Abstract
Objectives
By now, there are few data of the reference intervals (RIs) of SII, PLR, NLR, LMR and MLR. We aimed to establish RIs of SII, PLR, NLR, LMR and MLR for healthy persons.
Methods
A retrospective analysis on a cohort of ostensibly healthy, aged no <18 years old physical examinees who took health examination from January to December in 2013 was conducted to explore influences of age and gender on SII, PLR, NLR, LMR and MLR and to establish their RIs. And another cohort of 450 persons in our hospital from January to July in 2016 is included for validations of RIs.
Results
NLR, LMR and MLR were significantly different between gender groups (P=.010; P<.001; P<.001, separately), while SII and PLR were not (P=.137; P=.267, separately). While SII was not changed much between age groups (P=.842), PLR, NLR, LMR and MLR were significantly different (all with P<.001). RIs of SII, PLR, NLR, LMR and MLR were: SII: [161,701]; PLR: 18‐65 year‐old: [61,179]/>65 year‐old: [55,179]; NLR: 18‐65 year‐old male: [0.90,2.94]/18‐65 year‐old female: [0.85,3.06]/>65 year‐old male: [0.95,3.57]/aged >65 year‐old female: [0.83,3.30]; LMR: 18‐65 year‐old male: [2.50,7.50]/18‐65 year‐old female: [2.75,8.50]/>65 year‐old male: [2.16,7.41]/>65 year‐old female: [2.40,8.33]; MLR: 18‐65 year‐old male: [0.12,0.35]/18‐65 year‐old female: [0.10,0.32]/>65 year‐old male: [0.12,0.41]/>65 year‐old male: [0.11,0.33].
Conclusions
RIs of SII, PLR, NLR, LMR and MLR of people in central China were established and validated. It will benefit experimental design of the related studies and lead to better standardizations of SII, PLR, NLR, LMR and MLR for their clinical applications.
Keywords: reference interval, the lymphocyte‐to‐monocyte ratio, the monocyte‐to‐lymphocyte ratio, the neutrophil‐to‐lymphocyte ratio, the platelet‐to‐lymphocyte ratio, the systemic immune‐inflammation index
Abbreviations:
- PLR
The platelet‐to‐lymphocyte ratio
- NLR
The neutrophil‐to‐lymphocyte ratio
- MLR
The monocyte‐to‐lymphocyte ratio
- LMR
The lymphocyte‐to‐monocyte ratio
- SII
The systemic immune‐inflammation index
- RI
Reference Interval
1. Introduction
In recent years, platelet, neutrophil, lymphocyte and monocyte derived from the peripheral blood are significantly associated with tumor progression in various tumors.1, 2, 3, 4, 5 The platelet‐to‐lymphocyte ratio (PLR),6 the neutrophil‐to‐lymphocyte ratio (NLR),7 the lymphocyte‐to‐monocyte ratio (LMR)8 and the monocyte‐to‐lymphocyte ratio (MLR),9 based on neutrophil, lymphocyte, monocyte and/or platelet counts and known as systemic inflammatory biomarkers, are immune response‐related indicators. Preoperative PLR, NLR, LMR and/or MLR have been reported to be related to the prognosis of various cancers.1, 2, 3, 4 Many studies have also confirmed that they are related to the progression and prognosis of many other diseases, like cardiovascular diseases,10, 11 virus infectious diseases12, 13 and thrombosis‐related diseases.14, 15
What's more, a novel index, defined as the systemic immune‐inflammation index (SII), based on lymphocyte, neutrophil and platelet counts, has been developed recently. Bo Hu and his colleagues find that the SII is a promising independent predictive factor for prognosis of patients with hepatocellular carcinoma (HCC) after surgery.5 And it also has been proven to be related with gastric cancer,16 metastatic castration‐resistant prostate cancer (mCRPC),17 small cell lung cancer18 and esophageal squamous cell carcinoma.19
But, most of the aforementioned studies do not have taken SII, PLR, NLR, LMR and/or MLR of healthy controls (HCs) into account, and only pre‐procedural SII, PLR, NLR, LMR and/or MLR are analyzed.20 What's more, even by now, there are few data of the reference intervals (RIs) of SII, PLR, NLR, LMR and MLR. And since data of RIs of SII, PLR, NLR, LMR and MLR are scarce, we do not know the changes of pre‐procedural SII, PLR, NLR, LMR and MLR in different diseases, namely the change may be either dysfunctionally varied or reasonably‐reactively varied. To apply these indicators to clinical practice better and more standard, RIs of SII, PLR, NLR, LMR and MLR for healthy persons are absolutely in an urgent need of establishing. In addition, determinant roles of gender and age on SII, PLR, NLR, LMR and MLR have not been reported either and further studies are needed.
In this study, we conformed to the required procedures of Clinical and Laboratory Standards Institute (CLSI) document C28‐A3—Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline—Third Edition 21 and established RIs of SII, PLR, NLR, LMR and MLR for healthy persons in a posteriori and big‐data‐based way. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR are also explored.
2. Materials and Methods
2.1. Inclusion and exclusion of subjects
A retrospective analysis in the database of the laboratory information system (LIS) and the hospital information system (HIS) of the First Affiliated Hospital of Zhengzhou University to retrieve results of hematological testing performed on a cohort of ostensibly healthy, aged no <18 years old physical examinees who took health examination from January to December in 2013, which had been described before,20 was implemented. This study was approved by the ethics committee of the First Affiliated Hospital of Zhengzhou University.
Subjects with HBV, HCV, HIV or any other diagnosed virus infection, autoimmune diseases such as systemic lupus erythematosus (SLE), leukemia or any other blood system diseases, or any other organic disease (liver, spleen, et al.) were excluded; Subjects receiving the treatment of whole blood or any other component blood products transfusion were also excluded. Subects aged no <18 year‐old and with eligible blood sample were included and analyzed.
Subjects' inclusion and exclusion were conducted as flowchart showed:
2.2. Clinical data
All specimens were EDTA‐K2 anticoagulated and tested within 30 minutes of collection. Hematological parameters: total white cell count (WBC), red blood cell count (RBC), platelet count (PLT), differential white cell count (neutrophils, lymphocytes, monocytes, eosinophils and basophiles), hemoglobin (HGB), hematocrit (HCT), mean cell volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), red cell distribution width (RDW), thrombocytocrit (PCT), mean platelet volume (MPV) and platelet distribution width (PDW) were obtained based on the Coulter principle, using a Coulter LH 750 automated blood analyzer and related reagents (Beckman, California, USA), strictly in accordance with the instructions. The SII,5 PLR,6 NLR,7 LMR8 and MLR9 were calculated as follows:
2.3. Identification and validation
For identification of each RIs, values of SII, PLR, NLR, LMR and MLR of all subjects included were calculated based on those above‐mentioned formulas. The Tukey's (1977) rule was used again for values of each parameter and each stratification to insure reference values against outliers. Then 95% confidence intervals of each parameter and each stratification was counted as reference intervals.
Another cohort of 450 persons aged no <18 years old, ostensibly healthy physical examinees who also took health examination in our hospital from January to July in 2016 are prospectively included for validations of RIs of SII, PLR, NLR, LMR and MLR. All subjects for validation meet the standards that for subjects included for RIs establishing. RIs validated with outsider‐rate <0.10 (OR<0.10) is considered efficient and successfully established.
2.4. Statistics
The statistical analysis was performed using IBM SPSS Statistics version 21.0 software (IBM Corp., Armonk, NY, USA). The quality of data was validated throughout the study period by regular internal quality control (IQC) procedures and participation to External Quality Assessment Scheme (EQAS). Data were finally reported as median and interquartile range (IQR) or as mean ± standard deviation, appropriately. The normality of distributions was analyzed using the Kolgomorov‐Smirnov test. Comparisons of demographic and clinical parameters of two groups were performed using Chi‐square test, Student's t test (independent‐sample t test) or Mann‐Whitney U test and for comparisons of more than two groups Kruskal‐Wallis test followed by pairwise comparisons was used when appropriate. All P‐values of <.05 were considered statistically significant.
3. Results
3.1. General characteristics of main hematological parameters of 24 029 included subjects
As shown in the flowchart (Figure 1), 24 029 subjects in total, a cohort that were aged no <18 years old (18‐65 year‐old (adulets) or more than 65 year‐old (old‐adults)), ostensibly healthy, were finally included. Here, since the SII, PLR, NLR, LMR and MLR were calculated based on PLT, Neo, Lymph and Mo, we excluded subjects with any WBC, Neo, Lymph, Mo and/or PLT violating Tukey's (1977) [Q1−1.5×IQR, Q3+1.5×IQR] rule to confirm the reliability of basic data. General characteristics of main hematological parameters of 24 029 included subjects based on gender and age were summarized in Tables 1 and 2.
Figure 1.
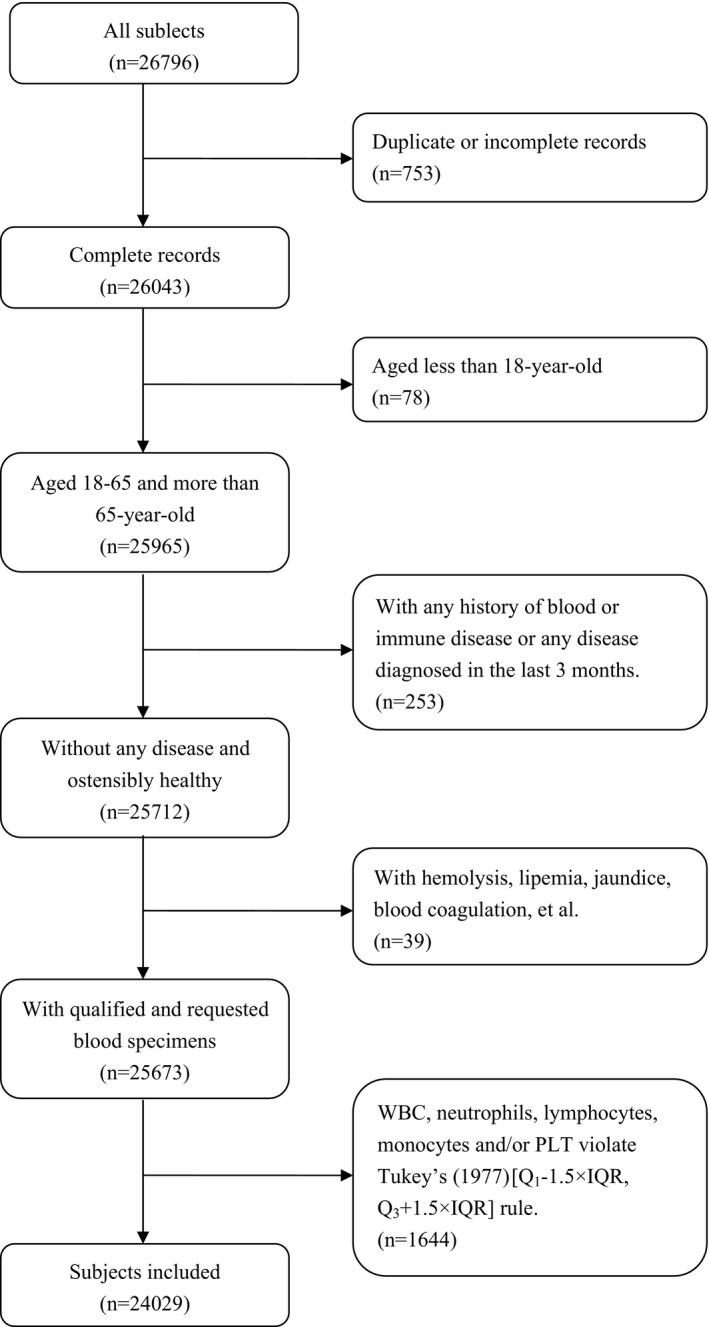
Flowchart presenting the steps of inclusion and exclusion of subjects
Table 1.
General characteristics of main hematological parameters of 24 029 included subjects based on gender
| Items | In total | Gender groups | P | |
|---|---|---|---|---|
| Male | Female | |||
| n | 24 029 | 12 660 | 11 369 | — |
| Age (y) | 44.23±14.21 | 45.14±13.99 | 43.21±14.38 | <.001 |
| WBC (×109/L) | 6.00 (5.10, 7.00) | 6.20 (5.30, 7.20) | 5.70 (4.90, 6.70) | <.001 |
| RBC (×1012/L) | 4.59 (4.27, 4.94) | 4.89 (4.64, 5.13) | 4.29 (4.09, 4.50) | <.001 |
| HGB (g/L) | 142 (131, 154) | 153 (146, 159) | 131 (125, 137) | <.001 |
| PLT (×109/L) | 213 (184, 246) | 208 (178, 240) | 220 (190, 253) | <.001 |
| Neo (×109/L) | 3.40 (2.80, 4.10) | 3.50 (2.90, 4.20) | 3.30 (2.60, 4.00) | <.001 |
| Lymph (×109/L) | 2.00 (1.60, 2.30) | 2.00 (1.70, 2.40) | 1.90 (1.60, 2.30) | <.001 |
| Mo (×109/L) | 0.40 (0.30, 0.50) | 0.40 (0.40, 0.50) | 0.40 (0.30, 0.40) | <.001 |
| Eo (×109/L) | 0.10 (0.06, 0.17) | 0.12 (0.07, 0.19) | 0.08 (0.05, 0.14) | <.001 |
| Ba (×109/L) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | <.001 |
| HCT | 0.428 (0.395, 0.460) | 0.458 (0.438, 0.477) | 0.395 (0.376, 0.413 | <.001 |
| MCV (fL) | 93.0 (90.2, 96.0) | 93.7 (91.0, 96.5) | 92.3 (89.4, 95.0) | <.001 |
| MCH (pg) | 31.0 (30.0, 32.0) | 31.3 (30.4, 32.3) | 30.7 (29.7, 31.6) | <.001 |
| MCHC (g/L) | 333 (326, 340) | 334 (327, 342) | 331 (325, 338) | <.001 |
| RDW (%) | 13.0 (13.0, 14.0) | 13.0 (13.0, 13.0) | 13.0 (13.0, 14.0) | <.001 |
| MPV (fL) | 8.0 (8.0, 9.0) | 8.0 (8.0, 9.0) | 9.0 (8.0, 9.0) | <.001 |
| PCT | 0.180 (0.160, 0.200) | 0.170 (0.150, 0.200) | 0.190 (0.160, 0.210) | <.001 |
| PDW (%) | 16.0 (16.0, 17.0) | 16.0 (16.0, 17.0) | 16.0 (16.0, 17.0) | <.001 |
n, sample number; —, unavailable.
Data are median (25th‐75th percentile) or mean±SD.
Table 2.
General characteristics of main hematological parameters of 24 029 included subjects based on age
| Items | In total | Age groups | P | |
|---|---|---|---|---|
| Adults (aged 18‐65) | Old‐adults (aged more than 65) | |||
| n | 24 029 | 21 999 | 2030 | — |
| Gender (M/F) | 12 660/11 369 | 11 524/10 475 | 1136/894 | .264 |
| WBC (×109/L) | 6.00 (5.10, 7.00) | 6.00 (5.10, 7.00) | 6.00 (5.10, 6.90) | .304 |
| RBC (×1012/L) | 4.59 (4.27, 4.94) | 4.61 (4.28, 4.95) | 4.42 (4.14, 4.70) | <.001 |
| HGB (g/L) | 142 (131, 154) | 143 (131, 154) | 138 (130, 148) | <.001 |
| PLT (×109/L) | 213 (184, 246) | 215 (185, 248) | 194 (164, 227) | <.001 |
| Neo (×109/L) | 3.40 (2.80, 4.10) | 3.40 (2.80, 4.10) | 3.40 (2.80, 4.20) | <.001 |
| Lymph (×109/L) | 2.00 (1.60, 2.30) | 2.00 (1.70, 2.30) | 1.90 (1.50, 2.30) | .061 |
| Mo (×109/L) | 0.40 (0.30, 0.50) | 0.40 (0.30, 0.50) | 0.40 (0.30, 0.50) | <.001 |
| Eo (×109/L) | 0.10 (0.06, 0.17) | 0.10 (0.06, 0.17) | 0.12 (0.07, 0.19) | .001 |
| Ba (×109/L) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | 0.03 (0.02, 0.04) | <.001 |
| HCT | 0.428 (0.395, 0.460) | 0.430 (0.396, 0.461) | 0.415 (0.390, 0.444) | .220 |
| MCV (fL) | 93.0 (90.2, 96.0) | 93.0 (90.1, 95.8) | 94.3 (91.4, 97.2) | <.001 |
| MCH (pg) | 31.0 (30.0, 32.0) | 31.0 (30.0, 32.0) | 31.4 (30.4, 32.3) | <.001 |
| MCHC (g/L) | 333 (326, 340) | 333 (326, 340) | 333 (326, 340) | <.001 |
| RDW (%) | 13.0 (13.0, 14.0) | 13.0 (13.0, 13.0) | 13.0 (13.0, 14.0) | .314 |
| MPV (fL) | 8.0 (8.0, 9.0) | 8.0 (8.0, 9.0) | 8.0 (8.0, 9.0) | <.001 |
| PCT | 0.180 (0.160, 0.200) | 0.180 (0.160, 0.210) | 0.160 (0.140, 0.190) | .198 |
| PDW (%) | 16.0 (16.0, 17.0) | 16.0 (16.0, 17.0) | 16.0 (16.0, 17.0) | <.001 |
n, sample number; —, unavailable.
Data are median (25th‐75th percentile) or mean±SD.
What's more, the reason for Tukey's (1977) rule, but not the D/R rule, was being used was that on a big‐data basis, values of each hematological parameters were closed leading to a relatively small ‘D’ while ranges of them were large leading to a relatively big ‘R’, which gave rise to none outlier observed and with a big sample size distribution of all parameters can be considered normal.
3.2. Influences of gender and age on SII, PLR, NLR, LMR and MLR
For the 24 029 subjects included, we firstly analysed the influences of gender on SII, PLR, NLR, LMR and MLR. NLR, LMR and MLR were significantly different between men and women (NLR: 1.72 (1.39, 2.17) vs 1.71 (1.35, 2.18), P=.010; LMR: 4.67 (3.80, 5.67) vs 5.25 (4.25, 6.33), P<.001; MLR: 0.21 (0.18, 0.26) vs 0.19 (0.16, 0.24), P<.001), while SII and PLR were not (SII: 358 (275, 466) vs 374 (282, 497), P=.137; PLR: 102 (85, 124) vs 115 (95, 140), P=.267) (Table 3). And then, the influences of age on SII, PLR, NLR, LMR and MLR were also analysed. While SII was not changed much between adults (aged 18‐65 year‐old) and old‐adults (aged more than 65 year‐old)(366 (278, 480) vs 366 (275, 488), P=.842), PLR, NLR, LMR and MLR were significantly different (PLR: 106 (88, 128) vs 139 (116, 169), P<.001; NLR: 1.71 (1.36, 2.17) vs 1.85 (1.46, 2.36), P<.001; LMR: 4.83 (4.00, 6.00) vs 5.00 (4.33, 8.00), P<.001; MLR: 0.21 (0.17, 0.25) vs 0.20 (0.13, 0.23), P<.001) (Table 4).
Table 3.
Comparison of SII, PLR, NLR, LMR and MLR between gender groups
| Items | In total | Gender groups | P | |
|---|---|---|---|---|
| Male | Female | |||
| n | 24 029 | 12 660 | 11 369 | — |
| SII | 366 (278, 481) | 358 (275, 466) | 374 (282, 497) | .137 |
| PLR | 108 (89, 132) | 102 (85, 124) | 115 (95, 140) | .267 |
| NLR | 1.72 (1.37, 2.18) | 1.72 (1.39, 2.17) | 1.71 (1.35, 2.18) | .010 |
| LMR | 5.00 (4.00, 6.00) | 4.67 (3.80, 5.67) | 5.25 (4.25, 6.33) | <.001 |
| MLR | 0.20 (0.17, 0.25) | 0.21 (0.18, 0.26) | 0.19 (0.16, 0.24) | <.001 |
n, sample number; —, unavailable.
Data are median (25th‐75th percentile) or mean±SD.
Table 4.
Comparison of SII, PLR, NLR, LMR and MLR between age groups
| Items | In total | Age groups | P | |
|---|---|---|---|---|
| Adults (aged 18‐65) | Old‐adults (aged more than 65) | |||
| n | 24 029 | 21 999 | 2030 | — |
| SII | 366 (278, 481) | 366 (278, 480) | 366 (275, 488) | .842 |
| PLR | 108 (89, 132) | 106 (88, 128) | 139 (116, 169) | <.001 |
| NLR | 1.72 (1.37, 2.18) | 1.71 (1.36, 2.17) | 1.85 (1.46, 2.36) | <.001 |
| LMR | 5.00 (4.00, 6.00) | 4.83 (4.00, 6.00) | 5.00 (4.33, 8.00) | <.001 |
| MLR | 0.20 (0.17, 0.25) | 0.21 (0.17, 0.25) | 0.20 (0.13, 0.23) | <.001 |
n, sample number; —, unavailable.
Data are median (25th‐75th percentile) or mean±SD.
3.3. Establishment of reference intervals of SII, PLR, NLR, LMR and MLR
Based on the above‐mentioned statistical results, stratification was not needed for RI of SII, while it was needed for that of PLR basing on age, but not on gender. And for RIs of NLR, LMR and MLR, stratification was needed basing on both age and gender. 95% CIs of values of each parameter and each stratification after eliminating outliers was counted as RIs (Table 5).
Table 5.
95% confidence intervals (reference intervals) of SII, PLR, NLR, LMR and MLR
| Items | Need for stratification | 95% CI (RIs); n | |||
|---|---|---|---|---|---|
| Aged 18‐65 year‐old | Aged >65 year‐old | ||||
| Male | Female | Male | Female | ||
| SII | No | [161, 701]; n=23 279 | |||
| PLR | Yes for age, but no for gender | [61, 179]; n=21 434 | [55, 179]; n=1966 | ||
| NLR | Yes for both age and gender | [0.90, 2.94]; n=11 213 | [0.85, 3.06]; n=10 198 | [0.95, 3.57]; n=1086 | [0.83, 3.30]; n=870 |
| LMR | Yes for both age and gender | [2.50, 7.50]; n=11 169 | [2.75, 8.50]; n=10 140 | [2.16, 7.41]; n=1102 | [2.40, 8.33]; n=876 |
| MLR | Yes for both age and gender | [0.12, 0.35]; n=11 230 | [0.10, 0.32]; n=10 136 | [0.12, 0.41]; n=1108 | [0.11, 0.33]; n=863 |
n, sample number; CI, confidence intervals.
3.4. Validation of reference intervals of SII, PLR, NLR, LMR and MLR
On the bases of Clinical and Laboratory Standards Institute (CLSI) document C28‐A3, we further included a cohort of 450 healthy persons to validate the reference intervals of SII, PLR, NLR, LMR and MLR. For SII (without stratification), PLR (with stratification for age), NLR (with stratification for both age and gender), LMR (with stratification for both age and gender) and MLR (with stratification for both age and gender), proportions of outsiders which are validation values below or beyond established RIs are all <10% (Table 6), which means RIs of SII, PLR, NLR, LMR and MLR are efficient and successfully established.
Table 6.
Validations of reference intervals of SII, PLR, NLR, LMR and MLR in a cohort of 450 healthy persons
| Item | Stratification | RIs | n | Range of validation data | Number of below the down‐limit | Number of above the upper‐limit | Number of outsider | OR |
|---|---|---|---|---|---|---|---|---|
| SII | no | [161, 701] | 450 | [140, 1147] | 8 | 27 | 35 | 0.0778 |
| PLR | Aged 18‐65 | [61, 179] | 235 | [53, 284] | 2 | 12 | 14 | 0.0596 |
| Aged more than 65 | [55, 179] | 215 | [44, 264] | 3 | 11 | 14 | 0.0651 | |
| NLR | Male aged 18‐65 | [0.90, 2.94] | 113 | [1.07, 4.06] | 0 | 8 | 8 | 0.0708 |
| Female aged 18‐65 | [0.85, 3.06] | 122 | [1.03, 5.14] | 0 | 5 | 5 | 0.0410 | |
| Male aged more than 65 | [0.95, 3.57] | 108 | [1.04, 4.29] | 0 | 4 | 4 | 0.0370 | |
| Female aged more than 65 | [0.83, 3.30] | 107 | [1.00, 5.24] | 0 | 6 | 6 | 0.0561 | |
| LMR | Male aged 18‐65 | [2.50, 7.50] | 113 | [2.67, 9.50] | 0 | 6 | 6 | 0.0531 |
| Female aged 18‐65 | [2.75, 8.50] | 122 | [1.17, 9.00] | 3 | 2 | 5 | 0.0410 | |
| Male aged more than 65 | [2.16, 7.41] | 108 | [1.29, 8.67] | 5 | 3 | 8 | 0.0741 | |
| Female aged more than 65 | [2.40, 8.33] | 107 | [1.29, 10.50] | 2 | 7 | 9 | 0.0841 | |
| MLR | Male aged 18‐65 | [0.12, 0.35] | 113 | [0.11, 0.38] | 1 | 5 | 6 | 0.0531 |
| Female aged 18‐65 | [0.10, 0.32] | 122 | [0.11, 0.86] | 0 | 5 | 5 | 0.0410 | |
| Male aged more than 65 | [0.12, 0.41] | 108 | [0.12, 0.78] | 0 | 9 | 9 | 0.0833 | |
| Female aged more than 65 | [0.11, 0.33] | 107 | [0.10, 0.78] | 1 | 5 | 6 | 0.0561 |
n, sample number; RIs, reference intervals; OR, outsider‐rate.
RIs validated with outsider‐rate <0.10 (OR<0.10) is considered efficient and successfully established.
4. Discussion
Studies about newly emerging systemic inflammatory biomarkers, including PLR, NLR, LMR and MLR wich are already red hot and SII which is becoming more and more desirable are and will be more highly focused. Since they are widely related to kinds of cancers and other diseases, RIs of SII, PLR, NLR, LMR and MLR are prerequisite and imperative for clinical application of these indicators.
In this study, we defined the RIs of SII, PLR, NLR, LMR and MLR by data of a cohort of ostensibly healthy, aged no <18 years old people from central China, and validated the RIs by data of another newly included cohort in line with the same conditions in a posteriori and big‐data‐based way followed the introductions of Clinical and Laboratory Standards Institute (CLSI) document C28‐A3.
As these indicators are all calculated by basic haematological parameters, their greater accessibility and lower cost ensure their position as indicators widely used in diagnosis, differentiating, and evaluating the prognosis of kinds of disease that will be well received. As we've mentioned before, most of the studies about SII, PLR, NLR, LMR and MLR do not have healthy controls (HCs), and only pre‐procedural SII, PLR, NLR, LMR and/or MLR are analyzed.20 Without RIs, it may be confused when a high or low indicator value group is mentioned and only comparison of high value group vis‐a‐vis low value group in certain disease may cover the real changes of these indicators in the certain disease.
Three kinds of pre‐procedural observed values, namely higher or lower than that in HCs and no big change, can be found and all could be a reasonably‐reactive variation.20 Although we define the RIs of these indicators, their medical decision levels still need to be further explored. Their sensitivities and specificities for certain disease, whether cancer or not, are also in urgent need of being intensively studied. It may be added, that as SII is with a unit of concentration (109/L), its clinical definition still needs a more scientific and reasonable delimitation.
The present study appears to be the first to report RIs of SII, PLR, LMR and MLR, and RI of NLR with stratification (Forget et al.22 reported RI of NLR in an adult, non‐geriatric, population in good health are between 0.78 and 3.53). Standard rules of Clinical and Laboratory Standards Institute (CLSI) document C28‐A3 are obeyed. However, there are also limitations to this study that should be emphasized. First, as the C28‐A3 mainly recommend rules of RIs establishing that meet the minimum requirements for reliability and usefulness (like for the minimum sample number asked),21 there is a lack of rules for RI establishing on a big‐data bassis and we adopt a plausible method as far as possible in accordance with the C28‐A3. Second, only adults aged no <18 are analyzed and only age and gender are pondered for stratification. As neutrophil, lymphocyte, monocyte and platelet all may change depending on age and gender, influences of age and gender on SII, PLR, NLR, LMR and MLR need further study. Third, this study is limited to people of central China and it may not be directly applicable to subjects from other region or clinical lab. What's more, as only data of healthy adults (18‐65 year‐old) and old‐adults (>65 year‐old)23 are analyzied, RIs of these parameters of teenagers, pre‐teens or other particular groups like pregnant women still needs further studying.
In summary, we establish RIs of SII, PLR, NLR, LMR and MLR of people in central China in a posteriori and big‐data‐based way. It will benefit experimental design of the related studies and lead to better standardizations of SII, PLR, NLR, LMR and MLR for their clinical applications.
Meng X, Chang Q, Liu Y, et al. Determinant roles of gender and age on SII, PLR, NLR, LMR and MLR and their reference intervals defining in Henan, China: A posteriori and big‐data‐based. J Clin Lab Anal. 2018;32:e22228 10.1002/jcla.22228
Funding Information
The work was supported by the Key Project on Science and Technology Research provided by Henan Province, China (No. 152102410067; No. 162102310142; No. 201403035).
Xianchun Meng and Qian Chang contribute equally to this study.
References
- 1. Nishijima TF, Muss HB, Shachar SS, Tamura K, Takamatsu Y. Prognostic value of lymphocyte‐to‐monocyte ratio in patients with solid tumors: a systematic review and meta‐analysis. Cancer Treat Rev. 2015;41:971‐978. [DOI] [PubMed] [Google Scholar]
- 2. Templeton AJ, McNamara MG, Eruga B, et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. J Natl Cancer Inst. 2014;106:u124. [DOI] [PubMed] [Google Scholar]
- 3. Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2014;23:1204‐1212. [DOI] [PubMed] [Google Scholar]
- 4. Xiang J, Zhou L, Li X, et al. Preoperative monocyte‐to‐lymphocyte ratio in peripheral blood predicts stages, metastasis, and histological grades in patients with ovarian cancer. Transl Oncol. 2016;10:33‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hu B, Yang XR, Xu Y, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20:6212‐6222. [DOI] [PubMed] [Google Scholar]
- 6. Smith RA, Bosonnet L, Ghaneh P, et al. The platelet‐lymphocyte ratio improves the predictive value of serum CA19‐9 levels in determining patient selection for staging laparoscopy in suspected periampullary cancer. Surgery. 2008;143:658‐666. [DOI] [PubMed] [Google Scholar]
- 7. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil‐lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181‐184. [DOI] [PubMed] [Google Scholar]
- 8. Merekoulias G, Alexopoulos EC, Belezos T, Panagiotopoulou E, Jelastopulu DM. Lymphocyte to monocyte ratio as a screening tool for influenza. PLoS Curr. 2010;2:N1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Warimwe GM, Murungi LM, Kamuyu G, et al. The ratio of monocytes to lymphocytes in peripheral blood correlates with increased susceptibility to clinical malaria in Kenyan children. PLoS ONE. 2013;8:e57320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yayla C, Akboga MK, Canpolat U, et al. Platelet to lymphocyte ratio can be a predictor of infarct‐related artery patency in patients with ST‐segment elevation myocardial infarction. Angiology. 2015;66:831‐836. [DOI] [PubMed] [Google Scholar]
- 11. Cicek G, Acikgoz SK, Bozbay M, et al. Neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio combination can predict prognosis in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2015;66:441‐447. [DOI] [PubMed] [Google Scholar]
- 12. Naess A, Nilssen SS, Mo R, Eide GE, Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2016; doi: 10.1007/s15010-016-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meng X, Wei G, Chang Q, et al. The platelet‐to‐lymphocyte ratio, superior to the neutrophil‐to‐lymphocyte ratio, correlates with hepatitis C virus infection. Int J Infect Dis. 2016;45:72‐77. [DOI] [PubMed] [Google Scholar]
- 14. Kurtipek E, Buyukterzi Z, Buyukterzi M, Alpaydin MS, Erdem SS. Endothelial dysfunction in patients with pulmonary thromboembolism: neutrophil to lymphocyte ratio and platelet to lymphocyte ratio. Clin Respir J. 2017;11:78‐82. [DOI] [PubMed] [Google Scholar]
- 15. Ferroni P, Riondino S, Formica V, et al. Venous thromboembolism risk prediction in ambulatory cancer patients: clinical significance of neutrophil/lymphocyte ratio and platelet/lymphocyte ratio. Int J Cancer. 2015;136:1234‐1240. [DOI] [PubMed] [Google Scholar]
- 16. Huang L, Liu S, Lei Y, et al. Systemic immune‐inflammation index, thymidine phosphorylase and survival of localized gastric cancer patients after curative resection. Oncotarget. 2016;7:44185‐44193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lolli C, Caffo O, Scarpi E, et al. Systemic immune‐inflammation index predicts the clinical outcome in patients with mCRPC treated with abiraterone. Front Pharmacol. 2016;7:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong X, Cui B, Wang M, Yang Z, Wang L, Xu Q. Systemic immune‐inflammation index, based on platelet counts and neutrophil‐lymphocyte ratio, is useful for predicting prognosis in small cell lung cancer. Tohoku J Exp Med. 2015;236:297‐304. [DOI] [PubMed] [Google Scholar]
- 19. Geng Y, Shao Y, Zhu D, et al. Systemic immune‐inflammation index predicts prognosis of patients with esophageal squamous cell carcinoma: a propensity score‐matched analysis. Sci Rep. 2016;6:39482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meng X, Wang W, Wei G, Chang Q, He F, Ming L. A high or a reasonably‐reactively elevated platelet‐to‐lymphocyte ratio, which plays the role? Platelets. 2016;27:491. [DOI] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute . Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline, 3rd edn CLSI document EP28‐A3c. Wayne, PA: Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 22. Forget P, Khalifa C, Defour J, Latinne D, Van Pel M, De Kock M. What is the normal value of the neutrophil‐to‐lymphocyte ratio? BMC Res Notes. 2017;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The World Health Organization, Ageing. http://www.who.int/topics/ageing/en/ Accessed January 18, 2016.


