In this study, the presepsin levels of the patients with or without neutropenia did not differ (Table 3). Among the patients with neutropenia, no difference in presepsin levels was detected between the patients with and without infections (Table 4). Recently, it was reported that monocytes are the main source of presepsin.10 Monocytes and macrophages, which generate presepsin, are activated in the condition with cytokine storm or during the recovery phase after myeloablative treatment. Patients with neutropenia have fewer granulocytes and usually accompanied with fewer numbers of monocytes. As fewer numbers of monocytes would make it less likely that any invading pathogens would be phagocytized by monocytes, it could result in the suppression of presepsin release, and hence, patients with neutropenia, who had infection, might have similar presepsin levels to uninfected patients (Table 4). When we compared the levels of presepsin between neutropenic patients without infections and non‐neutropenic patients without infections, the levels of presepsin showed the higher levels in neutropenic patients groups than non‐neutropenic patients, but not significant difference (data not shown).
On the other hand, it was reported that patients with hemophagocytic syndrome, in which macrophages are activated, had higher levels of presepsin than those without hemophagocytic syndrome.10 These results indicated that presepsin might have less diagnostic value in patients with neutropenia because of less phagocytosis. Some patients with neutropenia, including those with agranulocytosis, displayed high presepsin levels in the present study. These patients had infections or engraftment syndrome or were in the recovery phase after bone marrow suppression with anticancer drugs. Ogawa et al. reported that the serum level of the soluble type of CD14 increased significantly according to the grade of liver inflammation. We speculated that some tissue macrophages that were activated by infections and/or inflammation might destroy tissues and promoted the phagocytosis, resulted in increasing the release of presepsin.
When we divided the patients into the infection and the uninfected groups and plotted the ROC curves of PCT, presepsin, and CRP, PCT was the highest value of AUC. In the patients with neutropenia, this tendency was similar. PCT might be a more valuable biomarker than presepsin and CRP, and presepsin might have less diagnostic value in patients with neutropenia.
This study had some limitations. First, it was a retrospective analysis, and we were not able to obtain sufficient samples to allow us to measure the patients’ biomarker levels throughout the course of their fevers and the associated treatment. In addition, we could not obtain data about the patients’ biomarker levels before the onset of fever so we cannot draw any definitive conclusions based on our findings. A further large‐scale prospective study might be necessary to confirm the precise diagnostic value of these biomarkers, especially presepsin, for identifying infections.
In conclusion, we analyzed the utility of PCT, presepsin, and CRP in patients with hematological malignancies. Our results demonstrated that PCT might be a more valuable biomarker than presepsin and CRP and that presepsin might have less diagnostic value in patients with neutropenia. These biomarkers could not be used as diagnostic tools by themselves; however, they might be helpful for detecting infections when used in combination with other infectious biomarkers.
Authorship
Contribution: Y.E., K.K., N.A., and K.I. designed the study; T.M., N.T., and N.A. performed patients’ care; Y.E., K.K., A.I., Y.T., and K.I. analyzed data; and Y.E. and K.I. wrote the paper.
Acknowledgments
The authors would like to thank the physicians, and nurses who cared for patients, and Mr. Hiroyuki Uchida for his help in statistical analysis. This work was supported in part by Grants‐in‐Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sport, Science, and Technology (JSPS KAKENHI # 26461431) and by the National Cancer Center Research and Development Fund (26‐A‐24) to N.A.
Ebihara Y, Kobayashi K, Ishida A, et al. Diagnostic performance of procalcitonin, presepsin, and C‐reactive protein in patients with hematological malignancies. J Clin Lab Anal. 2017;31:e22147 10.1002/jcla.22147
References
- 1. Viscoli C. Management of infection in cancer patients: studies of the EORTC International Antimicrobial Therapy Group (IATG). Eur J Cancer. 2002;38(Supplement 4):82–87. [DOI] [PubMed] [Google Scholar]
- 2. Gustinetti G, Mikulska M. Bloodstream infections in neutropenic cancer patients: a practical update. Virulence. 2016;7:280–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rintala E, Irjala K, Nikoskelainen J. Value of measurement of C‐reactive protein in febrile patients with hematological malignancies. Eur J Clin Microbiol Infect Dis. 1992;11:973–978. [DOI] [PubMed] [Google Scholar]
- 4. Povoa P. C‐reactive protein: a valuable marker of sepsis. Intensive Care Med. 2002;28:235–243. [DOI] [PubMed] [Google Scholar]
- 5. Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker KL, Nylen ES, White JC, Muller B, Snider RH Jr. Clinical review 167: procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004;89:1512–1525. [DOI] [PubMed] [Google Scholar]
- 7. Limper M, de Kruif MD, Duits AJ, Brandjes DP, van Gorp EC. The diagnostic role of procalcitonin and other biomarkers in discriminating infectious from non‐infectious fever. J Infect. 2010;60:409–416. [DOI] [PubMed] [Google Scholar]
- 8. Urbonas V, Eidukaite A, Tamuliene I. The predictive value of soluble biomarkers (CD14 subtype, interleukin‐2 receptor, human leucocyte antigen‐G) and procalcitonin in the detection of bacteremia and sepsis in pediatric oncology patients with chemotherapy‐induced febrile neutropenia. Cytokine. 2013;62:34–37. [DOI] [PubMed] [Google Scholar]
- 9. Chenevier‐Gobeaux C, Borderie D, Weiss N, Mallet‐Coste T, Claessens YE. Presepsin (sCD14‐ST), an innate immune response marker in sepsis. Clin Chim Acta. 2015;450:97–103. [DOI] [PubMed] [Google Scholar]
- 10. Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori‐Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21:564–569. [DOI] [PubMed] [Google Scholar]
- 11. Endo S, Suzuki Y, Takahashi G, et al. Presepsin as a powerful monitoring tool for the prognosis and treatment of sepsis: a multicenter prospective study. J Infect Chemother. 2014;20:30–34. [DOI] [PubMed] [Google Scholar]
- 12. Koh H, Aimoto M, Katayama T, et al. Diagnostic value of levels of presepsin (soluble CD14‐subtype) in febrile neutropenia in patients with hematological disorders. J Infect Chemother. 2016;22:466–471. [DOI] [PubMed] [Google Scholar]
- 13. Chenevier‐Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens Y‐E. Presepsin (sCD14‐ST) in emergency department: the need for adapted threshold values? Clin Chim Acta. 2014;427:34–36. [DOI] [PubMed] [Google Scholar]
- 14. Nagata T, Yasuda Y, Ando M, et al. Clinical impact of kidney function on presepsin levels. PLoS ONE. 2015;10:e0129159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masaoka T. Management of fever of unknown origin in the neutropenic patient: the Japanese experience. Int J Hematol. 1998;68(Suppl 1):S9–S11. [PubMed] [Google Scholar]
- 16. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moon JM, Chun BJ. Predicting the complicated neutropenic fever in the emergency department. Emerg Med J. 2009;26:802–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura Y, Murai A, Mizunuma M, et al. Potential use of procalcitonin as biomarker for bacterial sepsis in patients with or without acute kidney injury. J Infect Chemother. 2015;21:257–263. [DOI] [PubMed] [Google Scholar]
- 19. Aimoto M, Koh H, Katayama T, et al. Diagnostic performance of serum high‐sensitivity procalcitonin and serum C‐reactive protein tests for detecting bacterial infection in febrile neutropenia. Infection. 2014;42:971–979. [DOI] [PubMed] [Google Scholar]
- 20. Koya J, Nannya Y, Ichikawa M, Kurokawa M. The clinical role of procalcitonin in hematopoietic SCT. Bone Marrow Transplant. 2012;47:1326–1331. [DOI] [PubMed] [Google Scholar]
- 21. Mussap M, Puxeddu E, Puddu M, et al. Soluble CD14 subtype (sCD14‐ST) presepsin in premature and full term critically ill newborns with sepsis and SIRS. Clin Chim Acta 2015;451:65–70. [DOI] [PubMed] [Google Scholar]


