Abstract
Background
Breast cancer (BC) is the most common neoplasm in women, with 5%‐10% patients showing a familial predisposition, where germline mutations in BRCA1/BRCA2 genes are found in –20% of cases. Next‐generation sequencing (NGS) is among the best available options for genetic screening, providing several benefits that include enhanced sensitivity and unbiased mutation detection. PALB2 (partner and localizer of BRCA2) is a cancer predisposing gene recently described that encodes a protein partner of BRCA2 involved in DNA double‐strand break repair and cell cycle control. The DNA damage response represents a key cellular event, targeted by innovative anticancer therapies, including those based on poly (ADP‐ribose) polymerase (PARP) inhibitors targeting PARP1 and PARP2 enzymes, activated by DNA damage and involved in single‐strand break and base excision repair.
Methods
Genomic DNA was isolated from 34 patient samples and four BC cell lines, as controls, and 27 breast cancer predisposing genes belonging to the BRCA1/BRCA2 and PARP pathways were sequenced by NGS.
Results
The panel described here allowed identification of several sequence variations in most investigated genes, among which we found a novel truncating mutation in PALB2.
Conclusions
The NGS‐based strategy designed here for molecular analysis of a customized panel of BC predisposing and related genes was found to perform effectively, providing a comprehensive exploration of all genomic sequences of the investigated genes. It is thus useful for BC molecular diagnosis, in particular for familiar cases where alterations in routinely investigated genes, such as BRCAs, result to be absent.
Keywords: Breast cancer, cancer gene panel, next‐generation sequencing, pathogenic variants
1. INTRODUCTION
Breast cancer (BC) is the most common cancer affecting women. In 5%‐10% of cases, a familial predisposition is found, up to 1/5th due to germline mutations in the BRCA1/BRCA2 genes.1 Women carrying BRCA mutations have a cumulative risk of 57%‐65% of developing BC by 70 years, in case of BRCA1, and 45%‐57% in case of BRCA2 mutations.2 Female BRCA mutation carriers also have an increased risk of developing ovarian cancer, with a cumulative risk by 70 years of 39%‐59% for BRCA1 and of 11%‐18% for BRCA2 carriers, respectively.3 Furthermore, BRCA1/BRCA2 carrier males have an increased risk of breast and prostate cancer.4 Genetic counseling and BRCA gene test are thus commonly recommended for BC patients with early onset, or with a significant family history, and their relatives.
Sequence variants in BRCA1 and BRCA2 genes characterized so far can be classified in three broad classes: single‐nucleotide changes, small insertion or deletion events (indels), and large genomic rearrangements (LGRs) occurring in proximity of Alu elements.5 BRCA2 gene contains less Alu sequences, which may explain how fewer rearrangements have been reported for this gene.6
Other non‐BRCA genes, such as ATM, BRIP1, PALB2, PTEN, and CHEK2, are reported to be medium‐to‐high‐penetrance susceptibility genes associated with hereditary BC.7, 8
PALB2 (partner and localizer of BRCA2, OMIM 610355) is located on chromosome 16p12.2 and is implicated in double‐strand break repair (DSB) and in cell cycle checkpoints. Recessive mutations in PALB2 are associated with Fanconi anemia, while dominant mutations are present in higher risk cases of breast and ovarian cancers, hereditary cancer syndromes, and familial pancreatic carcinomas.9, 10 The BC risk for female PALB2 mutation carriers ranges from 33% (95% CI, 25‐44) for those with no family history of BC to 58% (95% CI, 50‐66) for those with a family history.11 Given the growing number of genes involved in BC predisposition, comprehensive multiple gene sequencing in each single case is increasingly required to identify predisposing genetic factors.
Sanger sequencing is still considered the “gold standard” method in the diagnostic field, but next‐generation sequencing (NGS) is rapidly becoming a robust and effective alternative approach, thanks to its higher performance, increasing reliability and decreasing costs. Today, this technology is widely used for a variety of clinical genetic tests, including cell‐free DNA analysis for noninvasive prenatal testing, whole‐exome sequencing (WES), and targeted multigene panel sequencing for complex diseases, such as for example cardiomyopathies, epilepsy, congenital muscular dystrophy, and X‐linked intellectual disability.12, 13
The DNA damage response is a key pathway for the control of cell functions both in physiological and pathological conditions. Recently, innovative cancer therapies targeting this pathway are being used in clinical practice, including in particular those based on poly (ADP‐ribose) polymerase (PARP) inhibitors (PARPis).
PARP is a family of proteins with enzymatic scaffolding properties and recruiting ability for additional proteins required for DNA repair.14 PARP1 and PARP2, in particular, are critical for the function of base excision repair (BER). PARPis are mainly used for ovarian cancer therapy and are increasingly considered also against BRCA mutation‐associated and triple‐negative BCs, as sensitizers to DNA damaging chemotherapy.15 In addition, the use of inhibitory drugs is likely to be extended in the future also to target other mutated genes involved in DNA repair machinery. A necessary prerequisite, however, is the identification of the mutational spectra of several such genes in breast and other cancers. NGS is the best approach to allow a comprehensive view of the genomic status of a large number of genes in a single test. Here, we report the design and performances of a multigene custom panel for the molecular analysis of 27 BC risk‐associated genes, including BRCA1/BRCA2 and related DNA repair pathway, including PARP1 and PARP2 and components of their functional pathway. The use of this panel allowed the identification, among others, of 40 gene variations already associated with breast or other cancer risk, and one novel truncating mutation of PALB2 in a patient diagnosed with familiar BC not associated with BRCA gene mutations. These results show how simultaneous sequence analysis of multiple BC predisposing genes in a single patient increases the possibility to identify disease‐causing gene variants, a condition particularly important in all cases lacking mutations in commonly analyzed loci, as well as in mutation hot spots of such genes. The approach described here can also provide useful information to enlarge the spectrum of applications of current and future therapies based on PARPis and other drugs targeting the BRCA and PARP pathways.
2. MATERIAL AND METHODS
2.1. Samples
Samples from 34 patients and four cell lines were analyzed in total. Human samples derived from breast cancer patients observed from 1980 to 2012 at the Department of Clinical Medicine and Surgery, University of Naples Federico II, Naples, Italy. All patients were screened for family history of cancer and referred to the genetic counseling service conducted according to the model previously described.16 All the patients included in this retrospective analysis provided their informed consent in the framework of cancer genetic counseling program regulated and approved by the Local Ethic committee (University “Federico II” of Naples, Prot. 80/00 and 63/02). Samples were obtained in accordance with Declaration of Helsinki.
A blood EDTA sample was collected from each subject and genomic DNA was isolated from peripheral blood using either the Genomic DNA isolation kit (Norgen Biotek Corp., Thorold, ON, Canada), according to the manufacturer's instructions, or the salting out method.
As positive controls, DNA extracted from human breast carcinoma (Hs 578T; ATCC® HTB‐126™), acantholytic squamous cell carcinoma (HCC1806; TCC® CRL‐2335™), ductal carcinoma (HCC1937; ATCC® CRL‐2336™), and adenocarcinoma (MDA‐MB‐468; ATCC® HTB‐132™) cells were used.
Purity of DNA was assessed by spectrophotometry using NanoDrop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). DNA yields were determined using a double‐stranded DNA (dsDNA) broad range (BR) kit on a Qubit®fluorometer (Thermo Fisher Scientific). Integrity of DNA was evaluated by Bioanalyzer DNA 12000 kit (Agilent Technologies, CA, USA).
2.2. Targeted capture library preparation and sequencing
Library preparation was performed with Illumina Nextera Rapid Custom Capture Enrichment (NRCCE) protocol, using our custom panel designed to test 27 genes involved in DNA repair pathways, including BRCA1/BRCA2 and PARP1/PARP2 (Table S1). The Nextera Rapid Capture Custom Enrichment panel was designed using the Illumina Design Studio sequencing assay design tool; 50 ng genomic DNA was used as input and libraries were generated with the Nextera Rapid Capture Enrichment protocol, according to the manufacturer's instructions. Briefly, DNA samples were simultaneously tagmented by the Nextera transposome and ligated to sequencing adapters. Dual Nextera sequencing indices were then attached to each of the tagmented samples by amplification. Samples were then normalized and pooled for sequencing, such that each library contained 500 ng of each uniquely indexed sample. Then, two consecutive hybridizations were performed, the first one lasting 2 hours and a second one overnight (up to 15 hours) using custom oligonucleotide probes. Libraries were captured using streptavidin‐conjugated magnetic beads, and a second PCR amplification was performed. Following quantification with a Qubit High Sensitivity kit (Thermo Fisher Scientific), each library was analyzed with the Agilent 2200 TapeStation to determine the average size of the enriched fragments. Each library was normalized to 2 nM with dilutions in 10 mM Tris‐HCl buffer (pH 8.5) with 0.1% Tween 20, and the concentration was then adjusted to 16 pM before cluster generation and sequencing on a MiSeq instrument (Illumina, CA, USA). Paired‐end sequencing was performed using the MiSeq Reagent Kit v3, with a read length of 300 bases.
For validation of the results relative to BRCA1/BRCA2 genes, the TruSeq Custom kit, (Illumina) specific for the target regions of BRCA1 and BRCA2, was also used, following the manufacturer's instructions.
2.3. PCR and Sanger sequencing of PALB2 exon 12
For validation of the newly identified PALB2 c.3231delC mutation, Sanger sequencing of both DNA strands of the region of interest was performed, using the following primer pairs (same as for the PCR reaction): 5′‐ agcctatcggtcattgcttt ‐3′ (forward) and 5′‐ agggaatctggggtttgact ‐3′ (reverse) for exon 12. PCR was performed by FastStart Taq DNA Polymerase kit (Roche Life Science, Indiana, USA), and sequencing was carried out with a CEQ2000XL DNA analysis system apparatus (Beckman) by the Molecular Biology Service of Stazione Zoologica “Anton Dohrn” (Naples, Italy).
2.4. Bioinformatic analysis
Demultiplexing, FASTQ files generation, and alignment against the reference were performed with BWA software. Data were then processed with GATK's Unified Genotyper for variant calling to generate VCF files (variant files), which were further filtered and annotated against dbSNP (version 137 in this study).
Data were then analyzed with the optimized pipeline Isaac Enrichment App v2.1 from the Illumina's cloud genomics platform (https://basespace.illumina.com). Alignment was made against the human reference sequence build GRCh37/Hg19. Finally, the variant files were analyzed by VariantStudio Analysis software for annotation and filtering of the generated variants. Only passing filter data and read depth ≥50 were taken into account.
3. RESULTS
Thirty‐four patients, selected as previously described,17 and four cell lines, as positive controls, were analyzed in this study. With the aim of identifying gene sequence variations in BC samples from patients with and without BRCA gene mutations, we designed a multigene custom panel including 27 genes specifically involved in DNA damage repair pathways; 50 ng of input DNA was used for library preparation and sequencing as detailed in Material and methods. A detailed summary of characteristics, metrics, and information of the Nextera multigene panel used is shown in Table S1. On average, the sequence of selected genes was covered between 1700‐fold and 2500‐fold.
Four cell lines were analyzed as control samples, and the obtained results confirmed the genetic status reported on each technical sheet (ATCC, https://www.lgcstandards-atcc.org/). In detail, for HCC1937, we identified the BRCA1 c.5329dupC (p.Gln1777ProfsTer74) and TP53 c.916C>T (p.Arg306Ter); for MDA‐MB‐468, the PTEN c.253 + 1G>T (splice donor variant) and the TP53 c.818G>A (p.Arg273His); for HCC1806, the TP53 c.766_767insAA (p.Thr256LysfsTer90), and for Hs 578T, the TP53 c.469G>T (p.Val157Phe).
Globally, the designed test led to the identification of about 40 gene sequence variations, as detailed in Table S2, most of them being already described as BC predisposing ones, including all those previously identified in the same patients by Sanger sequencing of BRCA1 and BRCA2. In particular, we first evaluated sequence quality at the BRCA loci, finding 15 known variations in different patients. These were confirmed with independent tests performed by NGS with the TruSeq Custom amplicon kit and by Sanger sequencing (data not shown). Interestingly, among the several variations identified in the tested genes, a novel mutation in PALB2 gene (c.3231delC; p.Cys1078ValfsTer17) was found in one patient. The mutation occurs within exon 12 and causes a premature stop codon, with a deleterious effect of gene activity as predicted by MutationTaster tool.18 The mutation was confirmed by Sanger sequencing (Figure 1).
Figure 1.
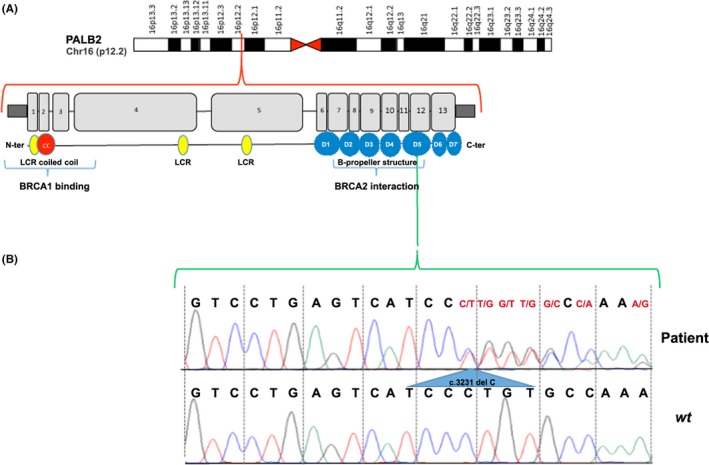
Identification of a novel mutation in the PALB2 gene. A, Schematic representation of PALB2 gene. B, Sanger sequencing electropherogram showing the mutated genotype c.3231delC in exon 12 of PALB2 gene in the patient (up) with respect to wt (down)
4. DISCUSSION
Cancer is a condition with significant genetic heterogeneity, for which NGS‐based testing has been demonstrated to be an efficient analytical method, as it allows simultaneous sequence analysis of multiple loci of interest in a single reaction.19 Indeed, commercial diagnostic panels for cancer, most of them investigating genes correlated with Lynch syndrome, cancerous syndromes, and other cancer types (such as kidney, pancreas, and colon), are already available for use in the clinical setting.
Two major high‐penetrance genes, BRCA1 and BRCA2, are involved in hereditary BC and when mutated raise the risk also of ovarian, and possibly other, cancer. When a family history of BC is present, it is conceivable to believe that a person has inherited a mutated gene linked to higher BC risk.20, 21, 22 For this reason, nowadays, genetic testing for screening and/or diagnostic purposes is widely used.
As new genes predisposing to BC are constantly being found, including also several ones involved in DNA damage repair,23 the use of NGS‐based test for panels of genes is increasing in clinical diagnostics to increase the possibility to detect a wider spectrum of mutations in BC patients, respect to those obtained when focusing on BRCA1/BRCA2 genes alone. On the other hand, identification of new germline mutations in additional “candidate” genes increases the required knowledge concerning cancer predisposition in humans, although at the same time, it raises several questions concerning patient management in such cases. Starting from these considerations, we focused here on an approach to screen in a single reaction a set of genes strictly implicated in the DNA repair pathway. To this end, we designed a custom NGS panel focusing on this class of genes, including also those not routinely screened. Results show that the analytical method devised performs well and can provide the required answers. Indeed, among the numerous variants identified, several are likely to be associated with BC risk (Table S2). For example, we found the XRCC1 rs1799782 (7 patients) with a prediction of damaging effects on gene function (SIFT, score 0, and Polyphen, score 0.995) but with no clinical annotation in the dbSNP database. Interestingly, this variant has been described to be associated with the response to platinum‐based neoadjuvant chemotherapy in patients with cervical cancer.24
Another drug response variant, found in 15 of the cases analyzed here, is TP53 rs1042522, that has been reported to represent independently minor, but cumulatively significant, increased risk for breast and endometrial cancer. The GG genotype is more likely resistant to first‐line chemotherapy in nonsmall cell lung cancer, in particular to the irinotecan plus cisplatin regimen, than CG or CC genotypes (60% vs 27%, P = .014). In addition, GG and CG genotypes were significantly correlated with a lower response rate (and worse progression) to a combination chemotherapy in gastric cancer, when compared to rs1042522 (CC) homozygotes (35.7 vs 66.7%, P‐value 0.019).25, 26
One patient carried the c.1366G>A transition in TERF2 (p.Glu456Lys, rs150757154), reported as a variation causing potential impaired function (prediction score ≥0.50) by Wang and colleagues.27 On the other hand, the BARD1 rs1048108 identified in 4 of 34 cases studied and has been associated with medium‐high risk of sporadic neuroblastoma28 and low BC risk,29 and the TOpBP1 rs55633281 found in 8 of 34 cases, located in close proximity to the transactivation domain of the encoded protein, might be critical for cellular functions under stress conditions.30 We also found several variants with conflicting interpretation of pathogenicity and/or lacking sufficient information for classification, such as BLM rs183176301 (2 of 34 cases), CHEK2 rs200050883 (1 of 34 cases), FAM175A rs12642536 (11 of 34 cases), and RAD51C rs773998134 (1 of 34 cases).
More interestingly, we found a new truncating mutation in PALB2 gene (c.3231delC) in one patient suffering with familiar BC but lacking BRCA1 or BRCA2 gene mutations (Figure 1 and Table S2). Interestingly, the BC risk for PALB2 mutation carriers may overlap that of BRCA2 mutation carriers.11 Catucci and colleagues31 screened 575 probands from Italian breast cancer families negative for BRCA12 mutations and found that 2.1% had deleterious mutations in PALB2. One of these was a nonsense mutation that was recurrent in the province of Bergamo in northern Italy (Q343X), and it has been suggested that mutations in this gene are population‐specific and occur with low frequencies in hereditary BC.32 These evidence suggest that screening for PALB2 gene mutations should be introduced in clinical practice, considering the high prevalence of such mutations in BRCA1/BRCA2‐negative BC patients, that suggest this gene as a strong candidate for clinical testing in such cases.33
It is worth mentioning that a novelty in the NGS gene panel test described here is represented by its specificity for DNA repair genes, including in particular PARP1 and PARP2 that are targets of new generation anticancer therapies with PARPis. These drugs have been demonstrated to be more effective in patients with germline BRCA1/BRCA2 mutation‐associated ovarian and, possibly, breast cancers.15, 34 Recently, the European Commission and the US Food and Drug Administration approved the use of the PARPi olaparib (Lynparza™, AstraZeneca) in two different clinical indications for patients with recurrent BRCA‐mutated advanced ovarian cancer.35, 36 Unfortunately, a significant number of such tumors becomes resistant to this drug, with different molecular mechanisms that include also loss of PARP1 catalytic activity, and thereby efficacy of the inhibitor. Cancer cells with normal levels of PARP1 protein but decreased enzyme activity, as noted by reduced level of endogenous PARylation, are in fact more resistant to PARPis,37 whereas HR‐deficient tumor cells with higher endogenous PARylation activity are more sensitive to these drugs.38 Furthermore, certain PARP1 gene variants with decreased catalytic activity could make cancer cells resistant to PARPis and certain SNPs, such as p.V762A39 or p.M129T and p.E251K,40 have been hypothesized to modify enzymatic activity. Of note, almost 50% of our patients carry the rs1136410 variant, in one case in homozygosis.
The genetic analysis of PARP1 and PARP2 genes on a higher number of patients, as well as identification of somatic mutations of these genes in cancer biopsies—both possible with the assay described here—will help clarify these key issues, thereby improving the clinical management of patients carrying such variations. The importance of testing DNA repair pathway genes to assess the clinical relevance of PARPis as targeted therapy is confirmed also by the fact that a clinical trial concerning the possible use of olaparib in cancer patients with genetic defects in DNA repair genes has been recently approved as is underway (NCT03375307). Patients eligible for this trial should present pathogenic variations in one or more DNA repair genes, most of which, including PALB2, can be analyzed with the assay described here.
Supporting information
ACKNOWLEDGMENTS
This work is supported by Italian Ministry of Education University and Research (PON03PE_00146_1 and Flagship Project InterOmics), Italian Association for Cancer Research (AIRC, Grant IG‐17426), and Genomix4Life Srl.
Guacci A, Cordella A, Rocco T, et al. Identification of a novel truncating mutation in PALB2 gene by a multigene sequencing panel for mutational screening of breast cancer risk‐associated and related genes. J Clin Lab Anal. 2018;32:e22418 10.1002/jcla.22418
Anna Guacci and Angela Cordella contributed equally to this work.
Contributor Information
Roberta Tarallo, Email: rtarallo@unisa.it.
Alessandro Weisz, Email: aweisz@unisa.it.
REFERENCES
- 1. Lux MP, Fasching PA, Beckmann MW. Hereditary breast and ovarian cancer: review and future perspectives. J Mol Med (Berl). 2006;84:16‐28. [DOI] [PubMed] [Google Scholar]
- 2. Mavaddat N, Peock S, Frost D, et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: results from prospective analysis of EMBRACE. J Natl Cancer Inst. 2013;105:812‐822. [DOI] [PubMed] [Google Scholar]
- 3. Antoniou A, Pharoah PD, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liede A, Karlan BY, Narod SA. Cancer risks for male carriers of germline mutations in BRCA1 or BRCA2: a review of the literature. J Clin Oncol. 2004;22:735‐742. [DOI] [PubMed] [Google Scholar]
- 5. Smith TM, Lee MK, Szabo CI, et al. Complete genomic sequence and analysis of 117 kb of human DNA containing the gene BRCA1. Genome Res. 1996;6:1029‐1049. [DOI] [PubMed] [Google Scholar]
- 6. Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet. 2001;10:705‐713. [DOI] [PubMed] [Google Scholar]
- 7. Couch FJ, Shimelis H, Hu C, et al. Associations between cancer predisposition testing panel genes and breast cancer. JAMA Oncol. 2017;3:1190‐1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Afghahi A, Telli ML, Kurian AW. Genetics of triple‐negative breast cancer: implications for patient care. Curr Probl Cancer. 2016;40:130‐140. [DOI] [PubMed] [Google Scholar]
- 9. Tischkowitz M, Xia B. PALB2/FANCN: recombining cancer and Fanconi anemia. Cancer Res. 2010;70:7353‐7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nepomuceno TC, De Gregoriis G, de Oliveira FMB, Suarez‐Kurtz G, Monteiro AN, Carvalho MA. The Role of PALB2 in the DNA Damage Response and Cancer Predisposition. Int J Mol Sci 2017;18:1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Antoniou AC, Casadei S, Heikkinen T, et al. Breast‐cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy JJ, McLeod HL, Ginsburg GS. Genomic medicine: a decade of successes, challenges, and opportunities. Sci Transl Med 2013;5:189sr184. [DOI] [PubMed] [Google Scholar]
- 13. Rabbani B, Mahdieh N, Hosomichi K, Nakaoka H, Inoue I. Next‐generation sequencing: impact of exome sequencing in characterizing Mendelian disorders. J Hum Genet. 2012;57:621‐632. [DOI] [PubMed] [Google Scholar]
- 14. Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG. PARP inhibition: PARP1 and beyond. Nat Rev Cancer. 2010;10:293‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Telli ML, Ford JM. PARP inhibitors in breast cancer. Clin Adv Hematol Oncol. 2010;8:629‐635. [PubMed] [Google Scholar]
- 16. Contegiacomo A, Pensabene M, Capuano I, et al. An oncologist‐based model of cancer genetic counselling for hereditary breast and ovarian cancer. Ann Oncol. 2004;15:726‐732. [DOI] [PubMed] [Google Scholar]
- 17. Arpino G, Pensabene M, Condello C, et al. Tumor characteristics and prognosis in familial breast cancer. BMC Cancer. 2016;16:924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwarz JM, Rodelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease‐causing potential of sequence alterations. Nat Methods. 2010;7:575‐576. [DOI] [PubMed] [Google Scholar]
- 19. Shah PD, Nathanson KL. Application of Panel‐Based Tests for Inherited Risk of Cancer. Annu Rev Genomics Hum Genet. 2017;18:201‐227. [DOI] [PubMed] [Google Scholar]
- 20. Ford D, Easton DF, Bishop DT, Narod SA, Goldgar DE. Risks of cancer in BRCA1‐mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994;343:692‐695. [DOI] [PubMed] [Google Scholar]
- 21. Breast Cancer Linkage Consortium . Cancer risks in BRCA2 mutation carriers. J Natl Cancer Inst. 1999;91:1310‐1316. [DOI] [PubMed] [Google Scholar]
- 22. Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA. 2017;317:2402‐2416. [DOI] [PubMed] [Google Scholar]
- 23. Ali R, Rakha EA, Madhusudan S, Bryant HE. DNA damage repair in breast cancer and its therapeutic implications. Pathology. 2017;49:156‐165. [DOI] [PubMed] [Google Scholar]
- 24. Kim K, Kang SB, Chung HH, Kim JW, Park NH, Song YS. XRCC1 Arginine194Tryptophan and GGH‐401Cytosine/Thymine polymorphisms are associated with response to platinum‐based neoadjuvant chemotherapy in cervical cancer. Gynecol Oncol. 2008;111:509‐515. [DOI] [PubMed] [Google Scholar]
- 25. Han JY, Lee GK, Jang DH, Lee SY, Lee JS. Association of p53 codon 72 polymorphism and MDM2 SNP309 with clinical outcome of advanced nonsmall cell lung cancer. Cancer. 2008;113:799‐807. [DOI] [PubMed] [Google Scholar]
- 26. Kim JG, Sohn SK, Chae YS, et al. TP53 codon 72 polymorphism associated with prognosis in patients with advanced gastric cancer treated with paclitaxel and cisplatin. Cancer Chemother Pharmacol. 2009;64:355‐360. [DOI] [PubMed] [Google Scholar]
- 27. Wang X, Szabo C, Qian C, et al. Mutational analysis of thirty‐two double‐strand DNA break repair genes in breast and pancreatic cancers. Cancer Res. 2008;68:971‐975. [DOI] [PubMed] [Google Scholar]
- 28. Capasso M, Devoto M, Hou C, et al. Common variations in BARD1 influence susceptibility to high‐risk neuroblastoma. Nat Genet. 2009;41:718‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu H, Zhang H, Sun X, He Y, Li J, Guo X. A cross‐sectional study of associations between nonsynonymous mutations of the BARD1 gene and breast cancer in Han Chinese women. Asia Pac J Public Health. 2013;25:8S‐14S. [DOI] [PubMed] [Google Scholar]
- 30. de Jesus Perez VA, Yuan K, Lyuksyutova MA, et al. Whole‐exome sequencing reveals TopBP1 as a novel gene in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;189:1260‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Catucci I, Peterlongo P, Ciceri S, et al. PALB2 sequencing in Italian familial breast cancer cases reveals a high‐risk mutation recurrent in the province of Bergamo. Genet Med. 2014;16:688‐694. [DOI] [PubMed] [Google Scholar]
- 32. Hellebrand H, Sutter C, Honisch E, et al. Germline mutations in the PALB2 gene are population specific and occur with low frequencies in familial breast cancer. Hum Mutat. 2011;32:E2176‐E2188. [DOI] [PubMed] [Google Scholar]
- 33. Janatova M, Kleibl Z, Stribrna J, et al. The PALB2 gene is a strong candidate for clinical testing in BRCA1‐ and BRCA2‐negative hereditary breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:2323‐2332. [DOI] [PubMed] [Google Scholar]
- 34. Lee JM, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation‐associated and BRCA‐like malignancies. Ann Oncol. 2014;25:32‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. LYNPARZA™ approved by the US Food and Drug Administration for the treatment of advanced ovarian cancer in patients with germline BRCA‐mutations [database on the Internet]. http://www.astrazeneca.com/ Media/Press‐releases/Article/20141219–lynparzaapproved. Accessed 19, December 2014.
- 36. LYNPARZA™ approved in the European Union as first‐in‐class treatment for advanced BRCA‐mutated ovarian cancer [database on the Internet]. http://www.astrazeneca.com/Media/Press-releases/Article/201420141218-lynparza-approved-in-the-european-union. 2015. Accessed December 18, 2014.
- 37. Oplustilova L, Wolanin K, Mistrik M, et al. Evaluation of candidate biomarkers to predict cancer cell sensitivity or resistance to PARP‐1 inhibitor treatment. Cell Cycle. 2012;11:3837‐3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gottipati P, Vischioni B, Schultz N, et al. Poly(ADP‐ribose) polymerase is hyperactivated in homologous recombination‐defective cells. Cancer Res. 2010;70:5389‐5398. [DOI] [PubMed] [Google Scholar]
- 39. Zaremba T, Ketzer P, Cole M, Coulthard S, Plummer ER, Curtin NJ. Poly(ADP‐ribose) polymerase‐1 polymorphisms, expression and activity in selected human tumour cell lines. Br J Cancer. 2009;101:256‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogino H, Nakayama R, Sakamoto H, Yoshida T, Sugimura T, Masutani M. Analysis of poly(ADP‐ribose) polymerase‐1 (PARP1) gene alteration in human germ cell tumor cell lines. Cancer Genet Cytogenet. 2010;197:8‐15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


