Abstract
Background
Serum creatinine, urea, and cystatin‐c are standardly used for the evaluation of renal function in the clinic. However, some patients have chronic kidney disease but still retain kidney function; a conventional serum index in these patients can be completely normal. Serum amino acid levels can reflect subtle changes in metabolism and are closely related to renal function. Here, we investigated how amino acids change as renal impairment increases.
Methods
Subjects were divided into three groups by renal function glomerular filtration rate: healthy controls, patients with chronic kidney disease with normal kidney function, and patients with chronic kidney disease with decreased kidney function group. We identified 11 amino acids of interest using LC‐MS/MS on MRM (+) mode.
Results
Statistical analysis indicated that alanine (ALA), valine (VAL), and tyrosine (TYR) decrease with renal function impairment, whereas phenylalanine (PHE) and citrulline (CIT) increase. We tried to construct a diagnostic model utilizing a combination of amino acids capable of identifying early chronic kidney disease patients. The accuracy, specificity, and sensitivity of the combining predictors were 86.9%, 84.6%, and 90.9%, respectively, which is superior to the reported values for serum creatinine, urea, and cystatin‐c.
Conclusion
Our data suggest that serum amino acid levels may supply important information for the early detection of chronic kidney disease. We are the first to establish a diagnostic model utilizing serum levels of multiple amino acids for the diagnosis of patients with early‐stage chronic kidney disease.
Keywords: amino acids, chronic kidney disease, ROC curve
1. Abbreviations:
- Ala
Alanine
- ARG
Arginine
- BCAA
Branched‐chain amino acid
- CIT
Citrulline
- CKD
chronic kidney disease
- GLY
Glycine
- LEU
Leucine
- MET
Methionine
- ORN
Ornithine
- PHE
Phenylalanine
- PRO
Proline
- SER
Serine
- TYR
Tyrosine
- VAL
Valine
2. INTRODUCTION
Kidney function is routinely evaluated using levels of serum creatinine1 and urea2 and cystatin‐c,3 which reflect the glomerular filtration rate. However, these measures may not be ideal for the assessment of renal function because of the compensatory ability of the kidney and the influence of muscle mass and dietary intake on kidney function.4 Cystatin‐C is a more sensible indicator of the glomerular filtration rate than creatinine,5 but it is affected by thyroid function. Renal biopsy is invasive and is not easily performed in the clinic; furthermore, it has a number of contraindications such as the tendency to hemorrhage, severe hypertension, and renal malformation. Moreover, in chronic kidney disease (CKD) patients, the incidence of biopsy‐related complications increases and whether patients should undergo renal biopsy is a debatable point.6 Routine urine analysis provides evidence of kidney damage and it is used for screening, but it is affected by other factors.
Amino acids are produced as important metabolites of human body, synthesized mainly in liver and kidney, filtered freely by the glomerulus, and near completely absorbed at the proximal tubule. The human kidney plays a vital role in maintaining amino acid homeostasis by balancing amino acids synthesis, release, and transport.7, 8 In the clinic, serum amino acid levels are usually used as nutritive index. With the deepening understanding of metabolites, serum amino acid levels are more and more used as disease biomarkers.9, 10, 11, 12, 13, 14, 15, 16 Other research has focuses on serum amino acids changes in patients with various kidney diseases including drug‐induced nephrotoxicity17, 18 and chronic renal failure.19, 20, 21
Here, we investigated how changes in serum amino acid levels correspond to stages of renal function impairment with the aim of identifying more sensitive, accurate, and convenient serum biomarkers for early detection of chronic kidney disease. If chronic kidney disease is detected earlier and progression slowed, patient prognosis and quality of life can be significantly improved. Improved biomarkers will allow for earlier intervention to prevent further progression of chronic kidney disease.
3. MATERIALS AND METHODS
3.1. Materials and chemicals
A NeoBase Non‐derivatized MSMS Kit was purchased from PerkinElmer (Turku, Finland) including isotope internal standards and quality control standards (Table.1). Methanol (LC–MS grade) was purchased from Thermofisher (Shanghai, China). Blood spot cards were purchased from BaoRong Science and Technology Ltd. (Hangzhou, China).
Table 1.
Analytes, internal standards, and control materials as determined using a NeoBase Non‐derivatized MSMS Kit
| Analyte | Internal standard | Control materials |
|---|---|---|
| ALA | 2H4‐ALA | ALA |
| ARG | 2H4,13C‐ARG | ARG |
| CIT | 2H2‐CIT | CIT |
| GLY | 15N,2‐13C‐GLY | GLY |
| LEU/ILE/PRO‐OH | 2H3‐LEU | LEU |
| MET | 2H3‐MET | MET |
| ORN | 2H6‐ORN | ORN |
| PHE | 13C6‐PHE | PHE |
| PRO | 13C5‐PRO | PRO |
| TYR | 13C6‐TYR | TYR |
| VAL | 2H8‐VAL | VAL |
ALA, Alanine; ARG, Arginine; CIT, Citrulline; GLY, Glycine; LEU, Leucine; ILE, Isoleucine; PRO, Proline; MET, Methionine; ORN, Ornithine; PHE, Phenylalanine; TYR, Tyrosine; VAL, Valine.
3.2. Study design and patients
This is a case‐control study consisted of different stages of chronic kidney disease patients and health control. Through determination of 11 kinds of serum amino acids to establish a diagnostic model for determination of early‐stage chronic kidney disease. And compare this model with conventional serum kidney index. The flow chart of the study design is shown in Figure 1.
Figure 1.
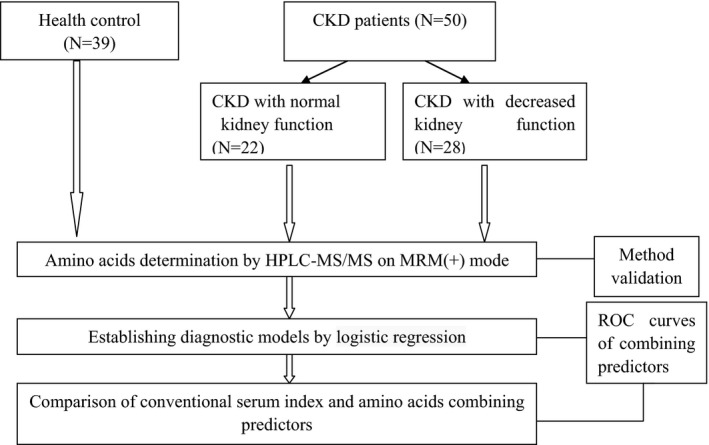
The flowchart of study design
Fifty chronic kidney disease patients were enrolled from the nephrology department at the First Affiliated Hospital of China Medical University between August 2015 and August 2016, the primary diagnosis was confirmed by biopsy of chronic glomerulonephritis or interstitial nephritis.
Chronic kidney disease staging criteria was based on Kidney Disease Outcomes Quality Initiative (K/DOQI) (Table 2). Subjects were excluded if they had metabolic disorders (hypertension or diabetes), severe liver disease, or small bowel disease. Patients were divided into two groups according to their glomerular filtration rate: patients with chronic kidney disease with normal kidney function group (CKD stage I) and patients with chronic kidney disease and decreased kidney function. A total of thirty‐nine healthy control subjects were enrolled from medical examination center during the same period. (Table 3) They were considered healthy on the basis of general medical examination. The health control subjects were excluded if (i) they had history of renal transplantation; (ii) they had urinary sediment or serum kidney function index abnormity; (iii) they had kidney imaging structural abnormalities; and (iv) they had history of hypertension or diabetes. The research was approved by Institutional Ethics and all subjects have obtained informed consent.
Table 2.
Stages of Chronic Kidney Disease in K/DOQI
| Stage | GFR (mL/min/1.73 m2) | Description |
|---|---|---|
| I | ≥90 | Kidney damage with normal or ↑ GFR |
| II | 60‐89 | Kidney damage with mild ↓ GFR |
| III | 30‐59 | Moderate ↓ GFR |
| IV | 15‐29 | Severe ↓ GFR |
| V | <15 | Kidney failure |
Table 3.
Clinical characteristics of health control and CKD patients
| Variables | Health control (N=39) | CKD with normal kidney function (N=22) | CKD with decreased kidney function (N=28) | P value |
|---|---|---|---|---|
| Gender | ||||
| Male | 20 | 14 | 15 | >.05 |
| Female | 19 | 8 | 13 | |
| Age (years) | 45.49±13.09 | 40.45±14.24 | 46.67±13.63 | >.05 |
3.3. Solution preparation
Internal standards and stock solutions were prepared by rehydrating the dried amino acids internal standard with 1.0‐mL NeoBase extraction solution and mixing adequately until totally dissolved. The daily working solution was prepared by diluting the stock solution to be 110 times; the working solution was stable 30 days when stored in a sealed vial at a temperature between 2 and 8°C.
3.4. Sample preparation
First, serum samples were applied to blood spot cards for convenient storage and transport. Next, amino acids were extracted from the serum using a NeoBase Non‐derivatized MSMS Kit. A 3.2‐mm‐diameter spot, equivalent to approximately 3 μL of serum, was punched into a 96‐well plate. One hundred μL of daily work solution was added to every well. The microplate was oscillated for 45 minutes at a temperature of 45±5°C. Seventy‐five μL solution of each sample was transferred to heat‐resistant microplate for analysis.
3.5. HPLC‐MS/MS conditions
All samples were analyzed on an API 4000 tandem triple‐quadrupole mass spectrometer (AB SCIEX, USA) in positive ion mode, controlled by Analyst® software version 1.6.1 and coupled with an HPLC system (SHIMADZU, Japan). The ion spray voltage, temperature, curtain gas, gas1, and gas2 were set at 5500 V, 550°C, 20L/min, 10L/min, and 10L/min, respectively. Selected MRM ion pairs and other mass spectrometric parameters are summarized in Table 4. The preliminary chromatographic separation was performed using a gradient change in flow rate as described in Table 5.
Table 4.
MRM ion pairs and mass spectrometric parameters
| Amino acids | Q1 | Q3 | CE | DP | EP | CXP |
|---|---|---|---|---|---|---|
| Pro | 116.1 | 70.1 | 25 | 21 | 15 | 5 |
| Val | 118.1 | 72.1 | 50 | 17 | 15 | 5 |
| Pro IS | 121.1 | 74.1 | 25 | 21 | 15 | 5 |
| Val IS | 126.1 | 80.1 | 50 | 17 | 15 | 5 |
| Leu | 132.1 | 86.1 | 52 | 13 | 15 | 5 |
| Orn | 133.1 | 70.1 | 45 | 24 | 15 | 5 |
| Leu IS | 135.1 | 89.1 | 52 | 13 | 15 | 5 |
| Orn IS | 139.1 | 76.1 | 45 | 24 | 15 | 5 |
| Met | 150.1 | 104.1 | 49 | 15 | 6 | 5 |
| Met IS | 153.1 | 107.1 | 49 | 15 | 6 | 5 |
| Phe | 166.1 | 120.1 | 48 | 18 | 15 | 5 |
| Phe IS | 172.1 | 172.1 | 48 | 18 | 15 | 5 |
| Arg | 175.1 | 175.1 | 60 | 30 | 15 | 5 |
| Cit | 176.1 | 176.1 | 44 | 23 | 7 | 5 |
| Cit IS | 178.1 | 178.1 | 44 | 23 | 7 | 5 |
| Arg IS | 180.1 | 180.1 | 60 | 30 | 15 | 5 |
| Tyr | 182.1 | 182.1 | 52 | 19 | 9 | 5 |
| Tyr IS | 188.1 | 188.1 | 52 | 19 | 9 | 5 |
| Gly | 76 | 76 | 46 | 17 | 5 | 5 |
| Gly IS | 78 | 78 | 46 | 17 | 5 | 5 |
| Ala | 90 | 90 | 48 | 21 | 10 | 5 |
| Ala IS | 94 | 94 | 48 | 21 | 10 | 5 |
IS, Internal Standard; PRO, Proline; VAL, Valine; LEU, Leucine; ORN, Ornithine; MET, Methionine; PHE, Phenylalanine; ARG, Arginine; CIT, Citrulline; TYR, Tyrosine; GLY, Glycine; ALA, Alanine.
Table 5.
Preliminary chromatographic separation
| Time(s) | Module | Event |
|---|---|---|
| 0.20 | Pumps | Total Flow |
| 0.22 | Pumps | Total Flow |
| 1.20 | Pumps | Total Flow |
| 1.21 | Pumps | Total Flow |
| 1.50 | Pumps | Total Flow |
| 1.51 | Pumps | Total Flow |
| 1.70 | Controller | Stop |
3.6. Amino acid determination
Eleven kinds of amino acids were identified using the API4000 LC‐MS/MS on MRM(+) mode. The concentration of every amino acid was calculated by comparing the response of analyte with the response of its internal standard in daily work solution.
3.7. Method validation
In this method, a NeoBase Non‐derivatized MSMS Kit was used for extraction and calculation. The linearity, specificity, sensitivity, accuracy, precision, recovery, samples’ residual, interference, and stability have been validated and described in the literature provided with the kit; however, we also investigated the accuracy and precision of the data by performing a replicate analysis of three batches of quality control samples at two concentration levels (low and high). The accuracy and precision were expressed as the mean percentage recovery and percent coefficient of variation (CV%), respectively (Table 6).
Table 6.
Within‐ and between‐batch precision of high‐quality control (HC) and low‐quality control (LC)
| HC | LC | |||
|---|---|---|---|---|
| Within‐batch precision (%) | Between‐batch precision (%) | Within‐batch precision (%) | Between‐batch precision (%) | |
| ALA | 5.897 | 3.057 | 3.948 | 9.815 |
| CIT | 5.692 | 3.184 | 4.438 | 2.182 |
| GLY | 5.903 | 3.235 | 4.294 | 3.34 |
| LEU | 5.713 | 3.338 | 4.114 | 2.825 |
| MET | 5.805 | 3.108 | 4.213 | 16.994 |
| PHE | 5.713 | 3.463 | 4.129 | 20.579 |
| PRO | 5.688 | 1.487 | 3.637 | 5.913 |
| TYR | 5.906 | 4.024 | 4.063 | 8.954 |
| VAL | 5.601 | 3.289 | 4.175 | 8.851 |
ALA, Alanine; CIT, Citrulline; GLY, Glycine; LEU, Leucine; MET, Methionine; PHE, Phenylalanine; PRO, Proline; TYR, Tyrosine; VAL, Valine.
3.8. Statistical analysis
Statistical analysis was performed using SPSS software, version 19.0. The data were numerical and checked its normality with Kolmogorov‐Smirnov test. The median and the percentile were used to describe abnormal distribution statistics; the Mann‐Whitney U tests were used for comparing differences among groups. Based on the above analysis, we built binary logistic regression diagnostic models, and ROC analysis was used for evaluation of diagnostic efficacy. The level of significance was taken as P<.05.
4. RESULTS
4.1. The serum levels of 11 amino acids in each of the three groups were measured; variation tendencies for each amino acid are listed and shown in Figure 2
Figure 2.
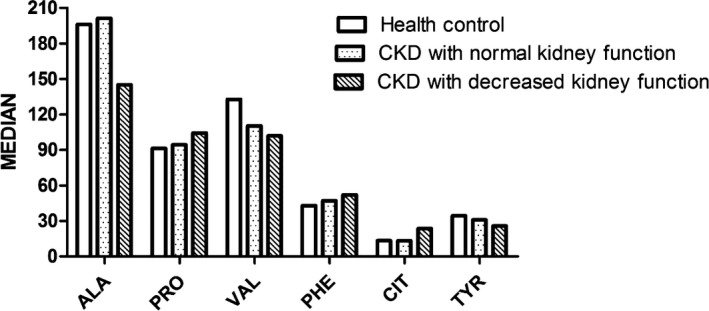
Ala, Pro, Val, Phe, Tyr, and Cit variations as renal impairment increases. As renal function declines, we found that serum levels of alanine, tyrosine, and valine also gradually decrease while phenylalanine and citrulline increase, citrulline kept the same level between health control and early stage of chronic kidney disease but increases markedly in chronic kidney disease with decreased kidney function
As renal function declines, we found that serum levels of alanine, tyrosine, and valine also gradually decrease while phenylalanine and citrulline increase, citrulline kept the same level between health control and early stage of chronic kidney disease, but increases markedly in chronic kidney disease with decreased kidney function.
4.2. Binary logistic regression was used to construct two diagnostic models to distinguish between groups
The classification equation between healthy controls and patients with CKD but normal kidney function is P=Eq.(A.1)=[1+e(−0.193Val+0.070Pro+0.310Phe+1.081)]−1; the classification equation to distinguish between healthy controls and patients with decreased kidney function is P=Eq.(B.1)=[1+e(−0.032Ala−0.077Val+0.277Phe+0.242Cit−1.477)]−1. ROC curves of combining predictors based on logistic model are shown in Figure 3. The area under the curve was 0.928 and 0.944, respectively, P<.01. The comparison of conventional serum index and amino acids combining predictors is shown in Table 7. We observed that in patients with CKD but normal kidney function, the sensitivity and specificity of measuring single amino acid serum levels is not ideally predictive. Therefore, we attempted to construct a diagnostic model using multiple amino acids (PHE, VAL, PRO, and TYR) to assess kidney function. This model evaluates the diagnostic efficiency.
Figure 3.
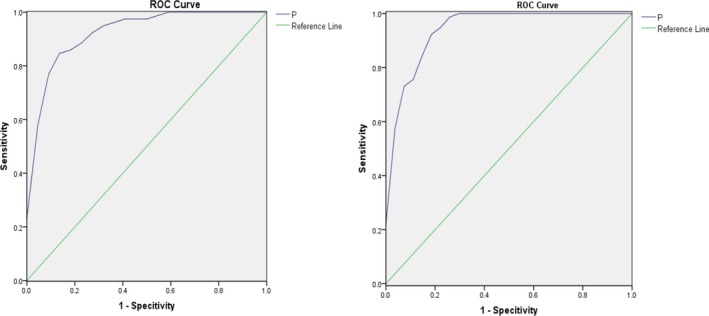
ROC curve of classification equation. (Left) Combining predictors distinguishing patients with CKD with normal kidney function and healthy controls. (Right) Combining predictors distinguishing patients with CKD with decreased kidney function and healthy controls
Table 7.
Comparison of conventional serum index levels and amino acids combination predictors
| CKD with normal kidney function | CKD with decreased kidney function | |||
|---|---|---|---|---|
| Accuracy | Sensitivity | Accuracy | Sensitivity | |
| Amino acids predictors | 86.9% | 90.9% | 89.4% | 85.2% |
| Cystatin‐c | 83.6% | 50.0% | 98.5% | 95.5% |
| Creatinine | 70.1% | 9.1% | 92.7% | 83.8% |
5. DISCUSSION
We were able to identify serum levels of 11 amino acids using an API4000 MSMS, including most amino acids that are metabolized in kidney. Free amino acids are stored in skeletal muscle and are metabolized mainly in the liver, kidney, and brain. The kidney plays an important role in amino acid metabolism, responsible for some amino acid synthesis, and transportation. Our results indicate that serum amino acid levels are closely associated with renal function, with some amino acids gradually increasing and others decreasing. Our diagnostic model was able to successfully distinguish between healthy subjects and patients with early CKD.
In the 1980s, there was a surge of interest in studying the relationship between amino acids and kidney disease. Previous studies about amino acids variation was almost about increase in citrulline and proline, and decrease in tyrosine and valine.20, 22 Our results confirmed these amino acids changes; in addition, we found that alanine and phenylalanine gradually but significantly decreased as kidney function impairment increased. Meanwhile, we are the first to successfully construct a diagnostic mode distinguishing healthy subjects and CKD patients with normal kidney function. From our results, we can conclude that amino acids variation is closely associated with renal function, as some amino acids gradually increase or decrease renal function impairment progresses. Alanine and valine levels decrease when kidney damage is present but renal function remains normal. While there was no significant difference in citrulline levels in patients with CKD with normal kidney function as compared to control subjects, citrulline did increase when kidney function decreased. Therefore, we concluded that multiple, different amino acids profiles are needed to accurately assess renal function.
According to the Kidney Disease Outcomes Quality Initiative, a diagnosis of early‐stage CKD requires evidence of kidney damage over 3 months including abnormalities in the composition of the blood or urine, pathological abnormalities, and abnormalities in imaging tests. The rates of abnormality in the conventional amino acid serum index are low and thus not useful for identifying patients with early stages of chronic kidney injury; however, using combinations of amino acids as predictors significantly elevated the accuracy rate, abnormity rate, sensitivity, and specificity. In addition, routine urine analysis provides a screening approach for kidney damage, but random urine is affected by many factors including menstrual blood contamination, urinary tract infection, and strenuous exercise among others.23 By contrast, serum biomarkers are more stable and fewer confounding factors.
Phenylalanine is an essential amino acid that cannot be synthesized by the human body. Phenylalanine hydroxylase is present in liver, kidney, and pancreas.24 (Figure 4). When chronic renal insufficiency occurs, phenylalanine concentrations progressively decrease.25 Isotope studies revealed that phenylalanine hydroxylation was impaired during chronic renal failure, thus resulting in decreases in tyrosine levels as well.26 By contrast, we found that phenylalanine increased slightly when CKD patients’ kidney function was normal and continued to increase progressively during CKD development. Thus, we concluded that phenylalanine could serve as a useful biomarker for kidney function.
Figure 4.
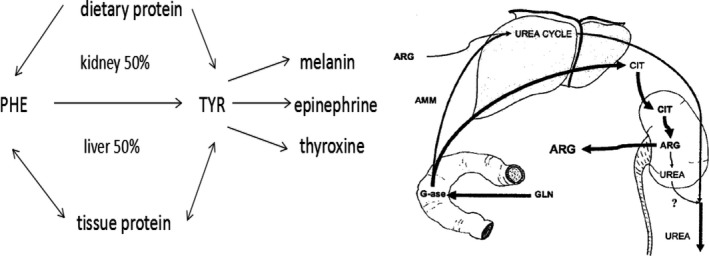
Citrulline and phenylalanine metabolism in kidney
Citrulline is a nonessential amino acid involved in the urea cycle (Figure 4).27, 28 Glutamine is metabolized by the gut into ammonia (NH3) and citrulline, thus CIT levels are closely associated with intestinal diseases such as short bowel syndrome. In recent years, use of CIT as a biomarker for rheumatoid arthritis has drawn attention.29 In patients with chronic renal failure, the kidney progressively filters less CIT; meanwhile less CIT is metabolized into arginine, so CIT levels increase in serum. In our research, we found no change in citrulline levels in patients with CKD with normal kidney function as compared with healthy controls. CIT levels did increase significantly when kidney function decreased indicating that during kidney function impairment, changes in citrulline levels occur later in the process.
Valine is nonessential amino acid and branched‐chain amino acid (BCAA). BCAAs have peculiar metabolism as they can avoid metabolism in the liver, but in skeletal muscle. Valine is converted into branched‐chain keto acids that supply energy for the kidney as well as other organs. It is not clear that how BCAAs metabolize in kidney as researchers found that there was no valine intake or release.19, 30 In patients with chronic renal failure, the kidney progressively filters less CIT; meanwhile less CIT is metabolized into arginine, so CIT levels increase in serum. In our research, we found no change in citrulline levels in patients with CKD with normal kidney function as compared with healthy controls. CIT levels did increase significantly when kidney function decreased indicating that during kidney function impairment, changes in citrulline levels occur later in the process.
Valine is nonessential amino acid and branched‐chain amino acid (BCAA). BCAAs have peculiar metabolism as they can avoid metabolism in the liver, but in skeletal muscle. Valine is converted into branched‐chain keto acids that supply energy for the kidney as well as other organs. It is not clear how BCAAs metabolize in kidney as researchers found that there was no valine intake or release.19, 30 In our study, patients had normal nutrition conditions and renal function compensation, at least in the group of patients with CKD with normal kidney function group. In this group, the significant decrease in valine levels is likely due to decreasing release of valine by skeletal muscle.
We also included proline in our diagnostic model. Proline in serum results from glutamate metabolism or the degradation of collagen; its oxidation process is conducted by proline dehydrogenase. Proline has been reported to play a role in tumor cell apoptosis and autophagy31 and there have been few reports of proline metabolism during kidney injury.
In conclusion, there have been numerous recent reports of amino acids as biomarkers, but few studies have focused on early‐stage CKD. We identified and measured serum levels of 11 amino acids in patients with CKD and constructed a diagnostic model utilizing a combination of amino acids. Our study included patients with early‐stage CKD as confirmed by renal biopsy, whose conventional serum amino acid index was almost normal. In this stage of disease, a panel that included a combination of amino acids was markedly different from health subjects. The diagnostic model is expected to be used for the early detection of CKD.
STUDY LIMITATIONS
This study measured 11 kinds of serum amino acids, other kinds of full‐spectrum amino acids can be measured if possible. This is a preliminary study for identification of early chronic kidney disease, the sample size can be enlarged and some cases to verify our diagnostic models.
CONFLICT OF INTEREST
The authors declare they have no conflict of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
Informed consent was obtained from all individual participants included in the study.
Li R, Dai J, Kang H. The construction of a panel of serum amino acids for the identification of early chronic kidney disease patients. J Clin Lab Anal. 2018;32:e22282 10.1002/jcla.22282
Funding information
This work was supported by National Natural Science Foundation of China (81501801).
REFERENCES
- 1. Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933‐1953. [PubMed] [Google Scholar]
- 2. Schuck O, Erben J, Nadvornikova H, et al. Residual kidney function and plasma urea concentration in patients with chronic renal failure. Int Urol Nephrol. 1990;22:573‐579. [DOI] [PubMed] [Google Scholar]
- 3. Randers E, Erlandsen EJ. Serum cystatin C as an endogenous marker of the renal function–a review. Clin Chem Lab Med. 1999;37:389‐395. [DOI] [PubMed] [Google Scholar]
- 4. Tomlanovich S, Golbetz H, Perlroth M, Stinson E, Myers BD. Limitations of creatinine in quantifying the severity of cyclosporine‐induced chronic nephropathy. Am J Kidney Dis. 1986;8:332‐337. [DOI] [PubMed] [Google Scholar]
- 5. Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H. Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol. 2000;54:203‐209. [PubMed] [Google Scholar]
- 6. Parrish AE. Complications of percutaneous renal biopsy: a review of 37 years’ experience. Clin Nephrol. 1992;38:135‐141. [PubMed] [Google Scholar]
- 7. Cynober LA. Plasma amino acid levels with a note on membrane transport: characteristics, regulation, and metabolic significance. Nutrition 2002;18:761‐766. [DOI] [PubMed] [Google Scholar]
- 8. Verrey F, Singer D, Ramadan T, Vuille‐dit‐Bille RN, Mariotta L, Camargo SM. Kidney amino acid transport. Pflugers Arch. 2009;458:53‐60. [DOI] [PubMed] [Google Scholar]
- 9. Miyagi Y, Higashiyama M, Gochi A, et al. Plasma free amino acid profiling of five types of cancer patients and its application for early detection. PLoS ONE. 2011;6:e24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Magnusson M, Lewis GD, Ericson U, et al. A diabetes‐predictive amino acid score and future cardiovascular disease. Eur Heart J. 2013;34:1982‐1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shingyoji M, Iizasa T, Higashiyama M, et al. The significance and robustness of a plasma free amino acid (PFAA) profile‐based multiplex function for detecting lung cancer. BMC Cancer. 2013;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Esther CR Jr, Turkovic L, Rosenow T. Metabolomic biomarkers predictive of early structural lung disease in cystic fibrosis. Eur Respir J 2016;48:1612‐1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu L, Gao Y, Cao Y, et al. Association of plasma arginine with breast cancer molecular subtypes in women of Liaoning province. IUBMB Life. 2016;68:980‐984. [DOI] [PubMed] [Google Scholar]
- 14. Kimura T, Hamase K, Miyoshi Y, et al. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease. Sci Rep. 2016;6:26137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klupczynska A, Derezinski P, Dyszkiewicz W, Pawlak K, Kasprzyk M, Kokot ZJ. Evaluation of serum amino acid profiles’ utility in non‐small cell lung cancer detection in Polish population. Lung Cancer 2016;100:71‐76. [DOI] [PubMed] [Google Scholar]
- 16. Rizner TL. Discovery of biomarkers for endometrial cancer: current status and prospects. Expert Rev Mol Diagn. 2016;16:1315‐1336. [DOI] [PubMed] [Google Scholar]
- 17. Boudonck KJ, Mitchell MW, Nemet L, et al. Discovery of metabolomics biomarkers for early detection of nephrotoxicity. Toxicol Pathol. 2009;37:280‐292. [DOI] [PubMed] [Google Scholar]
- 18. Kazubek‐Zemke M, Rybka J, Marchewka Z, Rybka W, Pawlik K, Dlugosz A. Preliminary study on application of urine amino acids profiling for monitoring of renal tubular injury using GLC‐MS. Postepy Hig Med Dosw(Online). 2014;68:1299‐1311. [DOI] [PubMed] [Google Scholar]
- 19. Tizianello A, De Ferrari G, Garibotto G, Gurreri G, Robaudo C. Renal metabolism of amino acids and ammonia in subjects with normal renal function and in patients with chronic renal insufficiency. J Clin Investig. 1980;65:1162‐1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ceballos I, Chauveau P, Guerin V, et al. Early alterations of plasma free amino acids in chronic renal failure. Clin Chim Acta. 1990;188:101‐108. [DOI] [PubMed] [Google Scholar]
- 21. Levillain O, Parvy P, Hassler C. Amino acid handling in uremic rats: citrulline, a reliable marker of renal insufficiency and proximal tubular dysfunction. Metabolism. 1997;46:611‐618. [DOI] [PubMed] [Google Scholar]
- 22. Gulyassy PF, Peters JH, Lin SC, Ryan PM. Hemodialysis and plasma amino acid composition in chronic renal failure. Am J Clin Nutr. 1968;21:565‐573. [DOI] [PubMed] [Google Scholar]
- 23. Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease. Lancet 2017;389:1238‐1252. [DOI] [PubMed] [Google Scholar]
- 24. Kopple JD. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr 2007;6(Suppl 1):1586S‐1590S; discussion 97S‐98S. [DOI] [PubMed] [Google Scholar]
- 25. Swendseid ME, Wang M, Vyhmeister I, et al. Amino acid metabolism in the chronically uremic rat. Clin Nephrol. 1975;3:240‐246. [PubMed] [Google Scholar]
- 26. Boirie Y, Albright R, Bigelow M, Nair KS. Impairment of phenylalanine conversion to tyrosine in end‐stage renal disease causing tyrosine deficiency. Kidney Int. 2004;66:591‐596. [DOI] [PubMed] [Google Scholar]
- 27. van de Poll MC, Soeters PB, Deutz NE, Fearon KC, Dejong CH. Renal metabolism of amino acids: its role in interorgan amino acid exchange. Am J Clin Nutr. 2004;79:185‐197. [DOI] [PubMed] [Google Scholar]
- 28. Marini JC, Didelija IC, Fiorotto ML. Extrarenal citrulline disposal in mice with impaired renal function. Am J Physiol Renal Physiol. 2014;307:F660‐F665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaore SN, Amane HS, Kaore NM. Citrulline: pharmacological perspectives and its role as an emerging biomarker in future. Fundam Clin Pharmacol. 2013;27:35‐50. [DOI] [PubMed] [Google Scholar]
- 30. Cano NJ, Fouque D, Leverve XM. Application of branched‐chain amino acids in human pathological states: renal failure. The Journal of nutrition. 2006;136(1 Suppl):299s‐307s. [DOI] [PubMed] [Google Scholar]
- 31. Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18:71‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]


