Abstract
Objective
To explore the role of serum periostin in patients with aneurysmal subarachnoid hemorrhage (aSAH).
Method
We conducted a retrospective study and 124 aSAH patients treated in Shenzhen People's hospital during March 1st 2015 to December 30th 2016 were included. Baseline information, neurological status and clinical outcome were recorded. Blood samples on admission were collected and enzyme linked immunosorbent assay (ELISA) kits were used to detect the serum level of periostin. Spearman's Correlation Analysis was used to analyze the correlation between periostin and clinical severity. Receiver operating characteristic (ROC) curve was performed to investigate variables’ prognostic value in patients with aSAH.
Results
The average age of patients included was 57.23 years old. Preliminary analysis revealed that serum periostin was significantly correlated with clinical severity. Patients with poor outcome at 12 months had higher level of periostin than patients with good outcome. Multivariate logistic regression analysis showed elevated level of periostin was significantly associated with poor outcome and the AUC was 0.85 for periostin in predicting poor outcome of patient with aSAH.
Conclusion
Elevated serum periostin concentrations are significantly associated with clinical severity and poor outcome of aSAH patients, which indicate serum periostin can be used as a prognostic biomarker in patients with aSAH.
Keywords: biomarkers, DCI, outcome, periostin, subarachnoid hemorrhage
1. INTRODUCTION
Aneurysmal subarachnoid hemorrhage (aSAH) is a fateful neurological disorder which affects millions of people and aSAH always results in high mortality and disability, as well as high medical expenses.1 It was estimated that the annual incidence of aSAH worldwide was about 9/100 000 person/year2 and as many as 29% of aSAH patients died in the first 3 month.3 What's more, more than 1/3 of aSAH patients suffered cognitive impairment and experienced a decline of quality of life.4 Cerebral vasospasm (CVS) and early brain injury (EBI) are regarded as the main causes of poor outcome of patients with aSAH.5 Accumulated evidences have showed that inflammatory response, oxidative stress and peripheral immune response are significantly associated with CVS6 and EBI.7 Recently, Biochemical markers involving in the pathologic process after SAH have been proven to be early diagnostic indexes for predicting clinical outcome of patients with aSAH.4, 8
Periostin is a 90 KD secreted protein that is broadly expressed in the body, including stomach, breast, uterus and thyroid tissue.9 Previous studies have identified periostin as a biomarker of inflammation10, 11 and oxidative stress12 and evidences also showed that periostin played a key role in promoting tumor progression under hypoxia.13, 14 What's more, Matsunaga E reported that periostin was expressed at higher level in primate cerebral cortex than mouse cerebral cortex and what's more, periostin exhibited neurite outgrowth activity.15 Periostin was observed in neurons and increased in astrocytes at 24 hours after transient middle cerebral artery occlusion (tMCAo), which indicated that periostin might play an important role in the pathologic process after stroke.16 Recently, study showed that serum periostin concentrations in patients with traumatic brain injury (TBI) was significantly higher than healthy individuals and level of periostin was significantly associated with clinical severity.17 While in SAH mouse model, periostin neutralization could alleviate EBI. 1 μg r‐periostin could significantly aggravated brain edema after SAH,18 which indicated that periostin may play a important role in pathologic process after SAH. While no study has revealed the role of serum periostin in patients with aSAH till now. So we performed this study to explore the role of periostin in patients with aSAH.
2. METHOD
2.1. Study population
This was retrospective study and all included patients were treated in the department of neurosurgery of Shenzhen People's hospital during March 1st 2015 to December 30th 2016. Patients were diagnosed as aneurysmal subarachnoid hemorrhage by head computed tomography (CT) and further confirmed by subsequent head CT angiography (CTA) or digital subtraction angiography (DSA). We excluded patients whose blood sample collection was more than 72 hours from symptom onset. Patients who were accompanied with infectious diseases, autoimmune disease or serve organ dysfunction 2 weeks before hospitalization and those who had history of neurological diseases were also excluded. All included patients received standard treatments which included hemostasis, reduce cerebral edema, prophylactic anti‐seizure and anti‐vasospasm before surgery. All participants or their relatives were informed and an consent was written for each patients. Our study was approved by the ethical committee at Shenzhen People's hospital.
2.2. Clinical assessment
Baseline information (sex, gender, comorbidities, etc.) of patients included were collected. Neurological assessment was conducted by using Glasgow Coma scale (GCS) and hunt‐hess grade. Fisher grade was accessed for each patients based on the presentation of head CT on admission. Head CT was routinely performed for all patients on the first day and at any time during hospital stay if patients suffered clinical deterioration. Delayed cerebral ischemia (DCI) was defined as previous described.19 All 12‐month follow‐up was performed by using telephone interviews and poor outcome was defined as Glasgow outcome scale (GOS) score 1‐3 and good outcome was defined as GOS score 4‐5.
2.3. Blood samples measurements
Two milliliters of blood samples were collected in early morning and immediately centrifuged at 2000 g for 15 minutes at −4°C. The serum was collected and stored at −80°C. Human Periostin ELISA Kit (RAB1075, SIGMA) was used to detect serum level of periostin according to the manufacture's instruction.
2.4. Statistical analysis
The descriptive analysis (mean, standard deviation, median) was used for continuous variables and percentage for categorical variables. t test or One‐Way ANOVA analysis was used for the comparisons between groups for continuous variables. Box‐plot was used to present levels of periostin between different groups. In order to assess the risk factors of poor outcome, variables were first analyzed by performing univariate logistic regression analysis and then multivariate logistic regression analysis would be used to analyzed the factors with P < .1. Receiver operating characteristic (ROC) curves were used to accessed the variables’ prediction ability. Statistical analyses were conducted by using SPSS 21.
3. RESULTS
3.1. Baseline information
Finally, there were 124 patients with aSAH included in this study. There were 78 females and 46 males with an overall average age of 57.23 years old. There were 41 patients with Hypertension, 16 patients with Diabetes and 24 current smokers. Among these included patients, there were 47 patients with Hunt‐hess grade III‐IV and 77 patients with Hunt‐hess grade I‐II on admission. The average GCS score was 11.84 (range from 3 to 15). There were 133 intracranial aneurysms with 94 located in anterior circulation artery and 39 in posterior circulation artery. Details of demographic information were showed in Table 1.
Table 1.
Baseline information of patients included
| Variables | N (%) |
|---|---|
| Female (%) | 78 (62.90) |
| Age (y) | 57.23 ± 8.77 |
| Accompanied diseases | |
| Hypertension | 41 (33.06) |
| Diabetes | 16 (12.90) |
| Smoking | 21 (16.94) |
| GCS | 11.84 ± 3.29 |
| Hunt‐hess | |
| I‐II | 77 (62.09) |
| III‐IV | 47 (37.91) |
| Fisher | |
| I‐II | 56 (45.16) |
| III‐IV | 68 (54.84) |
| Acute hydrocephalus | 13 (10.48) |
| Intraventricular hemorrhage | 32 (25.81) |
| Admission time (h) | 12.78 |
| Aneurysm locations | |
| Anterior circulation artery | 94 (75.81) |
| Posterior circulation artery | 29 (24.19) |
| Surgical approaches | |
| Clipping | 86 (69.35) |
| Coiling | 38 (30.65) |
3.2. Level of periostin correlated with clinical severity
Correlation analysis revealed that periostin was significantly correlated with clinical severity. Correlation coefficient was −0.44 between serum periostin level and GCS score on admission. Besides, periostin was positively correlated with Fisher grade and Hunt‐hess grade ((r = 0.25, 95% CI [0.07‐0.41], P < .01), (r = −.19, 95% CI [0.01‐0.35], P = 0.19, respectively). Details were presented in Figure 1.
Figure 1.
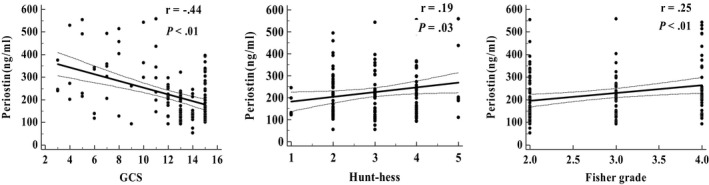
Correlation between periostin and clinical severity
3.3. Serum periostin concentrations in aSAH patients
There were 12 patients suffered with DCI during hospital stay and patients with DCI had higher level of serum periostin than those with non‐DCI (343.75 ± 64.65 vs 211.32 ± 112.04, P < .01). Figure 2. Besides, level of periostin was elevated in patients with poor outcome at 12 month than patients with good outcome (328.48 ± 121.40 vs 186.30 ± 85.69, P < .01).
Figure 2.
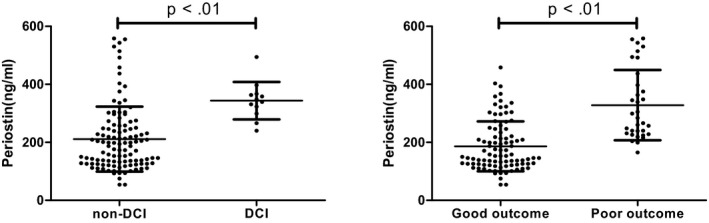
Level of serum periostin concentrations in aSAH patients
3.4. Prognostic effect of periostin on poor outcome
Multivariate regression analysis were used to analyze risk factors associated with poor outcome of patients with aSAH after using univariate analysis. The results showed that current smoking, DCI, high grade Fisher grade (III‐IV) and elevated level of periostin were significantly associated with poor outcome. Results of the mutivariate analyses are shown in Table 2. Besides, we also performed multivariate logistic regression analysis of occurrence of DCI and the results showed high serum concertration of periostin was an independent risk factor of DCI. Results were presented in Table 3. ROC was used to accessed the value of periostin in predicting poor outcome. The AUC of periostin, Hunt‐hess grade and Fisher grade in predicting poor outcome was 0.85, 0.73, 0.70, respectively, which indicated that periostin was an good diagnostic role in predicting poor outcome of aSAH patients (Figure 3 and Table 4).
Table 2.
Univariate and Multivariate logistic regression analysis of risk factors associated with poor outcome
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | 1.03 (0.99‐1.08) | .18 | ‐ | ‐ |
| Female | 0.44 (0.20‐0.99) | .05 | 0.94 (0.24‐3.67) | .93 |
| Hypertension | 0.99 (0.41‐2.33) | .96 | ‐ | ‐ |
| Diabetes | 0.91 (0.23‐3.59) | .89 | ‐ | ‐ |
| Current smoking | 5.21 (1.94‐13.99) | <.01 | 6.04 (1.22‐12.85) | .03 |
| Intraventricular hemorrhage | 1.96 (0.69‐3.91) | .51 | ||
| GCS | 0.85 (0.76‐0.95) | <.01 | 1.18 (0.95‐1.46) | .13 |
| Hunt‐hess grade III‐IV | 5.58 (2.14‐8.32) | <.01 | 3.02 (0.73‐12.41) | .13 |
| Fisher grade III‐IV | 2.56 (1.52‐4.29) | <.01 | 2.72 (1.13‐6.55) | .03 |
| acA | 1.48 (0.67‐2.98) | .71 | ‐ | ‐ |
| Clipping | 1.32 (0.53‐3.29) | .55 | ‐ | ‐ |
| DCI | 19.35 (3.96‐44.49) | <.01 | 9.10 (2.12‐17.99) | .04 |
| Periostin (ng/mL) | 1.01 (1.00‐1.02) | <.01 | 1.02 (1.01‐1.03) | <.01 |
Table 3.
Univariate and Multivariate logistic regression analysis of risk factors associated with DCI
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age | 1.12 (1.04‐1.20) | <.01 | 1.09 (1.00‐1.17) | .04 |
| Female | 0.56 (0.17‐1.84) | .34 | ‐ | ‐ |
| Hypertension | 1.15 (0.32‐4.07) | .83 | ‐ | ‐ |
| Diabetes | 5.72 (1.44‐12.75) | .01 | 4.70 (1.10‐20.69) | .04 |
| Current smoking | 0.42 (0.05‐3.43) | .42 | ‐ | ‐ |
| Intraventricular hemorrhage | 1.04 (0.99‐1.09) | .13 | ||
| GCS | 0.93 (0.79‐1.09) | .36 | ‐ | ‐ |
| Hunt‐hess grade III‐IV | 3.08 (0.91‐10.38) | .07 | 0.50 (0.10‐2.90) | .44 |
| Fisher grade III‐IV | 2.26 (1.06‐4.48) | .04 | 1.67 (0.70‐3.97) | .25 |
| Clipping | 1.45 (0.84‐3.48) | .91 | ‐ | ‐ |
| Periostin (ng/mL) | 1.01 (1.00‐1.02) | <.01 | 1.01 (1.01‐1.01) | .03 |
Figure 3.
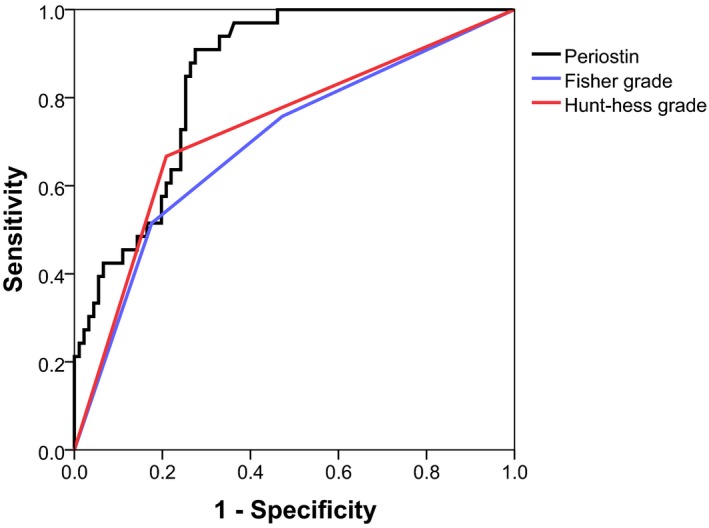
Values of periostin, Fisher grade and Hunt‐hess in predicting poor outcome in patients with aSAH
Table 4.
Diagnostic value of variables for poor outcome of aSAH patients
| Variables | Sensitivity (%) | Specificity (%) | AUC (95%CI) |
|---|---|---|---|
| Periostin | 90.91 | 72.53 | 0.85 (0.78‐0.91) |
| Fisher grade | 51.52 | 82.42 | 0.70 (0.61‐0.78) |
| Hunt‐hess grade | 66.67 | 79.12 | 0.73 (0.64‐0.81) |
4. DISCUSSION
Periostin is a extracellular matric protein which haven't been investigated in aSAH. In this retrospective study, we found that patients with poor oucome had higher level of periostin than patients with good outcome at 12 months. Level of periostin was negatively correlated with GCS on admission and positively correlated with hunt‐hess grade and fisher grade. Further analysis revealed that serum level of periostin was an independently associated with poor outcome and DCI. Additional analysis revealed that the AUC of periostin, Hunt‐hess grade and Fisher grade in predicting poor outcome was 0.85, 0.73, 0.70, respectively. Consequently, serum periostin could be used as an biomarker in patients with aSAH.
Previous studies have identified periostin was involved in process of inflammation, oxidative stress and it played a key role in promoting tumor progression under hypoxia. Periostin have been investigated as a biomarker in various diseases. Periostin was found to plays an important role in repairing the biological matrix of the lung and serum periostin can predict clinical progression both in asthma and idiopathic pulmonary fibrosis.20 Besides, periostin was found to involve in the progression of cancer and metastatic processes, as well as acting an regulator of epithelial mesenchymal transition (EMT).21 Serum periostin was elevated in patient with advanced non‐samll cell lung cancer and could be used as an reliable marker to predict patients’ chemotherapy response and survival.22 Few studies have explored the role of periostin in neurological diseases till now. Matsunaga et al reported that periostin was expressed at higher level in primate cerebral cortex than mouse cerebral cortex and periostin exhibited neurite outgrowth activity in brain tissues.15 Recent studies have revealed that periostin was overexpressed in neural stem cell (NSCs) following hypoxia ischemia and hypoxia could increased periostin expression.13, 23 Hypoxia, oxidative stress and inflammatory reaction could be involved in the pathological processes of traumatic brain injury (TBI)24 and stroke,25 so periostin was supposed to elevated in these neurological diseases. Periostin1 and periostin2 (its splicing variant) were both investigated in transient middle cerebral artery occlusion (tMCAo) model. The results showed that periostin 2, not periostin 1 was overexpressed at 24 hours after tMCAo. Exogenously injection of periostin 2 could reduce infarct volume, which indicated that periostin 2 exhibit anti‐hypoxia property and an protective effect.16 While on the contrary, previous study found that periostin was higher expressed in heart failure and inhibited expression of periostin could increase survival rate, which indicated that inhibition of periostin might be a potential theraphy for heart failure.26 Another study conducted by Xiao‐Qiao Dong et al revealed its role in traumatic brain injury. Serum periostin in patients with TBI was higher than patients in control group and level of periostin was significantly correlated with GCS. Our study showed similar results to this report. Patients who had DCI during hospital stay had higher level of periostin and periostin concentration was negatively correlated with GCS. While AUC of periostin in predicting 30‐day mortality of patients with TBI was 0.815. Periostin might play different role in pathological procession of TBI and SAH. Previous study demonstrated that anti‐periostin antibody could prevented post‐SAH neurobehavioral impariments and EBI. Recombinant‐periostin significantly aggravated EBI.18 However, serum periostin in patients with SAH hadn't been investigated yet and it was still unknown if periostin could be used as a prognostic biomarker in SAH. This was the first study to explored the role of periostin in patients with SAH. We found periostin was highly expressed in patients with poor outcome. Besides. AUC of periostin was 0.850 in predicting poor outcome at 12 months. Our study proved that perisotin could be used as a prognostic biomarker in patient with SAH.
There were some limitations in this retrospective study. Patients included in this study was relatively small and patients received both clipping and coiling were enrolled, which may caused extra biases because of different brain trauma caused by clipping and coiling. Besides, blood samples were routinely collected in the first morning and patients might treated with certain amount of liquid, which might have some effect on the level of serum periostin. Finally, in this study, we only explored preoperative level of periostin in patients with SAH and dynamic change of serum of periostin haven't been investigated till now.
In conclusion, aSAH patients with poor oucome had higher level of periostin than patients with good outcome at 12 months. Serum periostin was significantly correlated with GCS, Hunt‐hess grade and Fisher grade. AUC was 0.850 of periostin in predicting poor outcome at 12 months which indicated that periostin could be used as a prognostic biomarker in patients with aSAH.
Luo W, Wang H, Hu J. Increased concentration of serum periostin is associated with poor outcome of patients with aneurysmal subarachnoid hemorrhage. J Clin Lab Anal. 2018;32:e22389 10.1002/jcla.22389
REFERENCES
- 1. Ridwan S, Urbach H, Greschus S, von Hagen J, Esche J, Boström A. Health care costs of spontaneous aneurysmal subarachnoid hemorrhage for rehabilitation, home care, and in‐hospital treatment for the first year. World Neurosurg. 2017;97:495‐500. [DOI] [PubMed] [Google Scholar]
- 2. Bogason ET, Anderson B, Brandmeir NJ, et al. The epidemiology of admissions of nontraumatic subarachnoid hemorrhage in the United States. Neurosurgery. 2014;74:227‐229. [DOI] [PubMed] [Google Scholar]
- 3. Bender MT, Wendt H, Monarch T, et al. Small aneurysms account for the majority and increasing percentage of aneurysmal subarachnoid hemorrhage: A 25‐year, single institution study. Neurosurgery. 2017. [DOI] [PubMed] [Google Scholar]
- 4. Lu G, Wong MS, Xiong M, et al. Circulating microRNAs in delayed cerebral infarction after aneurysmal subarachnoid hemorrhage. J Am Heart Assoc. 2017;6:e005363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai J, Xu D, Bai X, et al. Curcumin mitigates cerebral vasospasm and early brain injury following subarachnoid hemorrhage via inhibiting cerebral inflammation. Brain Behav. 2017;7:e790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wu W, Guan Y, Zhao G, et al. Elevated IL‐6 and TNF‐alpha levels in cerebrospinal fluid of subarachnoid hemorrhage patients. Mol Neurobiol. 2016;53:3277‐3285. [DOI] [PubMed] [Google Scholar]
- 7. Savarraj J, Parsha K, Hergenroeder G, et al. Early brain injury associated with systemic inflammation after subarachnoid hemorrhage. Neurocrit Care. 2017;10:1‐9. [DOI] [PubMed] [Google Scholar]
- 8. Pan DS, Yan M, Hassan M, Fang ZB, Chen MT. Plasma 8‐iso‐Prostaglandin F2alpha, a possible prognostic marker in aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2017;469:166‐170. [DOI] [PubMed] [Google Scholar]
- 9. Idolazzi L, Ridolo E, Fassio A, et al. Periostin: The bone and beyond. Eur J Intern Med. 2017;38:12‐16. [DOI] [PubMed] [Google Scholar]
- 10. Izuhara K, Nunomura S, Nanri Y, et al. Periostin in inflammation and allergy. Cell Mol Life Sci. 2017;74:4293‐4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sanchez‐Garcia S, Habernau MA, Quirce S. Biomarkers in inflammometry pediatric asthma: utility in daily clinical practice. Eur Clin Respir J. 2017;4:1356160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu H, Chen L, Xie J, et al. Periostin expression induced by oxidative stress contributes to myocardial fibrosis in a rat model of high salt‐induced hypertension. Mol Med Rep. 2016;14:776‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo X, Xue H, Shao Q, et al. Hypoxia promotes glioma‐associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF‐beta and M‐CSFR. Oncotarget. 2016;7:80521‐80542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Li F, Gao F, et al. Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget. 2016;7:40148‐40159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Matsunaga E, Nambu S, Oka M, Tanaka M, Taoka M, Iriki A. Periostin, a neurite outgrowth‐promoting factor, is expressed at high levels in the primate cerebral cortex. Dev Growth Differ. 2015;57:200‐208. [DOI] [PubMed] [Google Scholar]
- 16. Shimamura M, Taniyama Y, Katsuragi N, et al. Role of central nervous system periostin in cerebral ischemia. Stroke. 2012;43:1108‐1114. [DOI] [PubMed] [Google Scholar]
- 17. Dong XQ, Yu WH, Du Q, et al. Serum periostin concentrations and outcomes after severe traumatic brain injury. Clin Chim Acta. 2017;471:298‐303. [DOI] [PubMed] [Google Scholar]
- 18. Liu L, Kawakita F, Fujimoto M, et al. Role of periostin in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2017;48:1108‐1111. [DOI] [PubMed] [Google Scholar]
- 19. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41:2391‐2395. [DOI] [PubMed] [Google Scholar]
- 20. O'Dwyer DN, Moore BB. The role of periostin in lung fibrosis and airway remodeling. Cell Mol Life Sci. 2017;74:4305‐4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lindsley A, Snider P, Zhou H, et al. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage‐restricted periostin enhancer. Dev Biol. 2007;307:340‐355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y, Yuan D, Yao Y, Sun W, Shi Y, Su X. Predictive and prognostic value of serum periostin in advanced non‐small cell lung cancer patients receiving chemotherapy. Tumour Biol. 2017;39:1393391297. [DOI] [PubMed] [Google Scholar]
- 23. Park SY, Piao Y, Jeong KJ, Dong J, de Groot JF. Periostin (POSTN) regulates tumor resistance to antiangiogenic therapy in glioma models. Mol Cancer Ther. 2016;15:2187‐2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McAteer KM, Corrigan F, Thornton E, Turner RJ, Vink R. Short and long term behavioral and pathological changes in a novel rodent model of repetitive mild traumatic brain injury. PLoS ONE. 2016;11:e160220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doden T, Sato H, Sasahara E, et al. Clinico‐radiological characteristics and pathological diagnosis of cerebral amyloid angiopathy‐related intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25:1736‐1745. [DOI] [PubMed] [Google Scholar]
- 26. Katsuragi N, Morishita R, Nakamura N, et al. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806‐1813. [DOI] [PubMed] [Google Scholar]


