Abstract
Background
Paraquat can cause severe injury to vascular endothelial cells and lead to coagulation dysfunction when it is taken into the blood by oral ingestion. In this study, we aim to find a routine coagulation index to serve as an indicator of outcome in patients with acute paraquat poisoning.
Methods
Between January 2012 and December 2016, 209 patients who attempted suicide by oral ingestion of paraquat were admitted to the emergency room. Routine coagulation indices, including plasma prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen (Fbg), thrombin time (TT), and D‐dimer were measured to analyze the trend of changes and their relationship with prognosis.
Results
The results showed that the PT and APTT values in the ≥30 mL group were significantly greater than those in the <30 mL group (both P < .01). Within 1 week of admission, PT and APTT values gradually decreased, while Fbg levels gradually increased. Univariate and multivariate Cox regression analysis indicated that sex, ingestion volume, and PT were independent predictors of mortality within 40 days. The cumulative survival rates differed significantly (P = .001) between patients with PT <12 seconds and PT ≥12 seconds.
Conclusions
Coagulation status in patients with PQ poisoning was closely related to prognosis. Routine monitoring of coagulation function, particularly PT in plasma, is helpful for analysis of the condition and prognosis of patients with PQ poisoning.
Keywords: coagulation function, endothelial cell, paraquat, poisoning, prothrombin time
1. INTRODUCTION
Paraquat (PQ, 1,1′‐dimethyl‐4,4′‐bipyridinium dichloride) is a nonselective, fast‐acting contact herbicide which is widely used in the Asia‐Pacific region, including China, Korea, and Sri Lanka.1, 2, 3 Due to its accessibility, paraquat poisoning occurs frequently. Intentional or accidental ingestion of PQ is considered a severe public health problem in developing countries. The high toxicity of PQ leads to a mortality rate of approximately 80% after confirmed exposure.4 Most patients die within a few days due to the lack of an effective antidote and treatment. Multiple organ failures, including hepatic and renal failure, acute respiratory distress syndrome, and cardiogenic shock, are the main causes of death.5, 6, 7, 8 PQ is absorbed rapidly within a few hours after ingestion, and is widely distributed into most organs, especially the lung. The lung is the organ that usually suffers the most severe damage, as paraquat is taken up against a concentration gradient in the lung.9 Plasma PQ concentration peaks at 2 hours after oral ingestion, and most of the ingested PQ is excreted in its original form from the kidneys within the first 24 hours.
Coagulation is a process by which blood changes from a liquid state to a gelatinous state. This hemostatic function of the body is completed through the action of platelets and the vascular endothelial system, as well as the processes of coagulation and fibrinolysis. Coagulation begins almost instantly after injury damages the endothelium lining of the blood vessel. Blood leaking from the injured endothelium initiates the process of change in platelets and results in the exposure of subendothelial tissue factor to plasma factor VII, which ultimately leads to fibrin formation. Platelets immediately form a plug at the site of injury, and then additional coagulation factors or clotting factors beyond factor VII respond in a complex cascade to form fibrin strands, which strengthen the platelet plug.10 The system of coagulation in humans has been extensively studied and is the best understood. The most common coagulation indices in clinical practice include plasma prothrombin time (PT), international normalized ratio (INR), fibrinogen (Fbg), activated partial thromboplastin time (APTT), and thrombin time (TT).
The mechanisms of coagulation include activation, adhesion, and aggregation of platelets along with deposition and maturation of fibrin. However, the mechanisms of paraquat poisoning are unclear to date. The generation of free radicals, oxidative stress, and secondary effects of oxidative stress have been reported to be associated with tissue injury. Redox reaction by reactive oxygen species and lipid peroxidation of cellular membranes are widely regarded as the mechanisms responsible for PQ intoxication.7, 11 Some studies have shown that PQ poisoning can cause the dysfunction of coagulation and fibrinolysis. Seok et al12 stated the manifestation and possible reasons for fibrinolysis dysfunction caused by acute PQ poisoning. In the past few decades, many studies have shown that acute PQ poisoning can lead to injury and dysfunction in the liver, kidney, ear, and other important organs.13, 14, 15 Moreover, many studies have shown that the severity of lung and vascular endothelial injury, as well as the inflammatory reaction, are time‐ and dose‐dependent with respect to PQ poisoning.16, 17, 18, 19 Some studies using animal models suggest that PQ poisoning can cause coagulation dysfunction, although there is a lack of detailed research on the effects and extent of PQ toxicity with respect to human coagulation function. While we already know that acute PQ poisoning can cause human blood coagulation dysfunction, the possible relationship between the ingestion dose and the severity of coagulation dysfunction are still unclear. In addition, there is less research evaluating the association between coagulation function and prognosis in patients with paraquat poisoning. Therefore, this study focuses on the possible mechanism and relationship between coagulation function and outcomes of paraquat intoxication.
2. MATERIALS AND METHODS
2.1. Patients
Patients were admitted to the emergency room of the First Affiliated Hospital, College of Medicine, Zhejiang University between January 2012 and December 2016. In total, 235 patients with acute oral intake of PQ within 24 hours of admission were enrolled in this study. Patients who met the following criteria were excluded: a history of hypertension (n = 15), hepatic failure (n = 4), renal failure (n = 1), tumor (n = 0), cardiac insufficiency (n = 2), diabetes (n = 4), and/or pregnancy (n = 0). Therefore, there were 209 remaining patients included in the present study.
This study was approved by the Institutional Research Ethics Committee of the First Affiliated Hospital of Zhejiang University and performed in accordance with the principles embodied in the Declaration of Helsinki. Written informed consent was obtained from all subjects prior to participation.
2.2. Treatment protocol
All patients were treated according to guidelines published by the China Physician Association (2013 version).20 All subjects received a single gastric lavage regardless of whether they had been previously lavaged after exposure. A few hours later after admission to the emergency room, hemoperfusion was performed and repeated once a day for at least 3 days. Hemofiltration was performed when acute renal failure occurred. Other treatment modalities such as immunosuppressive treatment (methylprednisolone, cyclophosphamide, and dexamethasone), prevention and treatment of inflammation, and protection of vital organs were implemented for each patient. In this study, we set the end of follow‐up to 45 days. The follow‐up period for each patient began at the time of admission and ended for overall survival at the occurrence of death, self‐withdrawal from the study, or on September 18, 2016, whichever came first.
2.3. Laboratory analysis
Blood samples were drawn within 24 hours after admission, and then continuously collected at least once a day in the following week. Routine laboratory analysis included coagulation indices such as prothrombin time, activated partial thromboplastin time, thrombin time, fibrinogen, and D‐dimer tests. All of the coagulation index tests were analyzed using a Sysmex CS‐5100 coagulation analyzer (Sysmex, Kobe, Japan).
2.4. Statistical analysis
Data were expressed as mean ± standard deviation or as a percentage. Each variable was compared with respect to death and survival. The mean values of each item between 2 groups were compared using Mann‐Whitney U test, and the chi‐squared test was used for comparing distributions among groups. Survival curves were estimated using the Kaplan‐Meier method and compared using the log‐rank test. Univariate and multivariate Cox regression analyses to determine predictors of 40‐day mortality were presented as hazard ratios with a 95% confidence interval. All tests were two‐tailed; P values <.05 were considered to indicate statistical significance. All statistical analyses were performed with IBM SPSS (version 20) for Microsoft Windows (SPSS, Inc., Chicago, IL, USA).
3. RESULTS
3.1. Patient characteristics
In our study, 209 patients with paraquat poisoning were enrolled. The clinical characteristics on admission of the patients are summarized in Table 1. Ingestion volume, PT, and APTT were significantly different between the groups. In addition, we divided the patients into 2 groups based on the ingestion volume of 30 mL. The PT and APTT values in the ≥30 mL group were significantly greater than in the <30 mL group, as can be seen in Figure 1A,B (both P < .01).
Table 1.
Characteristics of 209 paraquat poisoning patients on admission
| Variable | Death group (n = 90) | Survival group (n = 119) | P value |
|---|---|---|---|
| Age (y) | 37.88 ± 16.48 | 35.90 ± 13.62 | .736 |
| Gender(male/female) | 50/40 | 52/67 | .096 |
| Ingestion volume (mL) | 79.38 ± 115.55 | 14.77 ± 11.02 | <.001 |
| PT (s) | 13.97 ± 2.82 | 11.62 ± 1.14 | <.001 |
| APTT (s) | 42.26 ± 21.75 | 30.80 ± 11.81 | .007 |
| Fbg (g/L) | 2.23 ± 0.71 | 1.99 ± 0.55 | .051 |
| TT (s) | 32.07 ± 24.25 | 23.27 ± 11.80 | .354 |
| D‐dimer (μg/L FEU) | 1370.87 ± 1933.13 | 1227.49 ± 1698.92 | .951 |
Data are means ± SD for death group (n = 90) and survival group (n = 119) of paraquat poisoning patients. APTT, activated partial thromboplastin time; Fbg, fibrinogen; PT, prothrombin time; TT, thrombin time.
Figure 1.
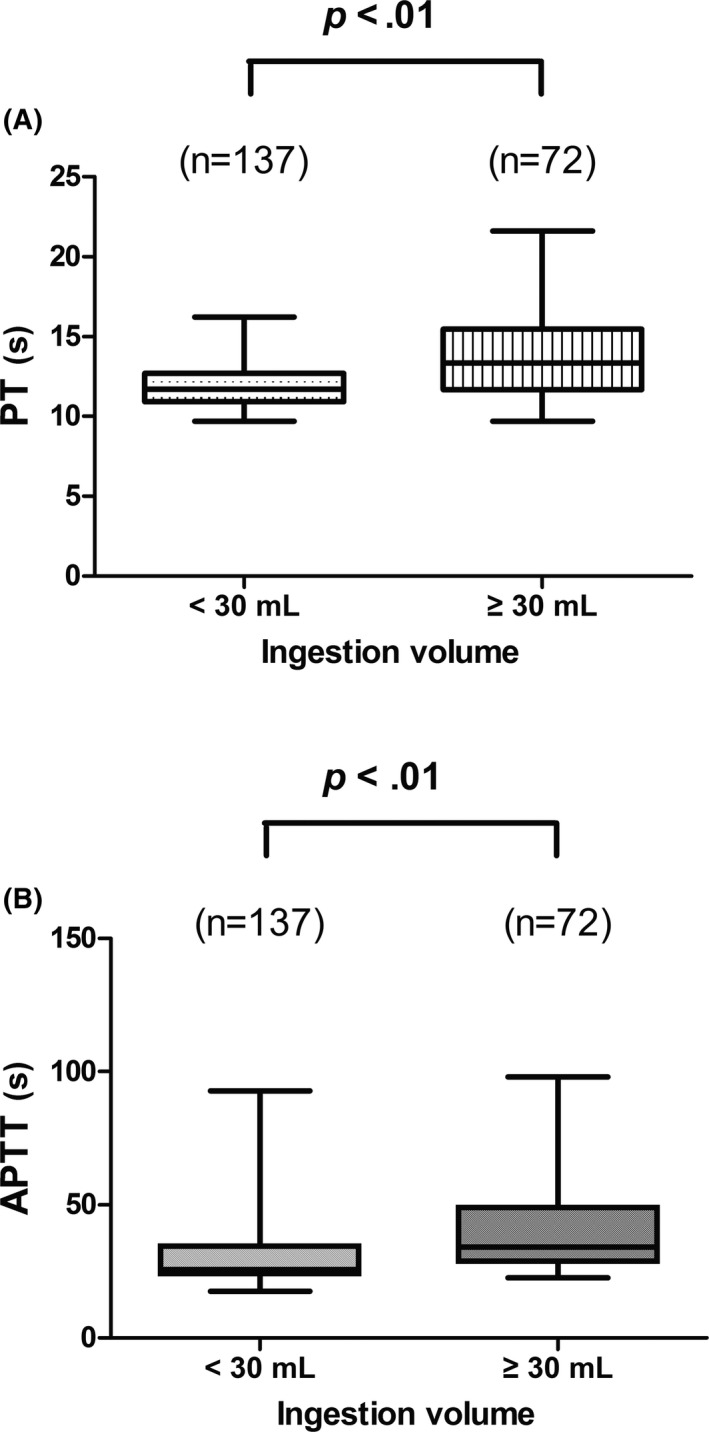
Comparison of prothrombin time and activated partial thromboplastin time with respect to the distinct ingestion volume groups
3.2. Dynamic changes in the coagulation index in the ensuing week after admission
Data on coagulation indices were collected continuously in these patients within 1 week of admission; the corresponding data and trend diagram are shown in Figure 2. A significant difference in dynamic changes with respect to PT, APTT, and Fbg were observed between the 2 groups (both, P < .05). Within 1 week of admission, PT and APTT values gradually decreased, while Fbg levels gradually increased.
Figure 2.
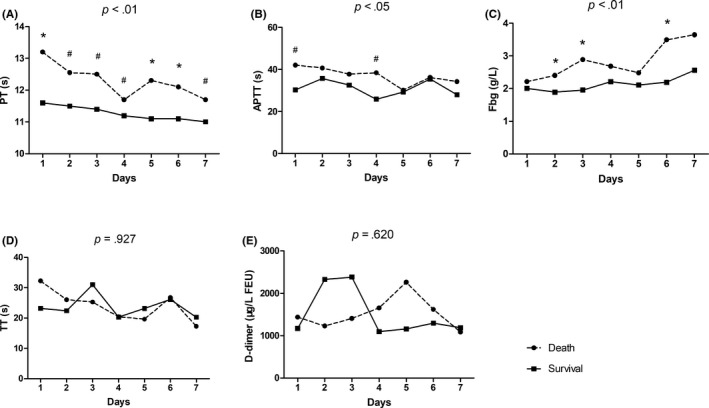
Dynamic changes in the coagulation index in patients with paraquat poisoning in the ensuing week after admission. *P < .01; #P < .05
3.3. Risk factor analysis for 40‐day mortality
As shown in Table 2, univariate and multivariate Cox regression analysis indicated that sex, ingestion volume, and PT were independent predictors of mortality within 40 days.
Table 2.
Risk factors for mortality within 40 days using Cox regression analysis
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.006 | 0.992‐1.021 | .389 | 1.025 | 0.992‐1.059 | .146 |
| Gender | 0.536 | 0.351‐0.819 | .004 | 0.119 | 0.031‐0.460 | .002 |
| Ingestion volume | 1.005 | 1.003‐1.006 | <.001 | 1.011 | 1.004‐1.018 | .002 |
| PT | 1.458 | 1.262‐1.684 | <.001 | 1.640 | 1.186‐2.268 | .003 |
| APTT | 1.019 | 1.002‐1.037 | .028 | 0.970 | 0.918‐1.024 | .266 |
| Fbg | 1.634 | 1.000‐2.669 | .050 | 1.775 | 0.577‐5.461 | .317 |
| TT | 1.012 | 0.995‐1.029 | .180 | 0.996 | 0.996‐1.028 | .825 |
| D‐dimer | 1.000 | 1.000‐1.000 | .364 | 1.000 | 1.000‐1.001 | .447 |
APTT, activated partial thromboplastin time; CI, confidence interval; Fbg, fibrinogen; HR, hazard ratio; PT, prothrombin time; TT, thrombin time.
3.4. Kaplan‐Meier survival test
We compared the survival time of patients using a 12‐second PT as a cut‐off value. It was found that patients with a PT value over 12 seconds had a poorer survival time as compared with those with a less than 12‐second PT in the follow‐up period (log‐rank χ2 = 11.474, P = .001) (Figure 3A). Similarly, we also compared the survival times between the groups divided by ingestion volume and gender. The results showed that the survival rate of the high dose group (≥30 mL) was much lower than that of the low dose group (<30 mL) (log‐rank, χ2 = 105.583, P < .001) (Figure 3C). The survival rate of male patients in PQ poisoning was lower than that of female patients (log‐rank, χ2 = 8.584, P = .003) (Figure 3B).
Figure 3.
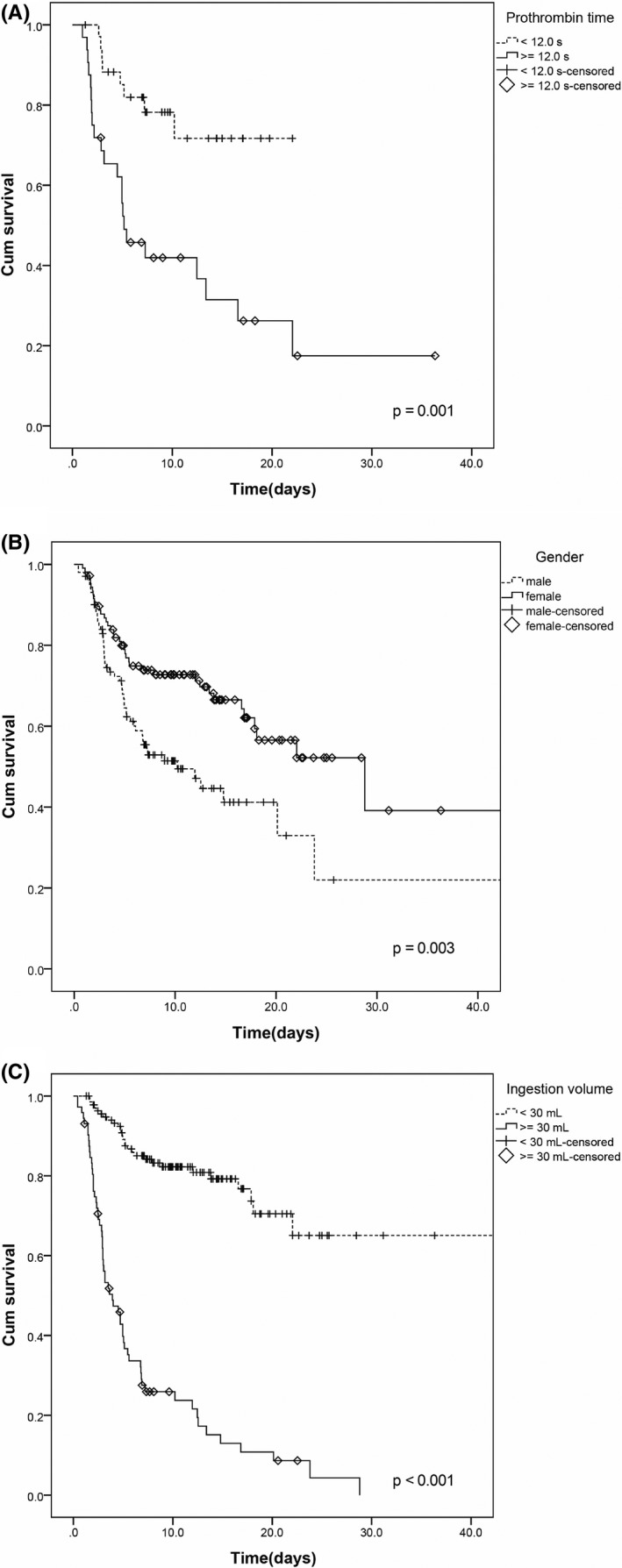
Kaplan‐Meier survival curves with respect to the groups divided by prothrombin time, sex, and ingestion volume in patients with paraquat poisoning
4. DISCUSSION
In recent years, most of the research on the clinical symptoms of patients with paraquat poisoning has focused on how to reduce lung injury and prevent multiple organ failure. In addition, various studies have focused on finding some indicators to predict clinical outcomes for paraquat poisoning patients. The studied indicators included amylase, white blood cells, pO2, and some fibrinolytic system markers. However, few studies have focused on the changes in routine coagulation function and their relationship with prognoses of paraquat poisoning patients. Therefore, our study aimed to find some coagulation parameters that could be used to predict the clinical prognoses or outcomes of patients with paraquat poisoning.
In much of the previous studies, it has been reported that one mechanism of paraquat‐induced toxicity is through the generation of free radicals as well as oxidative stress. Redox cycling and subsequent generation of highly reactive oxygen species (ROS) are the main features after ingestion.20, 21, 22, 23 When paraquat is metabolized by several enzyme systems, it generates a large quantity of superoxide (O2 −) which gives rise to the formation of the hydroxyl free radicals (HOs), leading to oxidative damage and apoptosis. 24, 25, 26 The concentration of PQ in the plasma of patients with PQ poisoning peaks within 2‐4 hours of oral administration,27, 28 and can remain at a relatively constant concentration for several days. As the lung is a highly vascularized tissue, with active uptake and enrichment of PQ in lung tissues during this period, the concentration of PQ in the lung continues to rise and reaches levels several times the plasma concentration.29 Therefore, the lung is usually the most seriously injured organ in PQ poisoning patients. Our results showed that both PT and APTT values (Figure 1) were significantly higher in the high dose ingestion (≥30 mL) group than in the low dose ingestion (<30 mL) group (both P < .01), suggesting that coagulation dysfunction is associated with dosage of PQ in poisoned patients. Similarly, it was also observed that daily PT values (Figure 2A) in the death group were significantly higher than those in the surviving group within 7 days of admission (P < .05), with significant differences between the 2 groups (P < .01). At the same time, observing the comparison of PQ intake dose between the 2 groups (Table 1), we can easily conclude that high doses of PQ may induce severe coagulation dysfunction, which may in turn affect the survival of patients with PQ poisoning (P < .001). Furthermore, using univariate and multivariate Cox regression analyses (Table 2), it was also found that PT was an independent risk factor predicting 40‐day mortality in patients with PQ poisoning. The explanation for this phenomenon may be related to injury of the pulmonary endothelial cells due to PQ, which results in the release of large amounts of tissue factor and von Willebrand factor into the blood to activate the extrinsic coagulation pathway. We suggest that this result may be similar to the mechanism by which other factors induce pulmonary endothelial cell injury, leading to dysfunction of the extrinsic coagulation pathway.30 In addition, high doses of PQ‐induced severe injury to the vascular endothelial cells in the lung tissue, which can encourage platelets to adhere, aggregate, and activate when exposed to blood flow. This in turn activates the coagulation system, consuming large amounts of coagulation factors and leading to dysfunction of coagulation. In addition to the excessive consumption of relevant clotting factors, another possible reason for the elevated PT and APTT values was reduced synthesis. Because the liver is the major synthetic organ for most clotting factors in the body, patients with high dose PQ poisoning usually have acute liver injury at the initial stage with varying degrees of hypohepatia. However, changes in liver function in patients with PQ poisoning were not the focus of our study.
Extensive pulmonary fibrosis often occurs in the second stage of PQ‐induced pulmonary toxicity, and may be a compensatory repair mechanism for the damaged alveolar epithelial cells.31 As an important feature of clinical progression in patients with PQ poisoning, acute respiratory distress syndrome is the most common reason for death in large numbers of patients with high dose oral poisoning within a few days. The study of Kubo et al32 showed that plasma D‐dimer levels were associated with mortality in patients with acute progression of idiopathic fibrosis, and that anticoagulant therapy could improve patient survival. Similarly, animal models of acute paraquat poisoning established by Liu Feng et al33 also showed that low‐molecular‐weight heparins combined with aspirin anticoagulant therapy for paraquat poisoning could significantly improve the coagulation dysfunction caused by paraquat and reduce paraquat‐induced acute lung injury. However, we did not find a clear relationship between changes in D‐dimer levels and patient survival in our study. This may be due to the difference between the previous studies and the status of disease progression in human pulmonary fibrosis induced by paraquat.
Univariate and multivariate Cox regression analyses showed that the PT value, PQ ingestion volume, and sex were independent risk factors for predicting 40‐day mortality. We can clearly observe from the Kaplan‐Meier survival curve analysis that 40‐day survival was significantly lower in patients with PT values above 12 s (Figure 3A). This may provide new treatment options and ideas for treating acute lung injury caused by paraquat poisoning. High ingestion volume showed a significant positive correlation with mortality (Figure 3C), and the survival in male group was significantly lower than that in female group (Figure 3B). We further compared PQ ingestion volumes between the groups divided by sex; the results showed that the ingestion volume of the male group (66.24 ± 14.05 mL) was significantly higher than that of the female group (30.19 ± 4.77 mL) (P = .003). This may be one of the reasons why the survival in male group was significantly lower.
There were some limitations in our study. First, this study was a retrospective analysis; we could thus only obtain results from routine coagulation index tests in patients with PQ poisoning from clinical data, but not information on the influence of PQ on the levels of clotting factors. In this regard, we will conduct further studies in our future work. Second, we did not know the exact PQ concentrations in the patients’ blood, as the PQ poisoning cases were included only on the basis of oral PQ intake history. While the predictive value of plasma PQ concentration has been reported in the literature on patients with acute PQ poisoning,4, 34 concentration values are not commonly available in the emergency room due to limited medical equipment.35
5. CONCLUSION
In summary, the coagulation status in patients with PQ poisoning was closely related to their prognoses. Routine monitoring of coagulation function, especially the PT value in plasma, is helpful for analysis of the condition and prognoses of patients with PQ poisoning.
Hu X, Guo R, Chen X, Chen Y. Increased plasma prothrombin time is associated with poor prognosis in patients with paraquat poisoning. J Clin Lab Anal. 2018;32:e22597 10.1002/jcla.22597
Funding information
This work was financially supported by the Medical Science and Technology Project of Zhejiang Province (2017KY337).
REFERENCES
- 1. Gawarammana IB, Buckley NA. Medical management of paraquat ingestion. Br J Clin Pharmacol. 2011;72:745‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seok SJ, Gil HW, Jeong DS, Yang JO, Lee EY, Hong SY. Paraquat intoxication in subjects who attempt suicide: why they chose paraquat. Korean J Intern Med. 2009;24:247‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee KH, Gil HW, Kim YT, Yang JO, Lee EY, Hong SY. Marked recovery from paraquat‐induced lung injury during long‐term follow‐up. Korean J Intern Med. 2009;24:95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila). 2008;46:515‐518. [DOI] [PubMed] [Google Scholar]
- 5. Pavan M. Acute kidney injury following Paraquat poisoning in India. Iran J Kidney Dis. 2013;7:64‐66. [PubMed] [Google Scholar]
- 6. Bismuth C, Garnier R, Baud FJ, Muszynski J, Keyes C. Paraquat poisoning. An overview of the current status. Drug Saf. 1990;5:243‐251. [DOI] [PubMed] [Google Scholar]
- 7. Dinis‐Oliveira RJ, Duarte JA, Sánchez‐Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13‐71. [DOI] [PubMed] [Google Scholar]
- 8. Kim YT, Jou SS, Lee HS, et al. The area of ground glass opacities of the lungs as a predictive factor in acute paraquat intoxication. J Korean Med Sci. 2009;24:636‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rannels DE, Kameji R, Pegg AE, Rannels SR. Spermidine uptake by type II pneumocytes: interactions of amine uptake pathways. Am J Physiol. 1989;257:346‐353. [DOI] [PubMed] [Google Scholar]
- 10. Furie B, Furie BC. Thrombus formation in vivo. J Clin Invest. 2005;115:3355‐3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran JM, Ortiz‐Ortiz MA, Ruiz‐Mesa LM, Fuentes JM. Nitric oxide in paraquat‐mediated toxicity: a review. J Biochem Mol Toxicol. 2010;24:402‐409. [DOI] [PubMed] [Google Scholar]
- 12. Seok SJ, Kim SJ, Gil HW, Yang JO, Lee EY, Hong SY. Tissue plasminogen activator and plasminogen activator inhibitor‐1 levels in patients with acute paraquat intoxication. J Korean Med Sci. 2011;26:474‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vale JA, Meredith TJ, Buckley BM. Paraquat poisoning: clinical features and immediate general management. Hum Toxicol. 1987;6:41‐47. [DOI] [PubMed] [Google Scholar]
- 14. Pond S. Manifestations and management of paraquat poisoning. Med J Aust. 1990;152:256‐259. [DOI] [PubMed] [Google Scholar]
- 15. Bismuth C, Hall A, Wong A. Paraquat ingestion exposure: symptomatology and risk In: Bismuth C, Hall AH, eds. Paraquat Poisoning: Mechanisms, Prevention, Treatment. New York, NY: Marcel Dekker; 1995:195‐210. [Google Scholar]
- 16. Modee J, Ivemark BI, Robertson B. Ultrastructure of the alveolar wall in experimental paraquat poisoning. Acta Pathol Microbiol Scand. 1972;80:54‐60. [DOI] [PubMed] [Google Scholar]
- 17. Vijeyaratnam GS, Corrin B. Experimental paraquat poisoning: a histological and electron‐optical study of the changes in the lung. J Pathol. 1971;103:123‐129. [DOI] [PubMed] [Google Scholar]
- 18. Sykes BI, Purchase IF, Smith LL. Pulmonary ultrastructure after oral and intravenous dosage of paraquat to rats. J Pathol. 1977;121:233‐241. [DOI] [PubMed] [Google Scholar]
- 19. Dearden LC, Fairshter RD, Morrison JT, Wilson AF, Brundage M. Ultrastructural evidence of pulmonary capillary endothelial damage from paraquat. Toxicology. 1982;24:211‐222. [DOI] [PubMed] [Google Scholar]
- 20. China Doctor Association of Emergency . Consensus of acute paraquat diagnosis and treatment. China J Crit Care Med. 2013;33:484‐490. [Google Scholar]
- 21. Yang W, Tiffany‐Castiglioni E. The bipyridyl herbicide paraquat induces proteasome dysfunction in human neuroblastoma SH‐SY5Y cells. J Toxicol Environ Health A. 2007;70:1849‐1857. [DOI] [PubMed] [Google Scholar]
- 22. Bonneh‐Barkay D, Reaney SH, Langston WJ, Di Monte DA. Redox cycling of the herbicide paraquat in microglial cultures. Brain Res Mol Brain Res. 2005;134:52‐56. [DOI] [PubMed] [Google Scholar]
- 23. Castello PR, Drechsel DA, Patel M. Mitochondria are a major source of paraquat‐induced reactive oxygen species production in the brain. J Biol Chem. 2007;282:14186‐14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mohammadi‐Bardbori A, Ghazi‐Khansari M. Alternative electron acceptors: proposed mechanism of paraquat mitochondrial toxicity. Environ Toxicol Pharmacol. 2008;26:1‐5. [DOI] [PubMed] [Google Scholar]
- 25. Blanco‐Ayala T, Anderica‐Romero AC, Pedraza‐Chaverri J. New insights into antioxidant strategies against paraquat toxicity. Free Radic Res. 2014;48:623‐640. [DOI] [PubMed] [Google Scholar]
- 26. Han J, Zhang Z, Yang S, Wang J, Yang X, Tan D. Betanin attenuates paraquat‐induced liver toxicity through a mitochondrial pathway. Food Chem Toxicol. 2014;70:100‐106. [DOI] [PubMed] [Google Scholar]
- 27. Proudfoot AT. Predictive value of early plasma paraquat concentrations In: Bismuth C, Hall AH, eds. Paraquat Poisoning: Mechanisms, Prevention, Treatment. New York, NY: Marcel Dekker; 1995:275‐283. [Google Scholar]
- 28. Proudfoot AT, Stewart MS, Levitt T, Widdop B. Paraquat poisoning: significance of plasma‐paraquat concentrations. Lancet. 1979;18:330‐332. [DOI] [PubMed] [Google Scholar]
- 29. Smith LL, Wright A, Wyatt I, Rose MS. Effective treatment for paraquat poisoning in rats and its relevance to treatment of paraquat poisoning in man. Br Med J. 1974;4:569‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang L, Wei J, Guo F, et al. Endosulfan activates the extrinsic coagulation pathway by inducing endothelial cell injury in rats. Environ Sci Pollut Res Int. 2015;22:15722‐15730. [DOI] [PubMed] [Google Scholar]
- 31. Smith P, Heath D. Paraquat. Crit Rev Toxicol. 1976;4:411‐445. [DOI] [PubMed] [Google Scholar]
- 32. Kubo H, Nakayama K, Yanai M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest. 2005;128:1475‐1482. [DOI] [PubMed] [Google Scholar]
- 33. Liu F, Jian XD, Zhang ZC, et al. Experimental anticoagulant therapy of acute lung injury induced by paraquat. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012;30:190‐193. [PubMed] [Google Scholar]
- 34. Senarathna L, Eddleston M, Wilks MF, et al. Prediction of outcome after paraquat poisoning by measurement of the plasma paraquat concentration. QJM. 2009;102:251‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weng CH, Hu CC, Lin JL, et al. Sequential organ failure assessment score can predict mortality in patients with paraquat intoxication. PLoS ONE. 2012;7:e51743. [DOI] [PMC free article] [PubMed] [Google Scholar]


