Abstract
Background
Serum free light chains detection assays are consistently meeting greater interest for the diagnosis and monitoring of monoclonal gammopathies and plasma cell dyscrasias. Nowadays, there are neither standardized methods nor reference material for the determination of free light chains; for this reason, it is important to compare two different assays used in clinical laboratory.
Methods
We evaluated 300 serum samples from patients with B‐cell disorders and compared the analytical performances of both assay. Each test was assayed on both testing platforms (Siemens Dade Behring BN II Nephelometer and SPAPLUS by The Binding Site). κ/λ ratios were determined and compared. Results were analyzed by Passing‐Bablok and Bland‐Altman plots to evaluate comparability of the two techniques and to determine bias.
Results
The reproducibility of both assays is acceptable, reaching minimum and desirable analytical goals derived from biological variability. However, values are not interchangeable between systems. This study shows that the two systems do not allow results to be transferred from one method to the other even if they display good agreement.
Conclusion
Our study highlights the importance of elaborating an international standard for free light chains quantification in order to offer homogeneous results as well as guarantee harmonization of values among laboratories. Moreover, the assays should be validated in specific patient groups to determine that they are clinically fit for purpose.
Keywords: comparative study, free light chains, multiple myeloma
Abbreviations:
- sFLC
serum free light chains
- FLCs
free light chains
- Igs
immunoglobulins
- SMM
smouldering multiple myeloma
- MM
multiple myeloma
- MCs
monoclonal components
1. Introduction
Immunoglobulin free light chains (FLCs) are normally produced in slight excess by B‐Cells in order to provide correct assembly of intact immunoglobulins (Igs). Nevertheless, only 60% of FLCs are correctly assembled into newly synthesized Igs, whereas the rest are released in the blood circulation giving rise to the serum polyclonal FLC pool. The excess is cleared by catabolic action of enzymes in the proximal tubules of the kidney.1
In physiological conditions, approximately 500 mg of serum FLCs (sFLC) is produced on a daily basis, with a half‐life ranging from 2 to 6 hours. Consequently, as sFLC concentrations are dependent on both their production and renal clearance, any over production as well as renal impairment may contribute to a shift in the normal sFLC concentration, giving rise to abnormal sFLC concentrations.2
Circulating sFLC assays are consistently meeting greater interest in clinical laboratory and many guidelines acknowledge their use in clinical practice for diagnosis, monitoring and follow‐up of monoclonal gammopathies.3, 4, 5, 6, 7, 8, 9, 10
The International Myeloma Working Group has recently included sFLC detection in clinical settings other than monoclonal gammopathies and plasma cell dyscrasias.9 The FLC ratio ≥100, from SLiM CRAB criteria (S: 60% or greater clonal plasma cells; Li: Involved/Uninvolved Light chains ≥100; M: MRI 1 or more focal lesion; C: calcium elevation; R: renal insufficiency; A: anemia; B: bone lesions), is a predictor of imminent progression of smouldering multiple myeloma (SMM) to overt multiple myeloma (MM) and that such patients should be regarded as having MM requiring therapy.11
Serum FLC are involved in a variety of pathological conditions related to natural and acquired immunity;12 therefore, it is plausible that sFLC testing may have clinical indications not yet fully understood.13
As a pioneer of the field, the Freelite assay shows poor post‐dilution linearity and relative imprecision, as well as increased probability of yielding false negative results due to antigen excess in patients with extremely high FLC concentration.14, 15
When using the Freelite assay, laboratories may be faced with several analytical problems including lot‐to‐lot variability of reagents, antigen excess, unrecognizable epitopes, excessive polymerization16, 17, 18 and different results obtained on different platforms as reports of the specific UK‐NEQAS.15, 16, 17, 18, 19
In order to overcome some of these problems, the N Latex assay by Siemens based on monoclonal antibodies was recently introduced to the worldwide market.20
Despite technological advances, there are still only three assays available on the worldwide market, and limited knowledge about their performance with the literature reporting little and conflicting data in regards to their reproducibility and the harmonization of results between methods.21, 22, 23, 24
The core of the problem is that both methods rely on different calibrators, different analytical methods and different references ranges for κ/λ ratio (Freelite 0.26‐1.65; N Latex 0.31‐1.56), that consequently give discordant results. This is despite Siemens assigning the value to N Latex calibrators by measuring FLC with Freelite assay to try to harmonize the FLC determination.
This issue was noted in a recently published article, in which the above mentioned sFLC assays were compared in a multicenter study. The authors conclude that both methods perform very differently, and they advise the use of the same method in routine testing, especially for patient monitoring.21, 25
Due to these discrepancies and confusion generated by conflicting reports, we considered it necessary to perform an accurate analysis of both methods. Our study aims to verify differences and compatibilities between the two methods on two different laboratory platforms. Recently, the IMWG guidelines have highlighted the importance of using an appropriate test for the correct interpretation of the κ/λ ratio in defining different degrees of SMM.26
2. Materials and Methods
This multicenter study was performed using samples obtained from two separate diagnostic centers in Italy (National Cancer Institute “Regina Elena”, Foundation “A. Gemelli” Catholic University of the Sacred Heart, Rome).
Random serum samples submitted for routine analysis from a total of 300 patients, 139 Female (mean age 66±12.6) and 161 Male (mean age 69±9.9) with B‐cell disorders: MM (206), Light Chain Multiple Myeloma (LCMM, 31), Amyloidosis (AL, 2), Monoclonal Gammopathy of Undetermined Significance (MGUS, 51), Plasmacytoma (5), Non‐secretory MM (3) and suspect MM with normal Immunofixation Electrophoresis (2) were collected on the basis of altered FLC ratio, after obtaining informed consent. All clinical diagnoses were determined by hematologists. Moreover, all patients had an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2.
A subset of 50 control samples was obtained from healthy blood donors who had previously been tested for the absence of monoclonal components (MCs), by serum protein electrophoresis, serum and urine Immunofixation Electrophoresis, and had a negative C‐Reactive Protein result. The collected samples were centrifuged at 2500 g for 10 minutes and serum divided in aliquots before being frozen at −80°C and stored until analysis. Samples were thawed only once, keeping them at room temperature and immediately analyzed. The analysis was performed by an operator without knowledge of the clinical history of the samples.
Each sample was tested in parallel on both the SPAPLUS (The Binding Site, Birmingham, UK) and Siemens Dade Behring BN II Nephelometer (Siemens Healthcare Diagnostics Ltd, Erlangen, Germany) analyzers, according to the manufacturer's instructions (hereafter referred to as Freelite, reference method, and N Latex FLC, test method) and all tests were carried out in the same laboratory with the same two analyzers. Normal κ FLC ranges are: 3.3‐19.4 mg/L (Freelite) and 6.7‐22.4 mg/L (N Latex); Normal λ FLC ranges are: 5.7‐26.3 mg/L (Freelite) and 8.3‐27 mg/L (N Latex).
Serum dilutions, where necessary, were performed according to the manufacturer's recommendations. κ/λ ratios were evaluated and compared.
For the repeatability of the new method, the rapid protocol scheme 3×5 (triple×5 days) was performed to verify the statement of the manufacturer, following the Clinical and Laboratory Standard Institute (CLSI) guideline EP‐15 A2. The intra‐assay imprecision was performed using the binding site controls at two different levels, Low (Human Kappa/Lambda Free SPAPLUS Control) and High (Human Kappa/Lambda Free SPAPLUS High Control) and were expressed as CV%.
This operation was done after controls were tested on each relative platform, and results were within the expected range. Inter‐assay imprecision was evaluated with commercial normal and pathological quality controls, on a daily basis. The study was assessed, during 20 days, using different reagent lots and calibrations.27 Method comparison was led according to CLSI EP‐09 A3 guideline.28
This study was approved by institutional ethical committee of the “Istituto Nazionale Dei Tumori Regina Elena” Rome, Italy and conducted according to the guidelines of the Declaration of Helsinki (1964).
2.1. Statistical analysis
The results were analyzed by Bland‐Altman plots, in order to evaluate comparability of the two methods and to estimate the differences. We decided to avoid log‐transformed data in order to have a more dynamic vision of results as a whole, so as to gain knowledge of dispersion. We compared the Freelite vs N Latex assay using Passing‐Bablok regression analysis with determination of the intercept, slope and coefficient of correlation. The scatter of difference was showed on Bland‐Altman Plots. Clinical concordance was assessed by creating a 3 by 3 contingency table accordingly to whether the patients would be classified as having abnormal or normal κ/λ ratio (normal range: 0.26‐1.65).9 The level of agreement was evaluated through Cohen's kappa statistics. Perfect agreement was set for kappa value ≥0.8; good agreement ranging from 0.6 to 0.8 and moderate agreement between 0.4 and 0.6.
All statistical analysis was performed using XLSTAT (Addinsoft SARL, New York, NY, USA). P<.05 was considered statistically significant.
3. Results
For the comparison study, we used 345 samples out of 350, because five of them were outliers and therefore excluded from the data set. The obtained FLCs values from each of the two methods include minimum κ value (0.4 mg/L Freelite assay, 0.6 mg/L N Latex) and maximum κ value (1489 mg/L Freelite assay, 800 mg/L N Latex); minimum λ value (0.5 mg/L Freelite assay, 0.3 mg/L N Latex) and maximum λ value (1756.1 mg/L Freelite assay, 1010 mg/L N Latex).
The intra‐assay imprecision of Binding Site quality controls measured on the Siemens Instrument showed a CV% of 1.78 at low control level of κ FLC (13.1 mg/L) and 1.10% at high control level (32.7 mg/L); while for λ FLC the CV% was 2.40 at low control level (11.9 mg/L) and 0.92% at high control level (31.6 mg/L).
The between‐run imprecision of Binding Site quality controls measured on the Siemens instrument for κ FLC was respectively 4.94% and 3.59% at concentration level of 12.7 and 32.2 mg/L, reaching minimum and desirable analytical goals derived from biological variability; while for λ FLC was 4.18% and 2.63% respectively at concentration levels of 11.5 and 31.5 mg/L, reaching minimum and desirable analytical goals.
The Passing‐Bablok linear regression analysis of κ FLC gave y=3.265+0.806x indicating there was a bias in the y‐intercept (95% CI did not include value zero (2.740‐3.898 mg/L)), and the slope (95% CI did not include value 1 (0.757‐0.850 mg/L)) as displayed in Figure 1.
Figure 1.
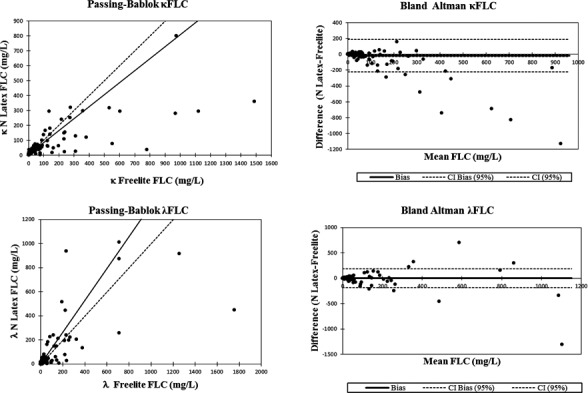
Passing‐Bablok and Bland‐Altman plots of kappa and lambda free light chains respectively
Results of κ FLC for some samples gave discrepancies between the two assays as indicated in Table 1.
Table 1.
(a) Kappa discordant data, (b) Lambda discordant data: values for the same samples are listed per method used for their quantification
| a) Kappa (mg/L) | b) Lambda (mg/L) | ||||
|---|---|---|---|---|---|
| Binding site | Siemens | % Difference | Binding site | Siemens | % Difference |
| 552 | 76 | 151.9 | 233 | 938 | −120 |
| 136 | 292 | −72.7 | 237 | 28.3 | 157 |
| 532 | 317 | 50.7 | 84.2 | 227 | −91.8 |
| 310 | 128 | 83 | 194 | 518 | −91 |
| 1121 | 293 | 117 | 379 | 135 | 95 |
| 969 | 279 | 111 | 168 | 8 | 182 |
| 156 | 15 | 164 | 1754 | 449 | 119 |
| 236 | 22.3 | 166 | 713 | 257 | 94.0 |
| 380 | 120 | 104 | 804 | 3010 | −116 |
| 234 | 107 | 7406 | 222 | 446 | −66.9 |
| 973 | 800 | 19.5 | 219 | 76.5 | 99.3 |
| 166 | 48 | 110 | 148 | 32.2 | 129 |
| 779 | 36 | 183 | 1540 | 268 | 140 |
| 2301 | 19 | 197 | 5976 | 1910 | 103 |
| 310 | 23.5 | 172 | 63.6 | 185 | −97.7 |
| 862 | 279 | 102 | 58.2 | 163 | −94.8 |
| 925 | 227 | 121 | 324 | 204 | 45.5 |
| 1488 | 674 | 75.3 | 1540 | 278 | 139 |
| 199 | 60.8 | 106 | 8085 | 602 | 172 |
| 604 | 293 | 69.3 | 1257 | 917 | 31.3 |
| 3758 | 1720 | 74.4 | 117 | 239 | 68.9 |
| 1525 | 284 | 137 | |||
| 712 | 1010 | −34.6 | |||
The scatter of differences through Bland‐Altman plot pointed out a significant systematic error between two methods (P=.002), showing a bias of −17.55 mg/L, a 95% CI ranging from −28.50 to −6.61 and a standard deviation of difference equal to 103.35 with a 95% limits of agreement from −220.12 to 185.01.
Concerning λ FLC the Passing‐Bablok analysis showed a linear regression equation (y=2.226+1.318x) with constant (95% CI intercept: 1.229‐3.238) and proportional systematic error (95% slope: 1.213‐1.436).
Bland‐Altman plot analysis did not reveal a significant bias between two methods (P=.722) with a mean of 1.83 mg/L (95% CI: −8.25 to 11.91), a standard deviation of 95.17 and 95% limits of agreement of −184.71 to 188.36.
Results of λ FLC for some samples gave discordant results between the two assays as shown in Table 1.
Concordance between two methods, assessed by Cohen's kappa test, displayed a good agreement with a value of 0.61 (Standard error: 0.04; 95% CI: 0.54‐0.69). The Freelite assay identified 164 patients with normal κ/λ ratio (0.26‐1.65) while N Latex assays 212 patients. Outside the upper limit (>1.65), 129 patients were classified by Freelite assay compared with 89 patients by N Latex assay. Fifty‐two patients showed a κ/λ ratio lower than 0.26 on the SPAPLUS while on the Siemens Dade Behring BN II there were 44 patients. An exact match was obtained for 77% of patients (23% discordant) (see Table 2).
Table 2.
Concordance between two methods assessed by Cohen's kappa test
| Binding site | Siemens | Total | ||
|---|---|---|---|---|
| <0.26 | 0.26‐1.65 | >1.65 | ||
| <0.26 | 33 | 19 | 0 | 52 |
| 0.26‐1.65 | 10 | 149 | 5 | 164 |
| >1.65 | 1 | 44 | 84 | 129 |
| Total | 44 | 212 | 89 | 345 |
4. Discussion
As serum FLC analysis is being more frequently requested in clinics, it is of equal importance to validate the analytical systems for diagnosing and monitoring disease states, but also to verify whether the two analytical system available show interchangeable results. This is of crucial importance for those patients requiring monitoring and follow‐up of MCs.
The consequence of the variability of the measurand is that unless a FLC immunoassay can recognize all molecular forms and conformations of the FLC with equimolar reactivity, the different forms will not produce the same FLC result for all patients.
The absence of a reference material, as well as the great difference found between the assays, strongly calls for the need of an international available standard calibrator, in order to standardize the two methods.
In terms of interchangeability, our study demonstrates that the two analyzers do not allow results to be transferred from one method to the other, as values are not totally overlapping and do not reach the perfect agreement.
As we do not know the precise value of the data (due to the lack of an international standard), we cannot define accuracy and thus, we cannot state that this method is more accurate than the N Latex assay.
Data display highest bias found between the assays. This may be due to the difference in methods used by the analyzers. In previous comparison studies, all samples were tested for measurement with Freelite and N Latex assays only on Siemens Dade Behring BN II Nephelometer.29, 30, 31 In this study, we compared the results obtained with the recommended manufacturer's instrumentation to assess if there was a better correlation between data. Our study therefore points to the importance of reaching an international standard, in order to offer interchangeable results among laboratories and instruments.
Although the two methods have different reference ranges for κ/λ ratio, the FLC range reported in Table 2 is justified by its presence in IMWG guidelines as global diagnostic reference range in clinical management.9, 11, 26, 32
At the same time, MCs are also highly variable, so one assay method may be more accurate to determine one type of component, whereas another may be less. As a result of the unpredictable values, they are of no use for monitoring the status of a patient if we use different assays during periodical testing.
Analytical problems related to sFLC quantification, such as lot‐to‐lot variability among reagents and analytical platforms, reduced post‐dilution sample recover and all consequential problems of non‐linearity, as well as the absence of parallelism between the polyclonal calibrator and the sample (mainly constituted of monoclonal FLCs) have been well described and emphasized. The Binding Site has recently published showing there is no longer the great lot‐to‐lot reagent variability for their polyclonal‐based assay.19 Siemens N Latex assay uses monoclonal antibodies and has published on reagent lot‐to‐lot variation.20
This was done in order to ensure all analytical issues are accurately evaluated when considering results obtained with this kind of assay, so they can be progressively overcome by the assay manufacturer. Even if the measurement range of FLCs is from 1‐100 000 mg/L, there is still a possibility of having an antigen excess phenomenon, with a concurring risk of missing diagnosis for a subset of diseases which actually require immediate therapeutic schemes.
Alternatively, mass spectrometry can readily identify a monoclonal FLC from the polyclonal background and identify the isotype of the light chain by top‐down mass spectrometry eliminating the need for reference ranges to determine if a monoclonal FLC is present.33
The development of this assay requires a collaboration between clinics and laboratory services to monitor the entire clinical status of each individual. While there is still a lack of studies concerning biological inter‐ and intra‐individual variability of FLC in serum, it is of increasing urgency to accomplish these analytical goals.34
For the N Latex FLC Siemens assay, it is important to find a significant cut‐off for High risk SMM patients11 and in minimal residue disease27 whom are monitored in their follow‐up testing, because the data reported as a critical threshold refer to the follow‐up testing with the Freelite Binding Site assay and the values cannot be absolutely superimposed. Concordance at these FLC ratio cut‐off points is extremely poor. Most patients with a Freelite FLC κ/λ ratio of 100 or 0.01 would have an N Latex FLC κ/λ ratio <100 and > 0.01, respectively. The clinical consequence is that these patients may or may not meet the criteria of MM requiring therapy depending on whether the FLC assay is performed at a diagnostic laboratory using Freelite or N Latex FLC reagents.15
When changing the analytical platform, it is necessary to assess the transferability of the reference intervals; so, for a predetermined relevant time period, the samples should be analyzed on both the current analyzer/method and the new analyzer/method.
Cigliana G, Gulli F, Napodano C, et al. Serum free light chain quantitative assays: Dilemma of a biomarker. J Clin Lab Anal. 2018;32:e22243 10.1002/jcla.22243
References
- 1. Dimopoulos MA, Kastritis E, Rosinol L, Bladé J, Ludwig H. Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008;22:1485‐1493. [DOI] [PubMed] [Google Scholar]
- 2. Tosi P, Tomassetti S, Merli A, Polli V. Serum free light‐chain assay for the detection and monitoring of multiple myeloma and related conditions. Ther Adv Hematol. 2013;4:37‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson KC, Alsina M, Atanackovic D, et al. NCCN guidelines insights: multiple myeloma, version 3. J Natl Compr Canc Netw. 2016;14:389‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahlis NJ. Darwinian evolution and tiding clones in multiple myeloma. Blood. 2012;120:927‐928. [DOI] [PubMed] [Google Scholar]
- 5. Bird J, Behrens J, Westin J, et al. UK Myeloma Forum (UKMF) and Nordic Myeloma Study Group (NMSG): guidelines for the investigation of newly detected M‐proteins and the management of monoclonal gammopathy of undetermined significance (MGUS). Br J Haematol. 2009;147:22‐42. [DOI] [PubMed] [Google Scholar]
- 6. Bird JM, Cavenagh J, Samson D, Mehta A, Hawkins P, Lachmann H. Guidelines on the diagnosis and management of AL amyloidosis. Br J Haematol. 2004;125:681‐700. [DOI] [PubMed] [Google Scholar]
- 7. Bird JM, Owen RG, D'sa S, et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br J Haematol. 2011;154:32‐75. [DOI] [PubMed] [Google Scholar]
- 8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117:4701‐4705. [DOI] [PubMed] [Google Scholar]
- 9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group guidelines for serum‐free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23:215‐224. [DOI] [PubMed] [Google Scholar]
- 10. Ludwig H, Miguel JS, Dimopoulos MA, et al. International Myeloma Working Group recommendations for global myeloma care. Leukemia. 2014;28:981‐992. [DOI] [PubMed] [Google Scholar]
- 11. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538‐e548. [DOI] [PubMed] [Google Scholar]
- 12. Brebner JA, Stockley RA. Polyclonal free light chains: a biomarker of inflammatory disease or treatment target? F1000 Med Rep. 2013;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Basile U, Mussap M. Rationale and prospects of serum immunoglobulin free light chain (FLC) determination in chronic inflammatory disease. Biochim Clin. 2013;37:357‐364. [Google Scholar]
- 14. Bosmann M, Kößler J, Stolz H, Walter U, Knop S, Steigerwald U. Detection of serum free light chains: the problem with antigen excess. Clin Chem Lab Med. 2010;48:1419‐1422. [DOI] [PubMed] [Google Scholar]
- 15. Jacobs JF, Tate JR, Merlini G. Is accuracy of serum free light chain measurement achievable? Clin Chem Lab Med. 2016;54:1021‐1030. [DOI] [PubMed] [Google Scholar]
- 16. Tate J, Bazeley S, Sykes S, Mollee P. Quantitative serum free light chain assay‐analytical issues. Clin Biochem Rev. 2009;30:131‐140. [PMC free article] [PubMed] [Google Scholar]
- 17. Jacobs JFM, Hoedemakers RMJ, Teunissen E, Van der Molen RG, Te Velthuis H. Effect of sample dilution on two free light chain nephelometric assays. Clin Chim Acta. 2012;413:1708‐1709. [DOI] [PubMed] [Google Scholar]
- 18. Vercammen M, Meirlaen P, Broodtaerts L, Broek IV, Bossuyt X. Effect of sample dilution on serum free light chain concentration by immunonephelometric assay. Clin Chim Acta. 2011;412:1798‐1804. [DOI] [PubMed] [Google Scholar]
- 19. Carr‐Smith HD, Jenner EL, Evans JA, Harding SJ. Analytical issues of serum free light chain assays and the relative performance of polyclonal and monoclonal based reagents. Clin Chem Lab Med. 2016;54:997‐1003. [DOI] [PubMed] [Google Scholar]
- 20. Te Velthuis H, Knop I, Stam P, et al. N Latex FLC—new monoclonal high‐performance assays for the determination of free light chain kappa and lambda. Clin Chem Lab Med. 2011;49:1323‐1332. [DOI] [PubMed] [Google Scholar]
- 21. Lock RJ, Saleem R, Roberts EG, et al. A multicentre study comparing two methods for serum free light chain analysis. Ann Clin Biochem. 2013;50(Pt 3):255‐261. [DOI] [PubMed] [Google Scholar]
- 22. Hoedemakers RM, Pruijt JF, Hol S, et al. Clinical comparison of new monoclonal antibody‐based nephelometric assays for free light chain kappa and lambda to polyclonal antibody‐based assays and immunofixation electrophoresis. Clin Chem Lab Med. 2011;50:489‐495. [DOI] [PubMed] [Google Scholar]
- 23. Pretorius CJ, Klingberg S, Tate J, Wilgen U, Ungerer JP. Evaluation of the N Latex FLC free light chain assay on the Siemens BN analyser: precision, agreement, linearity and variation between reagent lots. Ann Clin Biochem. 2012;49:450‐455. [DOI] [PubMed] [Google Scholar]
- 24. Campbell JP, Heaney JL, Shemar M, et al. Development of a rapid and quantitative lateral flow assay for the simultaneous measurement of serum κ and λ immunoglobulin free light chains (FLC): inception of a new near‐patient FLC screening tool. Clin Chem Lab Med. 2017;55:424‐434. [DOI] [PubMed] [Google Scholar]
- 25. Tate J, Mollee P. Towards improved measurement of serum free light chains: clinical and laboratory issues. Biochim Clin. 2013;37:395‐404. [Google Scholar]
- 26. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328‐e346. [DOI] [PubMed] [Google Scholar]
- 27. Clinical and Laboratory Standards Institute (CLSI) . User verification of performance for precision and trueness. CLSI document EP15‐A2. Wayne, PA: CLSI, April 2006. [Google Scholar]
- 28. CLSI . EP09‐A3‐Measurement Procedure Comparison and Bias Estimation Using Patient Samples; Approved Guideline, 3rd edn, 2013;33:1‐98. [Google Scholar]
- 29. Mahmood S, Wassef NL, Salter SJ, et al. Comparison of free light chain assays: Freelite and N latex in diagnosis, monitoring, and predicting survival in light chain amyloidosis. Am J Clin Pathol. 2016;146:78‐85. [DOI] [PubMed] [Google Scholar]
- 30. Kennard A, Hawley C, Tate J, et al. Comparison of Freelite™ and N Latex serum free light chain assays in subjects with end stage kidney disease on haemodialysis. Clin Chem Lab Med. 2016;54:1045‐1052. [DOI] [PubMed] [Google Scholar]
- 31. Di Noto G, Cimpoies E, Dossi A, et al. Polyclonal versus monoclonal immunoglobulin‐free light chains quantification. Ann Clin Biochem. 2015;52(Pt 3):327‐336. [DOI] [PubMed] [Google Scholar]
- 32. Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437‐1444. [PubMed] [Google Scholar]
- 33. Barnidge DR, Dispenzieri A, Merlini G, Katzmann JA, Murray DL. Monitoring free light chains in serum using mass Spectrometry. Clin Chem Lab Med. 2016;54:1073‐1083. [DOI] [PubMed] [Google Scholar]
- 34. Braga F, Infusino I, Dolci A, Panteghini M. Biological variation of free light chains in serum. Clin Chim Acta. 2013;415:10‐11. [DOI] [PubMed] [Google Scholar]


