Abstract
Background
Ongoing efforts in the development of HBsAg detection kits are focused on improving sensitivity and specificity. The purpose of this study was to evaluate an improved, highly sensitive quantitative assay, “Lumipulse HBsAg‐HQ”, a chemiluminescent enzyme immunoassay designed for a fully automated instrument, the “Lumipulse G1200”.
Methods
Serum samples for reproducibility, dilution, correlation, sensitivity, and specificity studies were obtained from patients at the Osaka University Hospital. Seroconversion and sensitivity panels were purchased from a commercial vender. Subtype, sensitivity panels, and HBsAg recombinant proteins with one or two amino acid substitutions were prepared in‐house.
Results
The coefficients of variation for the low, medium, and high concentration samples ranged from 1.93 to 2.55%. The HBsAg‐HQ reagent for dilution testing showed good linearity in the 0.005‐150 HBsAg IU/mL range and no prozone phenomenon. All 102 HBV carrier samples were positive by HBsAg‐HQ, while other commercial reagents showed one or more to be negative. In the seroconversion panel, the 14‐day blood sample was positive. The sensitivity against HBsAg‐HQ “ad” and “ay” subtypes was 0.025 ng/mL. Comparisons among the HBsAg‐HQ, HISCL, and Architect HBsAg reagents were performed using the Bland‐Altman plot. Specificity for 1000 seronegative individuals was 99.7%. HBsAg‐HQ detected 29 positive serum among 12 231 routinely obtained serum samples, which showed concentrations of 0.005‐0.05 HBsAg IU/mL.
Conclusions
According to these results, the Lumipulse HBsAg‐HQ assay, with a highly sensitive limit of detection of 0.005 IU/mL, may facilitate the development of a better management strategy for a considerable proportion of infected patients.
Keywords: chemiluminescent enzyme immunoassay, HBsAg, hepatitis B virus, immunoassay, Lumipulse
1. INTRODUCTION
Today, more than 400 million people worldwide are persistently infected with hepatitis B virus (HBV). To deal with this major health problem and to manage the therapy of HBV carriers, HBV DNA, hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), and hepatitis B core‐related antigen (HBcrAg) are used as HBV screening or monitoring markers.
HBsAg is the glycosylated envelope protein of the mature HBV virion and two noninfectious forms, the spherical and filamentous subviral particles. HBsAg is also the established serological marker for detecting acute HBV infection and monitoring chronic HBV infection. The HBV DNA level of hepatitis B carriers who are treated by nucleot(s)ide analogs can fall below the limit of detection. Quantitative HBsAg assay has been proposed recently to be useful for monitoring the course of chronic HBV infection, including the immune tolerance, immune clearance, and immune control/inactive carrier phases, as well as reactivated hepatitis B e antigen‐negative disease.1, 2 Therefore, HBsAg assays with high sensitivity coupled with sufficient specificity are required for diagnostic screening and monitoring, to minimize the number of false‐positive and false‐negative results of samples from patients undergoing treatment with nucleot(s)ide analogs.
The Japan Society of Hepatology recently established guidelines for the detection and medical treatment of HBV infection; three qualitative and three quantitative HBsAg assay reagents for HBsAg measurement were introduced in these guidelines.3
In this study, we evaluated the highly sensitive Lumipulse HBsAg‐HQ (HBsAg‐HQ) assay (Fujirebio Inc., Tokyo, Japan), which consists of a pretreatment solution for sample disruption and monoclonal antibodies against inner and outer epitopes of the HBsAg molecule.4, 5, 6, 7, 8 We evaluated the assay with regard to viral mutations, because studies have shown that mutations in the HBsAg gene that are located near the major “a” determinant or in the regulatory elements of surface genes may affect the antigenic profiles of hepatitis B viruses.9, 10, 11, 12
2. MATERIAL AND METHODS
2.1. Lumipulse HBsAg‐HQ reagents
Monoclonal antibodies against the inner and outer portions of purified HBsAg were prepared according to conventional procedures. The solid phase consists of ferrite particles (0.3 μm diameter; Nippon Paint Co. Ltd., Tokyo, Japan) conjugated to antibodies against the outer and inner portion of HBsAg and coated with bovine serum albumin (Oriental Yeast Co. Ltd., Tokyo, Japan). Commercial alkaline phosphatase (Oriental Yeast Co.Ltd) is conjugated to anti‐HBsAg antibodies against the outer epitopes of HBsAg, that is, the loop structure of the “a” determinant. The chemiluminescent substrate is 3‐(2′‐spiroadamantane)‐4‐methoxy‐4‐(3′‐phosphoryloxy) phenyl‐1, 2‐dioxetane (AMPPD disodium salt).
2.2. Specimens and panels
This study was approved by the Institutional Review Board of the Osaka University School of Medicine. Serum samples for the evaluation of assay reagents were obtained from hospitalized patients or outpatients at the Osaka University Hospital (the tenets of the Declaration of Helsinki were observed and the study had the approval of the Internal Review Board). Serum samples were collected from 102 HBV‐infected patients, who were determined to be HBV carriers and also were positive for other markers of HBV infection or had been undergoing follow‐up observations for hepatitis and cirrhosis. Three pooled control panels were prepared using routinely tested serum samples, classified as high, middle, and low concentrations panels.
Commercially available HBsAg seroconversion (PHA935A) and HBsAg sensitivity (PHA807) panels were purchased from SeraCare Life Science, Inc. (Buffalo, NY, USA).
2.3. HBsAg variants
HBsAg variants were prepared as follows: plasmids expressing wild‐type small HBsAg (genotype C, serotype adr, GenBank access number AB033550) and 22 types of small recombinant HBsAg variants were expressed in COS7 cells using the expression vector pcDNA3.1 and PolyMag neo as transfection reagents. The culture fluid was collected and used to assess the ability of the HBsAg‐HQ assay to detect HBsAg variants. The amino acid positions and substitutions for the 22 HBsAg variants are shown in Table 6, under the column with the heading “HBsAg variant”. The HBsAg variants No. 1 through No. 20 had one amino acid substitution and No. 21 and 22 had two amino acid substitutions.
Table 6.
Reactivity against HBsAg with amino acid substitutions
| No. | HBsAg variant | Lumipulse HBsAg‐HQ | Architect HBsAg QT | ||||
|---|---|---|---|---|---|---|---|
| IU/mL | COI | % | IU/mL | COI | % | ||
| Wild‐type | 6.773 | 1354.5 | – | 8.00 | 160.0 | – | |
| 1 | P111T | 1.698 | 339.5 | 25 | 9.04 | 180.8 | 113 |
| 2 | T118K | 0.320 | 63.9 | 5 | 12.06 | 241.2 | 151 |
| 3 | P120Q | 0.507 | 101.3 | 7 | 3.62 | 72.4 | 45 |
| 4 | P120T | 1.459 | 291.7 | 22 | 4.34 | 86.8 | 54 |
| 5 | I126S | 6.542 | 1308.4 | 97 | 6.01 | 120.2 | 75 |
| 6 | Q129H | 6.249 | 1249.8 | 92 | 7.23 | 144.6 | 90 |
| 7 | M133L | 8.184 | 1636.9 | 121 | 10.64 | 212.8 | 133 |
| 8 | F134A | 1.657 | 331.4 | 24 | 3.43 | 68.6 | 43 |
| 9 | P135S | 0.453 | 90.6 | 7 | 3.31 | 66.2 | 41 |
| 10 | K141E | 0.687 | 137.4 | 10 | 1.87 | 37.4 | 23 |
| 11 | P142L | 4.258 | 851.5 | 63 | 1.45 | 29.0 | 18 |
| 12 | P142S | 13.645 | 2729.1 | 201 | 3.30 | 66.0 | 41 |
| 13 | S143L | 4.833 | 966.7 | 71 | 4.62 | 92.4 | 58 |
| 14 | D144A | 4.157 | 831.3 | 61 | 5.07 | 101.4 | 63 |
| 15 | D144E | 5.080 | 1016.0 | 75 | 4.12 | 82.4 | 52 |
| 16 | G145R | 5.473 | 1094.5 | 81 | 6.21 | 124.2 | 78 |
| 17 | G145K | 2.877 | 575.4 | 42 | 3.57 | 71.4 | 45 |
| 18 | T148H | 2.491 | 498.2 | 37 | 10.20 | 204.0 | 128 |
| 19 | S154W | 4.312 | 862.5 | 64 | 4.92 | 98.4 | 62 |
| 20 | S155Y | 3.719 | 743.8 | 55 | 4.76 | 95.2 | 60 |
| 21 | I126S + G145R | 3.280 | 655.9 | 48 | 3.26 | 65.2 | 41 |
| 22 | D144A + G145R | 1.284 | 256.7 | 19 | 1.26 | 25.2 | 16 |
2.4. Assay protocol of HBsAg‐HQ
The HBsAg‐HQ quantitative assay, which is based on an assay reported previously,5, 8 was used to quantify HBsAg in serum specimens using the automated chemiluminescent enzyme immunoassay instrument (LUMIPULSE G1200; Fujirebio Inc.).6 This assay system has a number of improvements over the conventional, qualitative Lumipulse HBsAg assay: (i) the samples are first processed with a “pretreatment solution” that includes a surfactant to disrupt the HBV particles and linearize HBsAg, and (ii) the antibody conjugated to the ferrite particles was changed from a polyclonal antibody to two monoclonal antibodies (HBs5C3 and HBs163), one against the external structural region as determinant “a”, and the other against an internal epitope as a capture reagent.
The sample (100 μL) or calibrator (100 μL) was added to 20 μL of pretreatment solution and mixed. The mixture was added to the HBsAg‐HQ ferrite particle reagent (250 μL), and then incubated at 37°C for 10 minutes. The ferrite particles were separated magnetically and washed three times with a washing solution.
The particles were then added to 250 μL of the alkaline phosphatase‐conjugated anti‐HBsAg antibody reagent and incubated at 37°C for 10 minutes. After the particles were separated and washed, 200 μL of AMPPD was added and the mixture was incubated at 37°C for 5 minutes. The chemiluminescent emission of the mixture was then quantified. This two‐step assay was carried out using the automated chemiluminescent enzyme immunoassay instrument, LUMIPULSE G1200.
This study used five commercially available, quantitative and qualitative assays (Lumipulse HBsAg‐HQ, Architect HBsAg QT, HISCL HBsAg, Lumipulse HBsAg, and ADVIA Centaur HBsAg II) in accordance with the manufacturers’ instructions, as shown in Table 1, (partially modified from table 11 from The Japanese Society of Hypertension Guidelines for the management of hepatitis B virus infection.3 The Cobas ECLusys HBsAg II assay was not evaluated in this study.3 The dynamic range of each quantitative assay was as follows: HBsAg‐HQ, 0.005‐150 IU/mL, Architect HBsAg QT (ARCHITECT; Abbott Japan, Co. Ltd), 0.05‐250 IU/mL and HISCL HBsAg (HISCL; Sysmex Co. Ltd.), 0.03‐2500 IU/mL. The range of each qualitative assay was as follows: Lumipulse HBsAg (Fujirebio Inc.), 0.1‐2000 cut‐off index [COI] and ADVIA Centaur HBsAg II (ADVIA‐II; Siemens Healthcare Diagnostics, Erlangen, Germany), 0.1‐1000 Index.
Table 1.
Characteristics of assays used for quantitative HBsAg measurement
| Device Trade Name (abbreviation in this study) | Lumipulse HBsAg‐HQ | Architect HBsAg QT | HISCL HBsAg | Lumipulse HBsAg | ADVIA Centaur HBsAg II |
|---|---|---|---|---|---|
| Manufacturer | Fujirebio Inc. | Abbott Japan Co. Ltd. | Sysmex Co. Ltd. | Fujirebio Inc. | Siemens Healthcare Diagnostics Inc. |
| Principle of operation | CLEIA | CLIA | CLEIA | CLEIA | CLIA |
| Unit | IU/mL (quantitative) | IU/mL (quantitative) | IU/mL (quantitative) | COI (qualitative) | COI (qualitative) |
| Capture antibodies | Monoclonal (two) | Monoclonal (two) | Monoclonal (various) | Polyclonal | Monoclonal |
| Conjugate antibodies | Monoclonal (two) | Polyclonal | Monoclonal (various) | Monoclonal (two) | Monoclonal |
| Reaction time (min) | 30 | 30 | 17 | 30 | 30 |
| Sample volume (μL) | 100 | 75 | 20 | 100 | 100 |
| Positive criterion | ≥0.005 IU/mL | ≥0.05 IU/mL | ≥0.03 IU/mL | COI ≥ 1.0 | COI ≥ 1.0 |
| Measuring range (theoretical value range) | 0.005‐150 IU/mL (auto dilution) | 0.05‐250 IU/mL (manual/auto dilution) | 0.03‐2500 IU/mL (auto dilution) | 0.1‐2000 COI | 0.1‐1000 Index |
CLEIA, Chemiluminescent Enzyme Immuno Assay; CLIA, Chemiluminescent Immuno Assay.
2.5. Performance evaluation testing
Within‐run reproducibility (n = 10) of the HBsAg‐HQ assay was assessed using three serum control panels, as described above, and the mean, SD, and CV were calculated for each panel.
Serum samples with high concentrations of HBsAg, which were obtained from hospitalized patients or outpatients at Osaka University Hospital, were combined to yield a pool containing approximately 160 000 HBsAg IU/mL. This pool was used to prepare a dilution series, based on 2‐fold serial dilutions. The HBsAg titers of the dilution series were measured by the Lumipulse HBsAg‐HQ assay in order to evaluate reagent performance in terms of dilution linearity.
HBsAg specimens were prepared at 0.0052, 0.0041, 0.0033, 0.0021, 0.0016, 0.0011, 0.0008, 0.0006, and 0 IU/mL, diluted using a solution containing BSA, and HBsAg‐HQ testing was performed 20 times using these samples to measure the analytical sensitivity.
Serum samples from 102 HBV‐infected patients as described above were tested using the HBsAg‐HQ and four commercial HBsAg assay kits. The samples were judged to be positive according to each manufacturer's instructions and assays.
The analytical sensitivities of the five commercial HBsAg assays, including the HBsAg‐HQ, were tested against the HBsAg seroconversion panel, PHA935A (SeraCare Life Science, Inc.). Panel PHA935A consists of serial blood specimens from an individual donor during the seroconversion phase to HBV positivity, and includes specimens before HBsAg is detectable as shown in the technical sheet for PHA935A.
The reactivities of the five commercially available HBsAg assays were tested against the HBsAg subtype panel, PHA807 (SeraCare Life Science, Inc.), which comprises a series of specimens and was diluted using the HBsAg‐HQ diluent. Two dilution series of PH807 to the concentration of 0.1 ng/mL of HBsAg, and further dilution by a diluent (indicated as dil. in the Table below) were prepared.
Assessments of samples of wild‐type HBsAg and HBsAg with amino acid substitutions, as described above, were performed. The assay results were expressed in IU/mL and ratio (%) against wild‐type antigen as shown in Table 6.
The specificity study was performed using 1000 serum samples obtained from patients negative for HBsAg/anti‐HBs and anti‐HBc. Neutralization testing of three samples found positive with the HBsAg‐HQ assay was carried out following the manufacturer's instructions.
Serum samples were obtained from 46 HBV‐infected patients, and the comparison of HBsAg values obtained by the three assays was performed using the Bland‐Altman plot.
A total of 12 231 samples were collected from the routine testing program of the clinical laboratory in Osaka University hospital. These 12 231 samples were tested and the assay values were distributed into nine groups as follows: less than 0.005, 0.005‐0.05, 0.05‐0.5, 0.5‐5, 5‐50, 50‐500, 500‐5000, 5000‐15 000, and greater than 15 000 IU/mL HBsAg. Samples with low levels of HBsAg were retested and/or underwent neutralization testing following the manufacturer's instructions.
Confirmation of positive results from the HBsAg‐HQ reagent was carried out using the Lumipulse HBsAg‐HQ confirmation reagent. One tenth volume of neutralization solution was added to the specimen (A). As a control, a diluent of 1/10 volume not containing the neutralizing component was added to the specimen (B). Each mixture was left at 20‐30°C for 10‐60 minutes and the HBsAg‐HQ assay was then carried out. When the value of A/B was less than 50%, the test was determined to be positive.
2.6. Data analysis
Parametric methods with minimum two correlations were used for statistical analysis. A two‐tailed P‐value less than .05 was considered to be statistically significant.
3. RESULTS
3.1. Within‐run reproducibility and linearity of dilution
Within‐run reproducibility values (n = 10) for the HBsAg‐HQ assay were 2.55%, 2.31%, and 1.93% as the CVs for the low, middle, and high concentrations of HBsAg, respectively (Table 2). The linearity‐of‐dilution assessment was performed for diluted HBsAg samples and the results were linear between 0.005 and 150 IU/mL HBsAg. There was no prozone phenomenon (Figure 1).
Table 2.
Within‐run reproducibility
| Control panels | |||
|---|---|---|---|
| Low | Middle | High | |
| Mean (IU/mL) | 0.0103 | 1.1251 | 133.7333 |
| SD (IU/mL) | 0.0003 | 0.0260 | 2.5790 |
| CV (%) | 2.55 | 2.31 | 1.93 |
Figure 1.
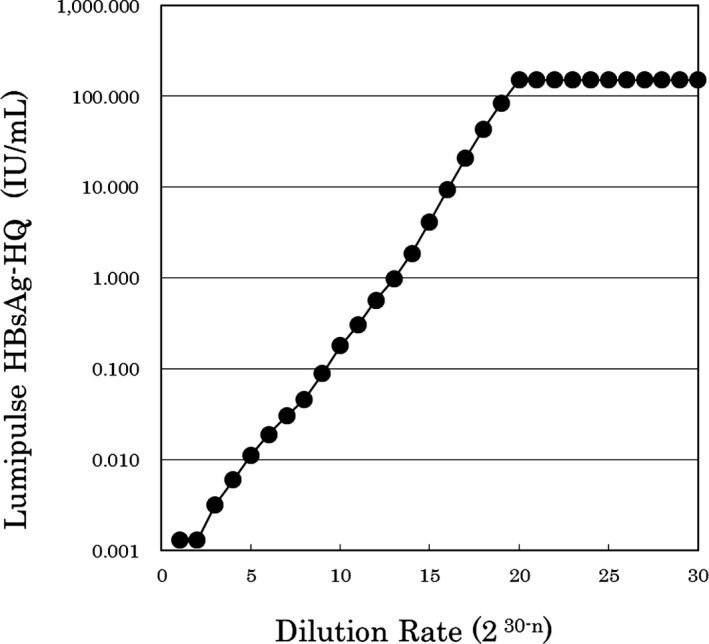
Dilution test
3.2. Analytical sensitivity against HBsAg assay kits
The LOD calculated by the ±3 SD method was 0.0011 IU/mL (Figure 2).
Figure 2.
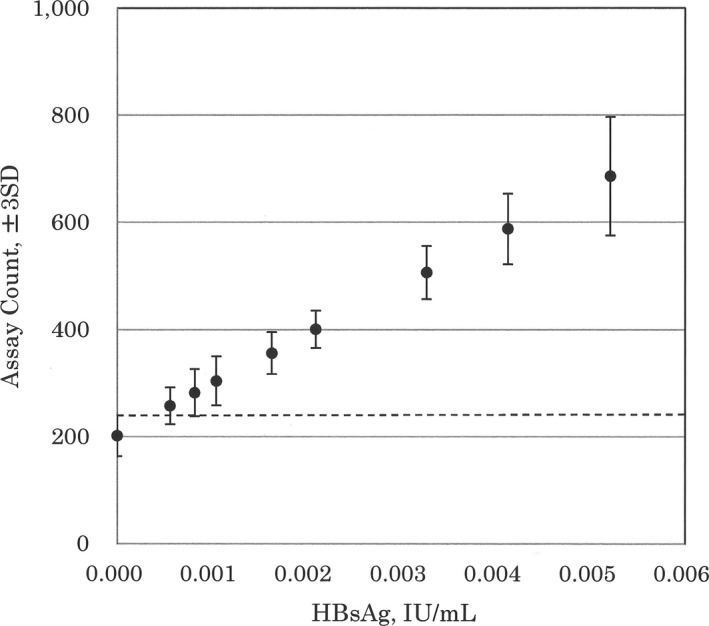
Analytical sensitivity against HBsAg‐HQ. The horizontal bar shows assay count corresponding to zero + 3SD
Regarding sensitivity testing, all 102 samples were positive by the HBsAg‐HQ assay. However, one sample (No. 1 of the patient series) was negative according to the HISCL, ARCHITECT, and ADVIA‐II assays and three samples (No. 1 to No. 3) were negative according to the Lumipulse HBsAg assay (Table 3).
Table 3.
Analytical sensitivity against samples obtained from 102 HBV‐infected individuals
| Sample No. | Lumipulse HBsAg‐HQ | Architect HBsAg QT | HISCL HBsAg | Lumipulse HBsAg | ADVIA Centaur HBsAg II |
|---|---|---|---|---|---|
| 1 | + | − | − | − | − |
| 2 | + | + | + | − | + |
| 3 | + | + | + | − | + |
| 4 | + | + | + | + | + |
| 5 | + | + | + | + | + |
| 6 | + | + | + | + | + |
| 7 | + | + | + | + | + |
| 8 | + | + | + | + | + |
| 9 | + | + | + | + | + |
| 10 | + | + | + | + | + |
| 11‐102 | + | + | + | + | + |
| Positive | 102 | 101 | 101 | 99 | 101 |
| Negative | 0 | 1 | 1 | 3 | 1 |
| Detection rate (%) | 100 | 99.0 | 99.0 | 97.1 | 99.0 |
The sample judged as positive was described as +, and the specimen judged as negative was done as –.
3.3. The sensitivity of detection of HBsAg during acute HBV infection
For sensitivity testing of the five commercial assays against the HBsAg seroconversion panel (PHA935A), the 14‐day blood sample was positive by the HBsAg‐HQ assay, the 21‐day sample was positive by the ARCHITECT, HISCL, and ADVIA‐II assays, and the 23‐day sample was positive by the Lumipulse HBsAg (Table 4).
Table 4.
Analytical sensitivity against the HBsAg seroconversion panel
| Samples | HBV DNA Roche PCR, copies/mL | Lumipulse HBsAg‐HQ | Architect HBsAg QT | HISCL HBsAg | Lumipulse HBsAg | ADVIA Centaur HBsAg II | |
|---|---|---|---|---|---|---|---|
| No. | Days since 1st Bleeda | ||||||
| 1 | 0 | BLD | − | − | − | − | − |
| 2 | 2 | BLD | − | − | − | − | − |
| 3 | 7 | BLD | − | − | − | − | − |
| 4 | 9 | 6 × 102 | − | − | − | − | − |
| 5 | 14 | 8 × 102 | + | − | − | − | − |
| 6 | 16 | 5 × 102 | + | − | − | − | − |
| 7 | 21 | 9 × 103 | + | + | + | − | + |
| 8 | 23 | 8 × 103 | + | + | + | + | + |
| 9 | 28 | 8 × 104 | + | + | + | + | + |
| 10 | 30 | 1 × 105 | + | + | + | + | + |
| 11 | 35 | 4 × 105 | + | + | + | + | + |
| 12 | 50 | 2 × 107 | + | + | + | + | + |
Serial blood specimens were obtained from an individual donor during the seroconversion stage to HBV positivity. The sample judged as positive was described as +, and the specimen judged as negative was done as –.
3.4. Analytical sensitivity against HBV subtypes “ad” and “ay”
The reactivity of the five assays against HBsAg “ad” and “ay” subtypes was 0.025 ng/mL of “ad” and 0.025 ng/mL of “ay” (Table 5). The ARCHITECT, HISCL, and ADVIA‐II assays detected 0.2 ng/mL but not 0.1 ng/mL of subtypes “ad” and “ay”. The Lumipulse HBsAg assay detected 0.3 ng/mL of “ad” and 0.4 ng/mL of “ay” (Table 5).
Table 5.
Analytical sensitivity against HBV subtypes “ad” and “ay”
| Subtype panel (PHA807) | Lumipulse HBsAg‐HQ | Architect HBsAg QT | HISCL HBsAg | Lumipulse HBsAg | ADVIA Centaur HBsAg II |
|---|---|---|---|---|---|
| PHA‐01 ad (3.1 ng/mL) | + | + | + | + | + |
| PHA‐02 ad (1.5 ng/mL) | + | + | + | + | + |
| PHA‐03 ad (1.0 ng/mL) | + | + | + | + | + |
| PHA‐04 ad (0.9 ng/mL) | + | + | + | + | + |
| PHA‐05 ad (0.7 ng/mL) | + | + | + | + | + |
| PHA‐06 ad (0.5 ng/mL) | + | + | + | + | + |
| PHA‐07 ad (0.4 ng/mL) | + | + | + | + | + |
| PHA‐08 ad (0.3 ng/mL) | + | + | + | + | + |
| PHA‐09 ad (0.2 ng/mL) | + | + | + | − | + |
| PHA‐10 ad (0.1 ng/mL) | + | − | − | − | − |
| PHA‐10 × 2 dil. (0.05 ng/mL) | + | − | − | − | − |
| PHA‐10 × 4 dil. (0.025 ng/mL) | + | − | − | − | − |
| PHA‐11 ay (2.6 ng/mL) | + | + | + | + | + |
| PHA‐12 ay (1.3 ng/mL) | + | + | + | + | + |
| PHA‐13 ay (0.9 ng/mL) | + | + | + | + | + |
| PHA‐14 ay (0.8 ng/mL) | + | + | + | + | + |
| PHA‐15 ay (0.6 ng/mL) | + | + | + | + | + |
| PHA‐16 ay (0.5 ng/mL) | + | + | + | + | + |
| PHA‐17 ay (0.4 ng/mL) | + | + | + | + | + |
| PHA‐18 ay (0.3 ng/mL) | + | + | + | − | + |
| PHA‐19 ay (0.2 ng/mL) | + | + | + | − | + |
| PHA‐20 ay (0.1 ng/mL) | + | − | − | − | − |
| PHA‐20 × 2 dil. (0.05 ng/mL) | + | − | − | − | − |
| PHA‐20 × 4 dil. (0.025 ng/mL) | + | − | − | − | − |
The sample judged as positive was described as +, and the specimen judged as negative was done as –.
3.5. Reactivity against HBsAg with amino acid substitutions
The reactivity of the HBsAg‐HQ assay against HBsAg variants was evaluated on 22 HBsAg recombinant proteins with one or two amino acid substitutions (Table 6). Wild‐type HBsAg was used as the control. The HBsAg‐HQ assay reacted with all the variants tested but the concentrations of HBsAg varied between the different mutations. In addition, eight recombinant proteins with different amino acid exchanged at the same site were prepared at positions 120, 142, 144, and 145, (samples No. 3 and 4, 11 and 12, 14 and 15, 16 and 17; respectively) and the reactivities of the HBsAg‐HQ and ARCHITECT assays were compared. HBsAg values were dependent upon the exchanged amino acid and the same tendency was observed in the two assays. The HBsAg‐HQ assay was evaluated for specificity on 1000 serum samples obtained from individuals who were not HBV carriers and who were seronegative for HBsAg/anti‐HBs and anti‐HBc antibody (Table 7). The following results were obtained: three samples were positive by the HBsAg‐HQ assay, one by the ARCHITECT and one by the Lumipulse HBsAg. The three samples positive by the HBsAg‐HQ reagent were negative according to the HBV DNA assay, ‘Cobas Taqman HBV Auto v2.0’ as well as other anti‐HBc reagents. Therefore, these were judged as false positives. Two other samples judged as positive by the Architect HBsAg QT reagent or Lumipulse HBsAg reagent might be false positives because they were negative according to the HBV DNA and anti‐HBc assays. There were no positive samples according to the ADVIA‐II assay. The HBsAg‐HQ assay was used to perform HBsAg neutralization testing on the three positive samples, following the manufacturer's instructions. These three samples showed less than 50% signal reduction, indicating that they were HBsAg negative.
Table 7.
Detection specificity of HBV‐negative seropanels
| Lumipulse HBsAg‐HQ | Architect HBsAg QT | Lumipulse HBsAg | ADVIA Centaur HBsAg II | |
|---|---|---|---|---|
| Positive | 3 | 1 | 1 | 0 |
| Negative | 997 | 999 | 999 | 1000 |
| Predictive rate (%) | 99.7 | 99.9 | 99.9 | >99.9 |
3.6. Bland‐Altman plot analysis
A Bland‐Altman plot between HBsAg‐HQ and HISCL showed a straight line rising to the right. This indicates that the correlation tendency of HBsAg‐HQ for HISCL is a little higher. Furthermore, the data of “Mean + 1.96 SD = 32.0 and Mean−1.96 SD = −19.3” suggested much higher values of HBsAg‐HQ at low HBsAg titers. In the same way, the data of “Mean + 1.96 SD = 35.6 and Mean−1.96 SD = −21.6” in the Bland‐Altman plot of HBsAg‐HQ and ARCHITECT, HBsAg values by HBsAg‐HQ were shown to be a little high. On the other hand, it was found that the values of HBsAg measured in the low range using HBsAg‐HQ were clearly higher than those of HISCL and ARCHITECT (Figure 3A).
Figure 3.
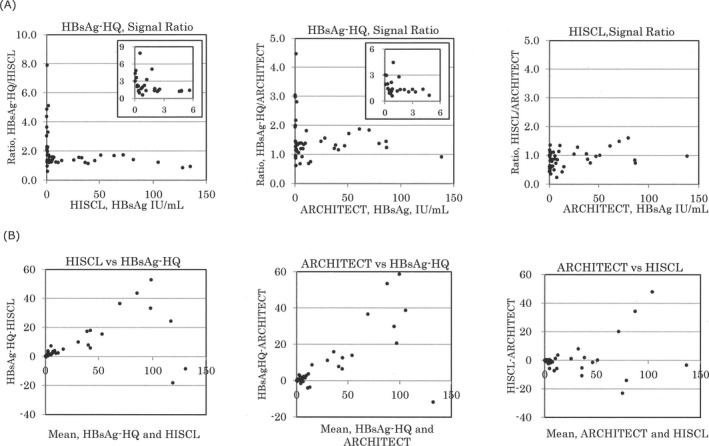
Correlation among HBsAg‐HQ, HISCL and ARCHITECT
In Figure 3B, the ratio of the HBsAg‐HQ to HBsAg values of HISCL and ARCHITECT was plotted for each specimen. In each combination, the ratio was particularly high in the low HBsAg range. The low HBsAg range is just before HBs seroconversion and it is possible that HBsAg and anti‐HBs neutralizing antibody coexist. The high HBsAg‐HQ measurement with the other HBsAg reagents shows that HBsAg might be captured using the antibody against the inner region the protein after pretreatment of the immune complex, in cases of coexistence of HBsAg and anti‐HBs neutralizing antibody.
3.7. Specificity of detection against HBV‐negative sera
A total of 12 231 routinely obtained clinical samples were tested by the HBsAg‐HQ assay to determine the distribution of the resultant values (Figure 4). Samples with low levels of HBsAg were retested and/or underwent HBsAg neutralization testing following the manufacturer's instructions. Twenty‐nine samples judged as positive by HBsAg‐HQ neutralization testing were positive or had been positive at an earlier point according to the HBV DNA reagent and/or anti‐HBc reagent (data not shown). The HBsAg‐HQ assay identified 1317 positive and 10 914 negative samples (less than 0.005 IU/mL). The highest number of positive samples (N = 484) were in the range of 500 to 5000 IU/mL HBsAg. Of the 1317 positive samples, 88 (6.7%) samples ranged from 0.005 to 0.05 IU/mL, HBsAg and 29 of the 88 samples (33.0%) were determined to be HBsAg‐positive on retesting and/or on HBsAg neutralization testing. Similarly, 61 of the 64 samples within the range of 0.05‐0.5 HBsAg IU/mL were confirmed to be positive.
Figure 4.
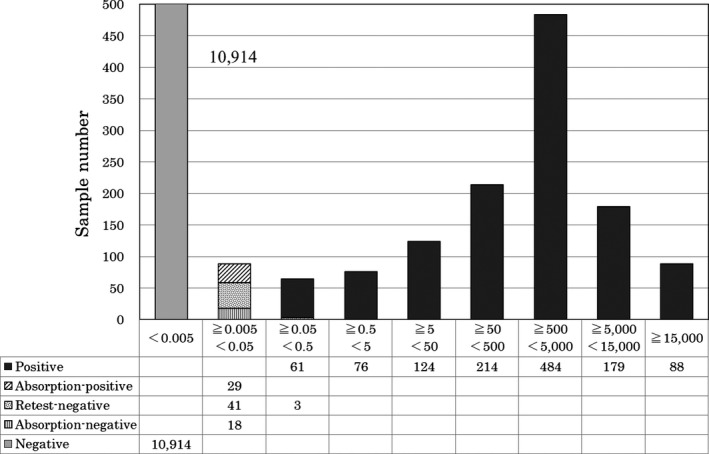
Distribution of HBsAg assay values by Lumipulse HBsAG‐HQ
In addition, the HBsAg concentration of 95 percentile of 10 976 specimens judged as negative including retesting or neutralization testing was 0.0014 IU/mL.
4. DISCUSSION
There are three HBsAg proteins, small (S), medium (M), and large (L). In addition to the virions, the sera of viremic patients contain 104‐ to 106‐fold more small (S) proteins (spherical and filamentous particles) than infectious Dane particles containing HBV DNA. From the diagnostic perspective, HBsAg assay reagents should be able to detect all three forms of circulating HBsAg, thereby increasing the probability of identifying infected individuals.
In attempts to achieve greater sensitivity of HBsAg detection, samples have been pretreated and pretreatment was shown to be effective for disrupting several forms of HBsAg.5, 8 In addition, the combination of a monoclonal antibody against the “a” determinant in the external region and a monoclonal antibody against an internal epitope have reduced the number of false‐negative results. The LOD of HBsAg‐HQ was 0.0011 IU/mL (Figure 2) and the 95 percentile of negative specimens was 0.0014 IU/mL. The sensitivity of the HBsAg‐HQ assay (0.005 IU/mL) is approximately 10‐ or more‐fold greater than that of other commercially available reagents.8 The number of HBV carriers among 29 positive samples whose HBsAg concentration was 0.005‐0.05 IU/mL after retesting and/or neutralization testing was 16. Four of those 16 individuals had continued as HBsAg negative from the past and four were HBsAg negative at the initial examination. If HBsAg reactivation occurs in someone whose HBsAg values had been judged to be completely negative, and their reactivation risk to be very low, there is the concern that medical treatment for hepatitis might be delayed. Therefore, we consider that the significance of finding positive samples in the group considered negative is high. Certainly, the special report “JSH guidelines for the management of hepatitis B virus infection” also says that a high sensitivity testing method should be used for the measurement of HBsAg.3
Therefore, this assay appears to be a convenient, accurate, and precise test for HBsAg. Furthermore, the highly sensitive HBsAg‐HQ assay, which uses a pretreatment step and antibodies against both inner and outer epitopes of the HBsAg molecule, reportedly detected positive results in HBsAg seroclearance cases, which were negative by a conventional HBsAg assay.7
Anti‐HBV therapy and passive‐active immunization (hepatitis B immune globulin and HBV vaccine) have been found to select HBsAg variants.13 Amino acid substitutions within the “a” determinant can lead to conformational changes and several commercially available assays show significantly lower reactivity with specific variants than with wild‐type HBsAg, which may be one of the factors accounting for false‐negative results.9, 10, 11, 12 We evaluated the reactivity of two quantitative assay kits against recombinant HBsAg antigens with one or two amino acid substitutions and obtained positive results against all recombinant antigens with both assays, with varying degrees of reactivity.
The relationship between the results of testing for HBsAg vs HBV DNA in HBV‐infected patients is complex and varies, depending on treatment settings; for instance, HBsAg‐positive but HBV DNA‐negative results were observed in patients after treatment with nucleoside/nucleotide analogs.3, 8 In addition, because HBsAg comprises both virions and subviral particles, the serum HBsAg level is an expression of transcriptionally active intrahepatic covalently closed circular DNA; whereas the serum HBV DNA level indicates the amount of virion production.14
Under nucleot(s)ide analog therapy, HBV DNA becomes negative according to the treatment. Furthermore, when HBsAg values decrease below the detection limit after HBs antibody and HBc antibody have been acquired, we believe that HBV and HBsAg are suppressed by neutralizing antibody against HBV. In some cases, HBV reactivation and fatal severe liver injury might occur.
Shinkai et al8 performed a clinical evaluation study of the HBsAg‐HQ reagent. The HBsAg‐HQ values were positive in 16 of 26 patients who were thought to have experienced HBsAg seroclearance according to the results from the CLIA reagent, with a quantitative sensitivity of 0.05 IU/mL. In that paper, there were some cases in which the HBsAg‐HQ values were 0.05 IU/mL or more but the HBsAg values with the CLIA HBsAg reagent were below 0.05 IU/ML during treatment with Entecavir. In addition, the correlation slopes of HBsAg‐HQ and the HISCL or ARCHITECT HBsAg reagent were 1.137 and 1.266, respectively, and HBsAg‐HQ showed high values for HISCL and ARCHITECT. From these results, we think that pretreatment of the sample in the HBsAg‐HQ test and use of the antibody against the inner region of HBsAg also contribute to the reduction of the influence of anti‐HBs antibody in the blood.
Also, in the study by Seto et al7 25.8% of 329 samples judged by the CLIA reagent as HBsAg seroclearance were positive with the HBsAg‐HQ reagent. The presence of a trace amount of HBsAg was shown in the HBV‐infected group classified as HBsAg seroclearance. We think that HBsAg‐HQ, which is more sensitive than other HBsAg assays, reduces the number of cases judged as HBV reactivation cases from a previous infection and we can detect reactivation at an earlier stage.
For the latest Hepatitis B treatment guideline (3rd edition, Aug 2017), measurement of HBs DNA is recommended for monitoring in immunosuppressive therapy, and substitution with high sensitivity HBsAg (sensitivity 0.005 IU/mL) should also be considered in accordance with the nature of the treatment.
Actually, in our study, two of the 29 specimens within the range of 0.005‐0.05 IU/mL, HBsAg were from HBV carriers that followed de novo hepatitis. De novo hepatitis may simply be unable to detect trace amounts of HBsAg due to the sensitivity of the assay reagent or because co‐existing anti‐HBs neutralizing antibodies mask HBsAg. More remarkably, 10 out of the 29 cases were judged as positive by the HBsAg‐HQ assay for the first time; it can be said that obviously we reduced the number of cases of de novo hepatitis.
In conclusion, a quantitative HBsAg value is a promising diagnostic tool for monitoring the course of HBV infection prior to or during antiviral therapy, because the elimination of HBsAg (HBsAg seroconversion and/or seroclearance) is the ultimate goal of anti‐HBV therapy. Therefore, we think that a highly sensitive HBsAg assay might enable the optimization of management strategies for a considerable proportion of patients with HBV infection.
AUTHOR CONTRIBUTIONS
All the authors have accepted the responsibility for the entire content of this submitted article and approved submission.
Deguchi M, Kagita M, Yoshioka N, et al. Evaluation of the highly sensitive chemiluminescent enzyme immunoassay “Lumipulse HBsAg‐HQ” for hepatitis B virus screening. J Clin Lab Anal. 2018;32:e22334 10.1002/jcla.22334
REFERENCES
- 1. Moucari R, Marcellin P. Quantification of hepatitis B surface antigen: a new concept for the management of chronic hepatitis B. Liver Int. 2011;suppl 1:122‐128. [DOI] [PubMed] [Google Scholar]
- 2. Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. 2011;53:2121‐2129. [DOI] [PubMed] [Google Scholar]
- 3. Drafting Committee for Hepatitis Management Guidelines and the Japan Society of Hepatology . JSH guidelines for the management of hepatitis B virus infection. Hepatol Res. 2014;44(suppl.1):1‐58. [DOI] [PubMed] [Google Scholar]
- 4. Deguchi M, Yamashita N, Kagita M, et al. Quantitation of hepatitis B surface antigen by an automated chemiluminescent microparticle immunoassay. J Virol Methods. 2004;115:217‐222. [DOI] [PubMed] [Google Scholar]
- 5. Matsubara N, Kusano O, Sugamata Y, et al. A novel hepatitis B virus surface antigen immunoassay as sensitive as hepatitis B virus nucleic acid testing in detecting early infection. Transfusion. 2009;49:585‐595. [DOI] [PubMed] [Google Scholar]
- 6. Choi SJ, Park Y, Lee EY, Kim S, Kim HS. Performance evaluation of LUMIPULSE G1200 autoimmunoanalyzer for the detection of serum hepatitis B virus markers. J Clin Lab Anal. 2013;27:204‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seto W, Tanaka Y, Wong DK, et al. Evidence of serologic activity in chronic hepatitis B after surface antigen (HBsAg) seroclearance documented by conventional HBsAg assay. Hepatol Int. 2012;7:98‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shinkai N, Matsuura K, Sugauchi F, et al. Application of a newly developed high‐sensitivity HBsAg chemiluminescent enzyme immunoassay for hepatitis B patients with HBsAg seroclearance. J Clin Microbiol. 2013;51:3484‐3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen WN, Oon CJ. Hepatitis B virus surface antigen (HBsAg) mutants in Singapore adults and vaccinated children with high anti‐hepatitis B virus antibody levels but negative for HBsAg. J Clin Microbiol. 2000;38:2793‐2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seddigh‐Tonekaboni S, Waters JA, Jeffers S, et al. Effect of variation in the common “a” determinant on the antigenicity of hepatitis B surface antigen. J Med Virol. 2000;60:113‐121. [DOI] [PubMed] [Google Scholar]
- 11. Weber B. Genetic variability of the S gene of hepatitis B virus: clinical and diagnostic impact. J Clin Virol. 2005;32:102‐112. [DOI] [PubMed] [Google Scholar]
- 12. Verheyen J, Neumann‐Fraune M, Berg T, Kaiser R, Obermeier M. The detection of HBsAg mutants expressed in vitro using two different quantitative HBsAg assays. J Clin Virol. 2012;54:279‐281. [DOI] [PubMed] [Google Scholar]
- 13. Lada O, Benhamou Y, Poynard T, Thibault V. Coexistence of hepatitis B surface antigen (HBs Ag) and anti‐HBs antibodies in chronic hepatitis B virus carriers: influence of “a” determinant variants. J Virol. 2006;80:2968‐2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thompson AJV, Nguyen T, Iser D, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51:1933‐1944. [DOI] [PubMed] [Google Scholar]


